Abstract
Laminin β2 is a component of laminin-521, which is an important constituent of the glomerular basement membrane (GBM). Null mutations in laminin β2 (LAMB2) cause Pierson syndrome, a severe congenital nephrotic syndrome with ocular and neurologic defects. In contrast, patients with LAMB2 missense mutations, such as R246Q, can have less severe extrarenal defects but still exhibit congenital nephrotic syndrome. To investigate how such missense mutations in LAMB2 cause proteinuria, we generated three transgenic lines of mice in which R246Q-mutant rat laminin β2 replaced the wild-type mouse laminin β2 in the GBM. These transgenic mice developed much less severe proteinuria than their nontransgenic Lamb2-deficient littermates; the level of proteinuria correlated inversely with R246Q-LAMB2 expression. At the onset of proteinuria, expression and localization of proteins associated with the slit diaphragm and foot processes were normal, and there were no obvious ultrastructural abnormalities. Low transgene expressors developed heavy proteinuria, foot process effacement, GBM thickening, and renal failure by 3 months, but high expressors developed only mild proteinuria by 9 months. In vitro studies demonstrated that the R246Q mutation results in impaired secretion of laminin. Taken together, these results suggest that the R246Q mutation causes nephrotic syndrome by impairing secretion of laminin-521 from podocytes into the GBM; however, increased expression of the mutant protein is able to overcome this secretion defect and improve glomerular permselectivity.
Basement membranes (BMs) are sheets of specialized extracellular matrix that underlie all endothelial and epithelial cells and surround all muscle cells, fat cells, and peripheral nerves. They influence cell proliferation, differentiation, migration, and survival. BMs are also involved in filtration, in tissue compartmentalization, and in maintenance of epithelial integrity. The kidney glomerular BM (GBM) is an unusually thick BM formed via fusion of distinct BMs assembled by podocytes and glomerular endothelial cells.1 The GBM, like all basement membranes, contains members of four classes of proteins: laminin, type IV collagen, nidogen, and sulfated proteoglycans.2
Laminins are heterotrimeric glycoproteins consisting of α, β, and γ chains. There are five α, four β, and three γ chains that assemble to form at least 15 different heterotrimers.3 The major laminin heterotrimer in the mature GBM is laminin α5β2γ1, or LM-521.4 During glomerulogenesis there are transitions in laminin chain gene expression such that the laminin α1β1γ1 (LM-111) and α5β1γ1 (LM-511) trimers present in the immature GBM are replaced by LM-521 during maturation.5,6
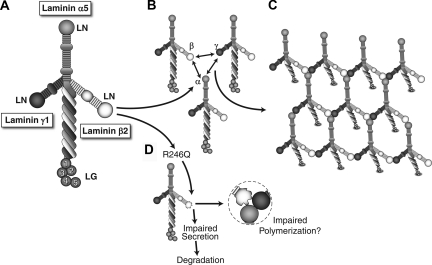
Laminin trimerization occurs in the endoplasmic reticulum (ER) and involves association of the three chains along their α-helical laminin coiled-coil domains to form the long arm7 (Figure 1A). Once trimers are secreted into the extracellular space, they polymerize to form a supramolecular network via interactions among the α, β, and γ short arm NH2 termini, known as LN domains8 (Figure 1, B and C). The large COOH-terminal laminin globular (LG) domain of α chains mediates laminin and BM interactions with cellular receptors. In this way, laminin polymerization both initiates basement membrane formation and provides signals to the adjacent cells.9
Figure 1.
Laminin trimers polymerize in the extracellular matrix via LN domain interactions. (A) Structure of a typical cruciform laminin αβγ heterotrimer, with some domain names indicated. (B, C) Mechanism for polymerization of laminin trimers. Polymerization requires interactions among the laminin N-terminal (LN) domains of the α, β, and γ chains. (D) The proposed mechanisms of the R246Q missense mutation causing congenital nephrotic syndrome. It may inhibit laminin secretion from podocytes, eventually leading to degradation intracellularly. In addition, the affected R246Q in the LN domain of laminin β2 may impair the polymerization of laminin trimers to form the GBM.
Mutations in five different laminin genes cause human disease.3 Of relevance here, Pierson syndrome (OMIM 609049) is caused by mutations in LAMB2, which encodes laminin β2.10 Also called microcoria-congenital nephrosis syndrome, Pierson syndrome is a rare autosomal recessive disease characterized by congenital nephrotic syndrome/diffuse mesangial sclerosis, distinct ocular abnormalities including microcoria (small pupils), muscular hypotonia, and impairment of vision and neurodevelopment.11–13 Children affected by Pierson syndrome usually die within days or weeks after birth from renal failure. However, with dialysis and therapeutic nephrectomy, a few have lived for up to 2 years. The features of the syndrome are consistent with the phenotype of mice lacking laminin β2, which exhibit congenital proteinuria and develop nephrotic syndrome,14 abnormal neuromuscular junctions,15–18 and abnormalities in the retina.19
Of the few LAMB2 mutations that have been described in Pierson syndrome, most are frameshift or nonsense mutations that prevent production of the full length β2 protein.10 Interestingly, R246W, a missense mutation affecting a highly conserved arginine in the NH2-terminal LN domain, greatly reduces the accumulation of β2 in the GBM (by approximately sevenfold), resulting in severe although slightly delayed kidney disease.10 This indicates that R246 is likely crucial for some aspect of LM-521 synthesis, secretion, or stability. More recently, Hildebrandt and colleagues reported the existence of LAMB2 missense mutations that cause congenital nephrotic syndrome, but with milder extrarenal features.20 One of these mutations, R246Q, affects the same conserved arginine, but the switch to glutamine is apparently a less severe alteration. These important findings show that LAMB2 can be the target gene in congenital nephrotic syndrome and that mutational analysis of LAMB2 should be considered when mutations cannot be found in NPHS1, NPHS2, or WT1.20
Here we investigated the mechanisms whereby the R246Q-LAMB2 missense mutation causes nephrotic syndrome, using a knockout/transgenic approach to replace endogenous mouse laminin β2 with different levels of R246Q mutant rat β2. Together with in vitro studies, our results suggest that in patients carrying the R246Q mutation, secretion of the mutant laminin trimer into the GBM is defective, leading to a paucity of laminin and/or an altered GBM laminin composition. Our studies also indicate that high-level expression of the mutant gene can overcome the secretion defect and maintain the structural integrity of the filtration barrier. We conclude that therapies that can increase either expression or secretion of the mutant form should improve selectivity of the defective glomerular filtration barrier.
RESULTS
Generation and Characterization of Podocyte-Specific R246Q Mutant Laminin β2 Transgenic Mice
To investigate the mechanisms whereby R246Q mutant laminin β2 (R246Q-LAMB2) causes congenital nephrotic syndrome, we sought to replace the endogenous wild-type mouse β2 in the GBM with the R246Q mutant rat form (technically R249Q due to rat versus human signal peptide length variation) using our previously described knockout/transgenic strategy.21,22 Briefly, transgenic expression of the full length wild-type rat β2 cDNA in podocytes (via the nephrin promoter) is sufficient to prevent proteinuria in Lamb2−/− mice. This is proof of principle that podocyte-derived LM-521 and expression from the nephrin promoter is sufficient to establish and maintain a normal filtration barrier.
Here we generated three independent lines of transgenic mice expressing a full length rat β2 cDNA with an engineered R246Q mutation. Transgene expression was assayed by both quantitative confocal immunofluorescence microscopy and in situ hybridization in transgenic Lamb2−/− animals so that endogenous mouse Lamb2 expression would not impact the results. As shown in Figure 2A, immunostaining of kidney sections with mouse monoclonal antibodies that recognize rat but not mouse laminin β2 showed that transgene-derived R246Q-laminin β2 was deposited into the GBM. Mice of line 1 exhibited the weakest expression, mice of line 3 exhibited the highest expression, and mice of line 2 exhibited an intermediate level, as indicated by both glomerular total fluorescence intensity and GBM segment intensity; this pattern was consistent in all transgenic animals tested. In parallel, transgene-derived mRNA was readily detected by in situ hybridization in lines 2 and 3 in a podocyte-specific pattern. Although no in situ signal was observed for line 1, we also could not detect endogenous mouse Lamb2 mRNA in glomeruli of control mice, indicating that low levels of gene expression are nevertheless sufficient for the required levels of wild-type LM-521 protein synthesis (Figure 2B). Next we compared the level of line 3 transgene expression to the level in Lamb2−/− mice expressing wild-type rat β2. Although in situ signals were higher in the line 3 mutant, rat β2 protein levels were higher in the wild-type β2 transgenic (Figure 2C). These data show that even with transcription levels higher than that of the wild-type transgene, mutant protein levels are lower. (For simplicity, lines 1, 2, and 3 transgenics will hereafter be referred to as TgLo, TgMed, and TgHi, respectively.)
Figure 2.
Quantitative confocal immunofluorescence and in situ hybridization reveals different levels of NEPH-R246Q-LAMB2 transgene expression in the three independent lines. (A) Confocal immunofluorescence intensities of transgene-derived rat R246Q-LAMB2 protein in the GBM showed the different expression levels in the three lines of transgenic mice (approximately 12 weeks). As shown in the histogram, similar differences were observed in sections through whole glomeruli (total fluorescence intensity) and across segments of GBM (GBM segment intensity). n = 15 glomeruli and 60 GBM segments for each line. **P < 0.001 and *P < 0.05 by t test. (B) Transgene-derived mRNA was readily detected by in situ hybridization in lines 2 and 3, but not in line 1 or in control (approximately 12 weeks). (C) Although the laminin β2 mRNA level was higher in the line 3 mutant compared with that in the wild-type rat β2 transgenic (Tg-WT) (approximately 12 weeks), R246Q-LAMB2 protein deposition in the GBM in line 3 was significantly reduced compared with that of wild-type LAMB2. n = 20 glomeruli and 80 GBM segments for each genotype in the histogram. **P < 0.001. Transgenes were on the Lamb2−/− background in all cases. Original magnifications: ×800 for immunofluorescence, ×200 for in situ hybridization.
Proteinuria in Lamb2−/−;NEPH-R246Q-LAMB2 Mice
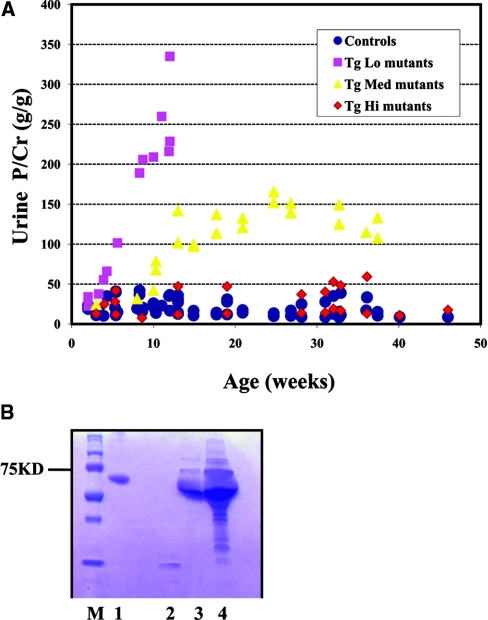
To assay the in vivo function of the R246Q-LAMB2 protein in the GBM, we analyzed mice carrying the transgenes on the Lamb2 null background in which all laminin β2 in the GBM must derive from the mutant transgene. (In all cases these mice also carried a muscle-specific wild-type rat β2 transgene (MCK-B2) that rescues the otherwise lethal neuromuscular junction defects.21) Because proteinuria is the earliest and most sensitive clinical indicator of glomerular dysfunction, proteinuria was closely monitored. The three lines of Lamb2−/−;NEPH-R246Q-LAMB2 mice (hereafter referred to as Tg mutants) were tested weekly during the first month, then monthly thereafter, for albumin/protein and creatinine in the urine. Some were followed to 9 to 12 months of age. TgHi mutants developed very mild proteinuria by 9 months, whereas the TgLo mutants developed mild proteinuria by 1 week and severe proteinuria by 3 months. The severity of proteinuria in the TgMed mutants was intermediate (Figure 3A) and first became detectable at 3 weeks. Importantly, even TgLo mutants showed far less proteinuria than their nontransgenic Lamb2−/− littermates (Figure 3B), and this was associated with greatly prolonged survival. Taken together, these results suggest that proteinuria in Tg mutants is inversely correlated with the level of R246Q-LAMB2 in the GBM, and that even the lowest observed expression provides significant benefit.
Figure 3.
Proteinuria in Lamb2−/−; NEPH-R246Q-LAMB2 mutant mice inversely correlates with the level of transgene expression. (A) Urinary protein/creatinine ratios at different ages in the three lines of Lamb2−/−;R246Q-LAMB2 mice and in control littermates. The high expressors (TgHi) developed only very mild proteinuria by 9 months, whereas the low expressors (TgLo) developed severe proteinuria by 3 months. The severity of proteinuria in TgMed mutants was intermediate. (B) Proteinuria in TgLo mutant mice at 1 month of age. Urine (1 μl) was analyzed by SDS-PAGE. Lane M, markers; lane 1, bovine serum albumin; lane 2, urine from a Lamb2+/− mouse; lane 3, urine from a Lamb2−/−;R246Q-LAMB2 mouse; lane 4, urine from a Lamb2−/− mouse.
Effects of R246Q-LAMB2 on GBM Composition
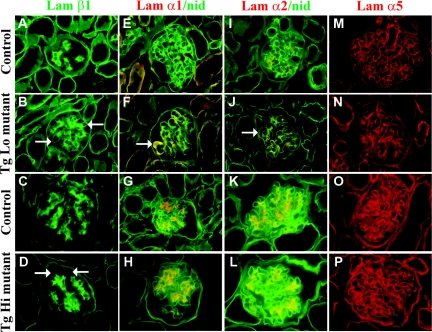
Laminin α, β and γ chains assemble with each other to form specific heterotrimers. During glomerular maturation, a developmental shift in the laminin components of the GBM occurs, from LM-111 to LM-511 to LM-521.7 In the absence of laminin β2, laminin β1 persists in the GBM,14 and laminins α1, α2, α3, β3, and γ2 are ectopically deposited.22 Similarly, in Alport syndrome there is abnormal accumulation of laminins α2 and β1,23,24 suggesting the existence of a compensatory response to abnormal GBM that might also be pathogenic. Therefore, we investigated how the replacement of wild-type laminin β2 with R246Q affected the GBM's laminin repertoire. We examined laminin chain deposition shortly after the onset of proteinuria using immunofluorescence co-localization assays. In normal kidneys the GBM lacked laminins β1, α1, and α2 (Figure 4, A, E, and I). In contrast, there was increased deposition of these laminins in the GBM of TgLo and TgMed mutants (Figure 4, B, F, and J; and data not shown). However, in TgHi mutants, although there was weak linear staining for laminin β1 in the GBM (Figure 4D), laminins α1 and α2 were found only in the mesangium (Figure 4, H and L). There were no detectable differences in laminin α5 (Figure 4, M through P; and data not shown). Together, these results indicate that increased deposition of R246Q-LAMB2 in the GBM was accompanied by decreased accumulation of potentially pathogenic ectopic laminins and that even the lowest levels of R246Q-LAMB2 prevent much of the ectopic deposition of laminin β1 previously reported in Lamb2−/− mice.
Figure 4.
Increased deposition of R246Q-LAMB2 in the GBM is accompanied by decreased accumulation of ectopic laminins (α1, α2, and β1 chains). Frozen sections of control and TgLo and TgHi Lamb2−/−;R246Q-LAMB2 mutant kidneys were stained for laminins at 2 weeks (TgLo) and 9 months (TgHi) of age. There was mildly increased deposition of laminin β1 in the GBM in R246Q mutants (more so in TgLo mutants; arrows in B and D) as compared with controls (A and C). The normally mesangial laminins α1 and α2 (red in E and G; I and K) were ectopically deposited in the TgLo mutant GBM (red in F and J, arrows), but not in TgHi (red in H and L), as revealed by nidogen colocalization (green in E through L). Compared with wild types (M and O), there was no obvious change in laminin α5 staining in the mildly proteinuric mutants (N and P). Magnification, ×600.
Expression of Slit Diaphragm (SD) Components
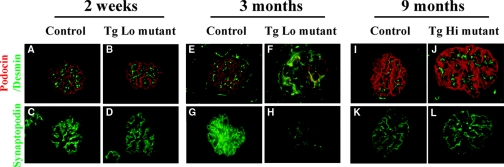
It has been proposed that the podocyte glomerular slit diaphragm is a major contributor to the glomerular barrier to plasma protein.25 Therefore, we examined slit diaphragm and foot process-associated proteins in Lamb2−/−;NEPH-R246Q-LAMB2 mice by immunofluorescence at early stages. TgLo and TgMed mutants had already developed mild proteinuria at 1 and 3 weeks of age, respectively, but expression and localization of podocin and synaptopodin appeared normal (Figure 5, A through D, and data not shown). There was no expression of desmin in mutant podocytes, indicating the lack of an abnormal program of gene expression in response to injury26 (Figure 5, A and B; and data not shown). Similarly, there was no change in podocin, synaptopodin, or desmin in TgHi mutants at 9 months, when they had developed mild proteinuria (Figure 5, I through L). These observations suggest that it is unlikely that proteinuria in Lamb2−/−;NEPH-R246Q-LAMB2 mice is due to changes in SD components. In contrast, adult TgLo and TgMed mutant mice with high levels of proteinuria did express desmin in podocytes and showed reduced levels of podocin and synaptopodin (Figure 5, E through H, and data not shown).
Figure 5.
Early stage proteinuria in LAMB2−/−; NEPH-R246Q-LAMB2 mice is unlikely due to changes in slit diaphragm composition. Frozen sections of control and Lamb2−/−;R246Q-LAMB2 kidneys at 2 weeks (TgLo), 3 months (TgLo), and 9 months (TgHi) of age were stained for podocin (red in A and B; E and F; I and J), desmin (green in A and B; E and F; I and J) and synaptopodin (C and D; G and H; K and L). Compared with controls (A, C, I, and K), there was no change in podocin, synaptopodin, or desmin in the mildly proteinuric mutants (B, D, J, and L). In contrast, in the highly proteinuric TgLo mutant at 3 months, desmin expression was activated in podocytes (green in F), and podocin (red in F) and synaptopodin (H) were greatly reduced. Magnification, ×600.
Glomerular Histologic and Ultrastructural Features
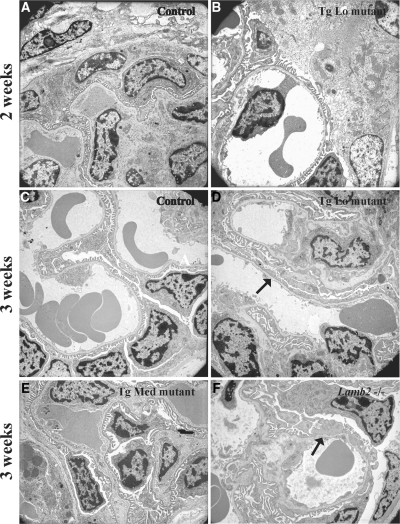
Conventional light and transmission electron microscopy (TEM) were used to examine renal histopathology in the Lamb2−/−;NEPH-R246Q-LAMB2 mice. Consistent with the data from immunofluorescence studies of SD components, there were no obvious ultrastructural abnormalities either in podocytes or in the GBM at up to 2 weeks after birth in TgLo mutants (Figure 6, A and B) and at 3 weeks in TgMed mutants (Figure 6, C and E), despite the proteinuria in both cases. In contrast, ultrastructural changes were noted at 3 weeks in TgLo mutants and in their nontransgenic Lamb2−/− littermates (Figure 6, D and F).
Figure 6.
Transmission electron microscopic analysis shows that podocyte foot process effacement in R246Q mutant mice lags the onset of proteinuria. Glomerular capillary segments from controls (A and C) and a 2-week-old TgLo mutant (B) showed normal interdigitated podocyte foot processes adjacent to the GBM. (D) Foot process effacement (arrow) was observed in the 3-week-old TgLo mutant. Ultrastructural analysis of the TgMed mutant at 3 weeks (E) revealed no obvious pathologic changes. (F) Widespread foot process effacement (arrow) was detected in the nontransgenic Lamb2−/− littermate at 3 weeks. Magnification, ×4360.
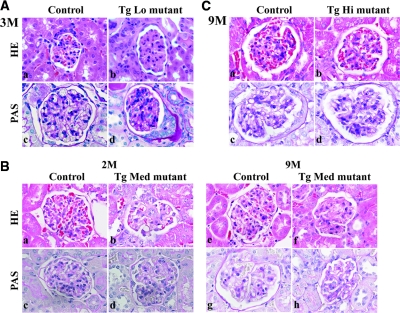
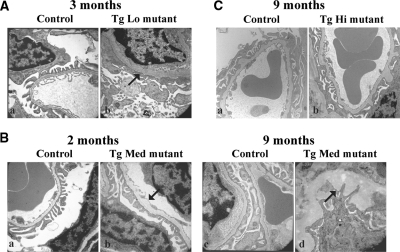
TgLo mutants developed severe proteinuria by 3 months, which was associated with mesangial sclerosis (Figure 7Ab), widespread foot process effacement, and GBM thickening (Figure 7Ad and Figure 8Ab). Similarly, in TgMed mutants, proteinuria increased with age, and light microscopic examination of H&E-stained kidney sections revealed more mesangial sclerosis and interstitial fibrosis (data not shown) at 9 months (Figure 7Bf) compared with 2 months (Figure 7Bb). PAS staining and electron microscopic examination showed more GBM thickening at 9 months (Figure 7Bh and Figure 8Bd) compared with 2 months (Figure 7Bd and Figure 8Bb), and some segments of the GBM were greatly expanded with a moth-eaten appearance at 9 months (Figure 8Bd). In addition, for both TgLo and TgMed mutants, there were abundant protein casts in the tubules when proteinuria became evident (data not shown). These data indicate a progression of disease over time with worsening severity of proteinuria and significant GBM thickening and glomerulosclerotic changes. In contrast to these findings, TgHi mutants showed no obvious histopathological changes in their kidneys at 9 months (Figure 7Cb and 7Cd and Figure 8Cb), other than occasional GBM outpocketing that was also present in TgHi controls (Figure 8C). We conclude that the increased transgene expression and the subsequent increased deposition of R246Q-LAMB2 in the GBM attenuate proteinuria in TgHi mutant mice, which correlates with maintenance of the integrity of the glomerular filtration barrier.
Figure 7.
Histological analysis of kidneys from the three mutant lines demonstrates that glomerulosclerosis and GBM thickening are attenuated by increased expression of R246Q-LAMB2. Paraffin sections of control and mutant kidneys were stained with H&E and PAS. (A) TgLo Lamb2−/−;R246Q-LAMB2 mice at 3 months. H&E staining showed mesangial matrix expansion in the mutant (Ab) compared with the control (Aa). PAS staining revealed GBM thickening in the mutant (Ad) as compared with the control (Ac). (B) Control (Ba, Bc, Be, and Bg) and TgMed Lamb2−/−;R246Q-LAMB2 mutant (Bb, Bd, Bf, and Bh) kidneys at 2 and 9 months (as indicated). At 2 months the mutant showed moderate mesangial sclerosis (Bb). As the mice aged, mesangial sclerosis became more severe (Bf), and there was also GBM thickening (Bh). (C) TgHi Lamb2−/−;R246Q-LAMB2 mutant kidneys at 9 months. H&E and PAS staining of paraffin sections from control (Ca, Cc) and TgHi mutant (Cb, Cd) kidneys revealed no obvious pathology. Magnification, ×600.
Figure 8.
The increased deposition of R246Q-LAMB2 in the GBM is associated with maintenance of the structural integrity of the glomerular filtration barrier. (A) Severe foot process effacement and GBM thickening (arrow) in the TgLo mutant (Ab) compared with the control (Aa). (B) Ultrastructural analysis of control (Ba and Bc) and TgMed mutant (Bb and Bd) kidneys showed much more significant GBM thickening (arrows) at 9 months (Bd) compared with that at 2 months (Bb). (C) Ultrastructural analysis of the glomerular filtration barrier in a TgHi control (Lamb2+/−;R246Q-LAMB2;Ca) and a TgHi mutant (Cb) showed occasional GBM outpocketing in both, but similar podocyte architecture. Magnification, ×15,000.
Impaired Secretion of R246Q-LAMB2 In Vitro
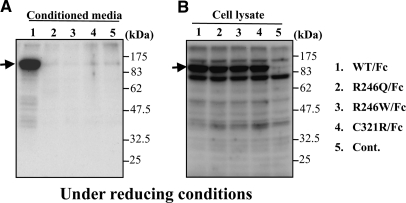
To investigate the mechanisms whereby the R246Q missense mutation inhibited accumulation of laminin β2 in the GBM, despite the apparently high level of transcription (Figure 2), we attempted to synthesize an NH2-terminal fragment of rat laminin β2 containing the R246Q mutation in transfected HEK 293 cells using a system in which the LN and LEa domains were fused to a human Ig Fc domain. Constructs encoding the wild-type fragment, as well as the pathogenic R246W and C321R mutations, were also transfected. Although the wild-type fusion protein was secreted into the medium, the mutant fusion proteins were not (Figure 9A). Analysis of cell lysates showed that the mutant proteins were synthesized, but they remained inside the cells (Figure 9B). These biochemical studies suggest that these mutations are pathogenic because they result in impaired laminin secretion. Together with our transgenic mouse studies, we conclude that the R246Q mutation impairs secretion of LM-521 into the GBM, resulting in subnormal GBM laminin levels and glomerular filtration barrier defects in these mice, as well as in patients carrying the mutation. However, high-level expression of the R246Q mutant in TgHi mutants mostly overcomes the effects of the secretion defect.
Figure 9.
Secretion of mutant laminin β2 fragment/Ig Fc fusion proteins from HEK 293 cells is inhibited. (A) Mutant (lane 2, R246Q; lane 3, R246W; lane 4, C321R) LAMB2/Fc fusion proteins were not secreted from transfected HEK 293 cells, whereas wild type (lane 1) was efficiently secreted. (B) Both wild-type and mutant laminin β2 fusion proteins were synthesized and could be detected in cell lysates (lanes 1 to 4). Negative control, lane 5.
DISCUSSION
Congenital nephrotic syndrome is a genetically heterogeneous disorder. The majority of cases have been attributed to mutations in NPHS1, NPHS2, and WT1. Pierson syndrome, characterized by congenital nephrotic syndrome, microcoria, and neurologic defects, has been associated primarily with null mutations in LAMB2.10,11 In contrast, LAMB2 missense mutations, such as R246Q, cause congenital nephrotic syndrome with milder extrarenal features.20 These findings underscore the importance of LAMB2 mutations in the pathogenesis of congenital nephrotic syndrome,27 but the mechanisms whereby these mutations cause proteinuria have been obscure.
Our results suggest that the mechanism responsible for nephrotic syndrome in patients with the R246Q mutation involves severely impaired laminin secretion. This hypothesis is supported by both our in vitro and in vivo data. In vitro, secretion of mutant LAMB2 fragments was severely inhibited compared with that of wild-type LAMB2 fragments. In vivo, we generated three lines of transgenic mice in which R246Q-LAMB2 mRNA was expressed in podocytes at three different levels, two of which significantly exceeded the endogenous Lamb2 gene's expression level (Figure 2). (Despite the high expression levels compared with the endogenous Lamb2 gene's expression level, we found no evidence of intracellular retention of rat laminin β2, either in the mutant or wild-type LAMB2 transgenics.) Lamb2−/− mice exhibit proteinuria that was attenuated in a dose-dependent fashion by deposition of R246Q-LAMB2 into the GBM. That only the highest expressor could prevent proteinuria (at least for many months) is consistent with a surmounting of the secretion defect. In addition, our results suggest that mutant R246Q-LAMB2 is functional once incorporated into the GBM, although further studies will be required to determine whether or not it functions in a manner equivalent to wild-type LAMB2.
An important question from these findings is how the R246Q mutation causes defective secretion of LAMB2 into the GBM. Maturation of secreted proteins takes place in the ER, where folding of nascent polypeptide chains and posttranslational modifications important for proper structure and function occur.28 Mutations resulting in protein sequence variations can lead to synthesis of misfolded or misrouted proteins unable to reach their destinations.29–32 Misfolded proteins can be retained in the ER and degraded or can aggregate into insoluble structures to cause diverse diseases: cystic fibrosis,33 α1-antitrypsin deficiency,34 retinitis pigmentosa,35 and Alzheimer's disease36 are some examples. Furthermore, in patients with congenital nephrotic syndrome of the Finnish type, some missense NPHS1 mutations cause nephrin to become trapped in the ER.37 Similarly, the underlying molecular pathomechanism causing defective LAMB2 secretion may involve misfolding of the R246Q mutant and its retention in the ER or degradation (Figure 1D). If true, it is conceivable that chemical chaperones may have important therapeutic implications.
Chemical chaperones are low molecular weight compounds that can assist protein folding and rescue trafficking-defective misfolded but otherwise functional mutant proteins, thereby arresting or reversing disease.38 Chemical chaperones are being investigated for the treatment of cystic fibrosis,33,39 α1-antitrypsin deficiency,40 nephrogenic diabetes insipidus,41 Gaucher's disease,42 Fabry's disease,43 and congenital nephrotic syndrome.44 Patients carrying LAMB2 missense mutations may be good candidates for treatment with chemical chaperones, a possibility we are currently investigating using our mice.
A second mechanism causing proteinuria in the R246Q mutant mice may be the altered GBM laminin composition, including the likely reduced levels of LM-521 and the accumulation of ectopic laminin chains that is also apparent in nontransgenic Lamb2−/− mice.22 In support of this, the increased accumulation of R246Q-LAMB2 in the GBM of the TgHi mutants was accompanied by diminished ectopic laminin chain accumulation and proteinuria. One likely explanation for ectopic laminin accumulation is attempted compensation for either subnormal levels of or functional defects in LM-521. That proteinuria preceded widespread foot process and slit diaphragm abnormalities argues against a primary podocyte defect, but the eventual loss of podocin, synaptopodin, and likely other foot process components and activation of desmin expression indicate a podocyte injury that surely contributes to increasing proteinuria.
Finally, that even in the TgHi mutant mice mild proteinuria still developed (after 9 months) renders it likely that a third mechanism may contribute to the pathogenesis of proteinuria. Laminin polymerization is important for maintaining the structural integrity of the basement membrane. In addition, laminin polymerization can induce reorganization of receptors and cytoskeletal components.9 Laminin trimers form a three-dimensional, mesh-like polymer due to interactions among the LN domains of their α, β, and γ subunits (see Figure 1, B and C).45,46 In the mutant, the affected R246, conserved from nematodes to humans, is in the LN domain, which is crucial for trimer polymerization.8 Therefore, our findings raise the possibility that mutant LAMB2-containing trimers may not be functionally perfect, as they may have an impaired ability to polymerize in the extracellular matrix to form and maintain the GBM (Figure 1D). Future biochemical studies will allow us to test this possibility.
CONCISE METHODS
Construction of Mutant Rat Laminin β2 Constructs
The R246Q, R246W, and C321R human laminin β2 mutations were separately engineered into analogous sites of the rat cDNA as c.746_747delinsAG, c.745_747delinsTGG, and c.970T>C (respectively) by site-directed mutagenesis (QuickChange XL; Stratagene, LA Jolla, CA). The R246Q mutant cDNA, along with a 3′ SV40 polyadenylylation signal sequence, was cloned under the control of the mouse 4.2-kb nephrin promoter (NEPH) for expression in podocytes in vivo,47 as previously done with the wild-type β2 cDNA.21
Generation of Podocyte-Specific R246Q-Laminin β2 Transgenic Mice
All animal experiments conformed to the NIH Guide for the Care and Use of Laboratory Animals. The NEPH-R246Q-LAMB2 transgene was digested and gel-purified away from plasmid vector sequences and then microinjected into the pronuclei of single-celled B6CBAF2/J embryos by the Washington University Mouse Genetics Core. For genotyping, tail DNA of founders and their transgenic offspring was subjected to PCR using the following primers: 5′-GAAGCAGCAGAATGAGTTCACAC-3′ (nephrin forward) and 5′-ATACGAAGTTATTCGAAGTCGAG-3′ (vector/loxP sequence between the nephrin promoter and the β2 cDNA).
To allow for functional studies on the Lamb2−/− background, Lamb2−/−;MCK-B2;NEPH-R246Q-LAMB2 mice were generated by standard breeding. The MCK-B2 transgene drives expression of wild-type rat laminin β2 in skeletal muscle and rescues the neuromuscular junction defects in Lamb2−/− mice that normally cause lethality at 3 weeks of age.21 Mice were genotyped as described previously.21
Urinalysis
Urine was collected from mice at various ages by manual restraint. Equal volumes of urine (1 μl) were run on precast 4 to 20% SDS polyacrylamide gels (Invitrogen) stained with Coomassie blue. For quantitation, urinary protein and creatinine concentrations were measured with a COBAS MIRA plus analyzer (Roche Diagnostics).
Light and Electron Microscopy
For light microscopy, kidneys were fixed in 10% buffered formalin, dehydrated through graded ethanols, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin & eosin (H&E) and PAS by standard methods. For TEM, tissues were fixed, embedded in plastic, sectioned, and stained as described previously.14
Antibodies and Immunofluorescence
Mouse mAbs D5 and D7 that recognize the rat laminin β2 COOH-terminal coiled-coil domain48 were purchased from the Developmental Studies Hybridoma Bank (Iowa City, IA). Rat anti-mouse laminin α2 mAb 4H8-2 was purchased from ALEXIS Biochemicals (Axxora). Mouse anti-human desmin clone D33, which cross-reacts with mouse desmin, was purchased from DAKO (Carpinteria, CA). Rat anti-mouse nidogen clone ELM1 was purchased from Millipore (Billerica, MA). Rabbit anti-mouse laminin α5 LEb and L4b domains (antiserum 8948) were described previously.5 Other primary antibodies were gifts from generous colleagues: rabbit anti-mouse laminin β1 and β2 LF domains49 from Takako Sasaki (Max Planck Institute for Biochemistry, Martinsried, Germany); rat anti-mouse laminin α1 mAb 8B350 from Dale Abrahamson (University of Kansas Medical Center, Kansas City, KS); rabbit anti-mouse podocin from Corinne Antignac (Necker Hospital, Paris); rabbit anti-mouse nidogen from Albert Chung (University of Pittsburgh, Pittsburgh, PA); mouse anti-human synaptopodin clone G1, which also recognizes mouse synaptopodin, from Peter Mundel (University of Miami, Miami, FL). Alexa 488– and Alexa 594–conjugated secondary antibodies were purchased from Invitrogen. Immunofluorescence analysis was performed as described previously.21
Confocal Microscopy and Quantitative Image Analysis
Confocal microscopy was used to quantify the levels of laminin β2 deposition in the GBM in the three R246Q mutant and in the one wild-type rat LAMB2 transgenic lines. In the individual comparative studies, 8-μm kidney cryosections of the different genotypes were placed on the same slide and immunolabeled with the same mixture of primary antibodies (D5 and D7) and Alexa 488–conjugated secondary antibody. The slides were then examined under a Nikon TE-2000 scanning laser confocal microscope (Melville, NY), and Z-series images were captured at 0.3-μm intervals. For each genotype, 15 to 20 glomeruli were scanned, and the images were captured on the same day using the same laser intensity, confocal aperture, and gain. Raw confocal images taken from the mid-regions of the glomeruli were imported into Image-J software. Total pixel density within a fixed circle, which was slightly smaller than the smallest glomerular field, was used to measure glomerular immunofluorescence intensity. In addition, the fluorescence intensity across a peripheral GBM segment in each glomerular quadrant was measured. The mean intensity for each genotype was compared statistically using a t test.
In Situ Hybridization
DIG-labeled riboprobes were generated by in vitro transcription of cDNA encoding mouse laminin β2 (nt 1245-2048 of NM_008483) and rat laminin β2 (nt 4882-5581 of NM_012974). Frozen sections (10 μm) were fixed with 4% paraformaldehyde, washed with DEPC-treated PBS, soaked in acetylation solution (0.1 M triethanolamine, pH 8.0, containing 0.25% acetic anhydride), washed with DEPC-treated PBS, incubated with hybridization buffer (50% formamide, 5× SSC, 5× Denhardt's, 250 μg/ml baker's yeast RNA, 500 μg/ml salmon sperm DNA), and hybridized with 0.2 to 0.4 ng/μl cRNA in hybridization buffer overnight at 65°C. After hybridization, slides were washed four times with 0.2× SSC at 65°C for 45 minutes each, with a final wash at room temperature. Slides were then covered with B1 buffer (0.1 M Tris, pH 7.5, 0.15 M NaCl) for 5 minutes, blocked for 1 hour with 10% heat-inactivated goat serum in B1 buffer, and exposed to anti-DIG antibody in 1% heat-inactivated goat serum in B1 buffer overnight at 4°C. The following day, slides were washed in B1 buffer, soaked in B3 buffer (0.1 M Tris, pH 9.5, 0.1 M NaCl, 50 mM MgCl2) for 5 minutes, exposed to developing solution (B3 buffer with 0.4 mM 5-bromo-4-chloro-3-indolyl phosphate and 0.4 mM nitroblue tetrazolium salt) for 6 to 24 hours in the dark at room temperature, washed with 10 mM Tris, pH 7.5, 0.1 mM EDTA, and mounted in glycerol.
Expression of Fc-Tagged Laminin β2 Fragments In Vitro
We used a mammalian expression vector, Fc/pcDNA3.1Zeo, containing a human Fc-Tag to which wild-type and site-directed mutant rat laminin β2 fragments containing the LN and LEa domains were fused. The expression constructs were transfected into HEK 293 cells in 24-well plates using Lipofectamine 2000 (Invitrogen). The next day, growth medium was replaced with 500 μl of serum-free DMEM. After 4 days, conditioned media were harvested and clarified by sequential centrifugation at 500 rpm for 5 minutes and 10,000 rpm for 10 minutes. To obtain cell lysates, cells were lysed with 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10 mM EDTA, 1% NP-40, and protease inhibitor cocktail (Sigma). The lysates were clarified by centrifugation at 10,000 rpm for 10 minutes. Conditioned media and lysates were immunoblotted with anti-human IgG1 Fc antibody (Jackson ImmunoResearch, West Grove, PA). Bound antibodies were visualized with the ECL Western blotting detection system (GE Healthcare Bio-Sciences).
DISCLOSURES
None.
Acknowledgments
We thank Gloriosa Go, Jeanette Cunningham, and Jennifer Richardson for technical assistance; generous colleagues for antibodies; the Mouse Genetics Core for generation and care of mice; and the Washington University Center for Kidney Disease Research (P30DK079333) and the Research Center for Auditory and Visual Studies (P30DC004665) core facilities for valuable assistance. Mice were housed in a facility supported by NIH C06RR015502. This research was supported by NIH grants R01DK078314, R01GM060432, and R21DK074613 to J.H.M. Y.M.C. was supported by NIH grants T32DK007126 and K08DK089015.
Part of this material was presented in abstract form at the 2008 Annual Meeting of the American Society of Nephrology; November 4 through 9, 2008; Philadelphia, PA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Abrahamson DR: Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol 100: 1988–2000, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sasaki T, Fassler R, Hohenester E: Laminin: The crux of basement membrane assembly. J Cell Biol 164: 959–963, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miner JH, Yurchenco PD: Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol 20: 255–284, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Miner JH: Renal basement membrane components. Kidney Int 56: 2016–2024, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR: The laminin alpha chains: Expression, developmental transitions, and chromosomal locations of alpha1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel alpha3 isoform. J Cell Biol 137: 685–701, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miner JH, Sanes JR: Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: Sequence, distribution, association with laminins, and developmental switches. J Cell Biol 127: 879–891, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miner JH: Building the glomerulus: A matricentric view. J Am Soc Nephrol 16: 857–861, 2005 [DOI] [PubMed] [Google Scholar]
- 8. McKee KK, Harrison D, Capizzi S, Yurchenco PD: Role of laminin terminal globular domains in basement membrane assembly. J Biol Chem 282: 21437–21447, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Colognato H, Winkelmann DA, Yurchenco PD: Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol 145: 619–631, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A: Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13: 2625–2632, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Zenker M, Tralau T, Lennert T, Pitz S, Mark K, Madlon H, Dotsch J, Reis A, Muntefering H, Neumann LM: Congenital nephrosis, mesangial sclerosis, and distinct eye abnormalities with microcoria: An autosomal recessive syndrome. Am J Med Genet A 130A: 138–145, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Zenker M, Pierson M, Jonveaux P, Reis A: Demonstration of two novel LAMB2 mutations in the original Pierson syndrome family reported 42 years ago. Am J Med Genet A 138: 73–74, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, Barrow M, Blahova K, Bockenhauer D, Cheong HI, Maruniak-Chudek I, Cochat P, Dotsch J, Gajjar P, Hennekam RC, Janssen F, Kagan M, Kariminejad A, Kemper MJ, Koenig J, Kogan J, Kroes HY, Kuwertz-Broking E, Lewanda AF, Medeira A, Muscheites J, Niaudet P, Pierson M, Saggar A, Seaver L, Suri M, Tsygin A, Wuhl E, Zurowska A, Uebe S, Hildebrandt F, Antignac C, Zenker M: Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat 31: 992–1002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP: The renal glomerulus of mice lacking s-laminin/laminin beta 2: Nephrosis despite molecular compensation by laminin beta 1. Nat Genet 10: 400–406, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Knight D, Tolley LK, Kim DK, Lavidis NA, Noakes PG: Functional analysis of neurotransmission at beta2-laminin deficient terminals. J Physiol 546: 789–800, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nishimune H, Sanes JR, Carlson SS: A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature 432: 580–587, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP: Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature 374: 258–262, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Patton BL, Chiu AY, Sanes JR: Synaptic laminin prevents glial entry into the synaptic cleft. Nature 393: 698–701, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Libby RT, Lavallee CR, Balkema GW, Brunken WJ, Hunter DD: Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. J Neurosci 19: 9399–9411, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, Ozaltin F, Nurnberg G, Becker C, Hangan D, Pohl M, Kuwertz-Broking E, Griebel M, Schumacher V, Royer-Pokora B, Bakkaloglu A, Nurnberg P, Zenker M, Hildebrandt F: Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int 70: 1008–1012, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Miner JH, Go G, Cunningham J, Patton BL, Jarad G: Transgenic isolation of skeletal muscle and kidney defects in laminin beta2 mutant mice: Implications for Pierson syndrome. Development 133: 967–975, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jarad G, Cunningham J, Shaw AS, Miner JH: Proteinuria precedes podocyte abnormalities in Lamb2−/− mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest 116: 2272–2279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kashtan CE, Kim Y, Lees GE, Thorner PS, Virtanen I, Miner JH: Abnormal glomerular basement membrane laminins in murine, canine, and human Alport syndrome: Aberrant laminin alpha2 deposition is species independent. J Am Soc Nephrol 12: 252–260, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Abrahamson DR, Prettyman AC, Robert B, St John PL: Laminin-1 reexpression in Alport mouse glomerular basement membranes. Kidney Int 63: 826–834, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Yaoita E, Kawasaki K, Yamamoto T, Kihara I: Variable expression of desmin in rat glomerular epithelial cells. Am J Pathol 136: 899–908, 1990 [PMC free article] [PubMed] [Google Scholar]
- 27. Hinkes BG, Mucha B, Vlangos CN, Gbadegesin R, Liu J, Hasselbacher K, Hangan D, Ozaltin F, Zenker M, Hildebrandt F: Nephrotic syndrome in the first year of life: Two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics 119: e907–919, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Dobson CM: Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol 15: 3–16, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Dobson CM: Protein misfolding, evolution and disease. Trends Biochem Sci 24: 329–332, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Forloni G, Terreni L, Bertani I, Fogliarino S, Invernizzi R, Assini A, Ribizzi G, Negro A, Calabrese E, Volonte MA, Mariani C, Franceschi M, Tabaton M, Bertoli A: Protein misfolding in Alzheimer's and Parkinson's disease: Genetics and molecular mechanisms. Neurobiol Aging 23: 957–976, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Radford SE, Dobson CM: From computer simulations to human disease: Emerging themes in protein folding. Cell 97: 291–298, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Sanders CR, Nagy JK: Misfolding of membrane proteins in health and disease: The lady or the tiger? Curr Opin Struct Biol 10: 438–442, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Howard M, Welch WJ: Manipulating the folding pathway of delta F508 CFTR using chemical chaperones. Methods Mol Med 70: 267–275, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Lomas DA, Evans DL, Finch JT, Carrell RW: The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature 357: 605–607, 1992 [DOI] [PubMed] [Google Scholar]
- 35. Saliba RS, Munro PM, Luthert PJ, Cheetham ME: The cellular fate of mutant rhodopsin: Quality control, degradation and aggresome formation. J Cell Sci 115: 2907–2918, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Koo EH, Lansbury PT, Jr., Kelly JW: Amyloid diseases: Abnormal protein aggregation in neurodegeneration. Proc Natl Acad Sci U S A 96: 9989–9990, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu L, Done SC, Khoshnoodi J, Bertorello A, Wartiovaara J, Berggren PO, Tryggvason K: Defective nephrin trafficking caused by missense mutations in the NPHS1 gene: Insight into the mechanisms of congenital nephrotic syndrome. Hum Mol Genet 10: 2637–2644, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Cohen FE, Kelly JW: Therapeutic approaches to protein-misfolding diseases. Nature 426: 905–909, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Powell K, Zeitlin PL: Therapeutic approaches to repair defects in deltaF508 CFTR folding and cellular targeting. Adv Drug Deliv Rev 54: 1395–1408, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Burrows JA, Willis LK, Perlmutter DH: Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci U S A 97: 1796–1801, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morello JP, Salahpour A, Laperriere A, Bernier V, Arthus MF, Lonergan M, Petaja-Repo U, Angers S, Morin D, Bichet DG, Bouvier M: Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest 105: 887–895, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW: Chemical chaperones increase the cellular activity of N370S beta-glucosidase: A therapeutic strategy for Gaucher disease. Proc Natl Acad Sci U S A 99: 15428–15433, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fan JQ, Ishii S, Asano N, Suzuki Y: Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med 5: 112–115, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Liu XL, Done SC, Yan K, Kilpelainen P, Pikkarainen T, Tryggvason K: Defective trafficking of nephrin missense mutants rescued by a chemical chaperone. J Am Soc Nephrol 15: 1731–1738, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Cheng YS, Champliaud MF, Burgeson RE, Marinkovich MP, Yurchenco PD: Self-assembly of laminin isoforms. J Biol Chem 272: 31525–31532, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Schittny JC, Yurchenco PD: Terminal short arm domains of basement membrane laminin are critical for its self-assembly. J Cell Biol 110: 825–832, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eremina V, Wong MA, Cui S, Schwartz L, Quaggin SE: Glomerular-specific gene excision in vivo. J Am Soc Nephrol 13: 788–793, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Sanes JR, Engvall E, Butkowski R, Hunter DD: Molecular heterogeneity of basal laminae: Isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol 111: 1685–1699, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sasaki T, Mann K, Miner JH, Miosge N, Timpl R: Domain IV of mouse laminin beta1 and beta2 chains. Eur J Biochem 269: 431–442, 2002 [DOI] [PubMed] [Google Scholar]
- 50. St John PL, Wang R, Yin Y, Miner JH, Robert B, Abrahamson DR: Glomerular laminin isoform transitions: Errors in metanephric culture are corrected by grafting. Am J Physiol Renal Physiol 280: F695–F705, 2001 [DOI] [PubMed] [Google Scholar]