Abstract
The interaction between IgG and Fc-γ receptors in glomeruli contributes to the development of several types of proteinuric glomerular disease, but the involvement of immunological mechanisms in hypertensive renal injury is incompletely understood. Here, we investigated serum IgG levels in SHR-A3 rats, which develop hypertensive injury, and compared them with the injury-resistant SHR-B2 line. At 18 weeks old, SHR-A3 rats had serum total IgG levels nearly twice those of SHR-B2 rats, although subclass IgG2b was undetectable in SHR-A3 rats compared with mean levels (± SEM) of 80.7 ± 12.8 mg/dl (18 weeks) and 116.6 ± 19.0 mg/dl (30 weeks) in SHR-B2 rats. In addition, these two strains had significantly different serum levels of IgG1, IgG2a, and IgG2c; differences persisted at 30 weeks for all subclasses except IgG2a. Genetic mapping revealed that a locus on chromosome 6 linked to IgG subclass levels that affected IgG1, IgG2b, and IgG2c but not IgG2a. The mapped haplotype block contains IgH, suggesting regulation of three of four serum IgG subclass levels in cis. Resequencing revealed variation in the sequence of the Fc portion of the IgG heavy chain, which predicts important functional changes. To examine whether there is any relationship between this haplotype block and susceptibility to renal injury, we examined the effect of SHR-A3 and SHR-B2 alleles at this block on albumin excretion in an F2 intercross. Albuminuria doubled with inheritance of SHR-A3 alleles. In summary, allelic variation in IgH or nearby genes may modulate the susceptibility to hypertensive renal injury in SHR-A3 rats.
The pathogenesis of several forms of renal glomerular disease and proteinuria involves interaction between IgG and Fc-γ receptors in glomeruli.1,2 Genetic variation in this pathway has been demonstrated to be an important modifier of susceptibility to glomerulonephritis in humans.1 IgG plays a central role in immune injury by activating the Fc-γ receptor and initiating the complement cascade. Mice, rats, and humans each possess four distinct IgG subclasses defined by the Fc portion of their heavy chain sequences. Although closely related, these subclasses have distinct properties, varying in their capacity to activate complement3,4 and their binding affinity for Fc receptors.5,6
The spontaneously hypertensive rat strain (SHR) was produced by inbreeding of Wistar rats with selection on the trait of elevated BP.7,8 Although all of the SHR animals are descended from two founder Wistar animals, segregation of lines during inbreeding resulted in lines that share hypertension, but the SHR-A3 line notably differs in experiencing susceptibility to brain and renal injury resulting from elevated BP. Inbreeding of the progenitor Wistar line resulted in the normotensive Wistar-Kyoto (WKY) strain. The WKY strain is highly susceptible to renal injury and proteinuria resulting from nephrotoxic serum, and a major modifier of this susceptibility is associated with variation in the Fc receptor locus.1,9 Hypertension also induces renal injury that involves activation of immune signaling components and that can be modified by treatment with immunosuppressants.10–12 Therefore, in this study we examined the role of the IgG/Fc-γ receptor pathway as a potential mediator of renal injury in the injury-prone SHR-A3 rat. We have observed that the Fcgr3 variation contributing to renal injury in WKY rats1 is present in both renal injury-prone SHR-A3 and injury-resistant SHR-B2 rats. In contrast, these two inbred rat lines are highly distinct in their serum IgG subclass levels. Further we have used genetic mapping approaches to determine that serum IgG levels are genetically determined and that a narrowly defined haplotype block containing the IgH locus controls serum IgG levels. Differences in detected serum IgG subclass levels in SHR-A3 occur alongside extensive alteration in the gene coding sequence of the IgG heavy chain exons from which the specific IgG subclass heavy chains are translated.
RESULTS
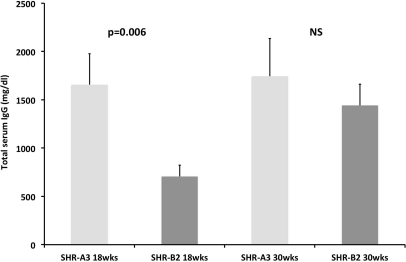
Serum total IgG levels in SHR-A3 and SHR-B2 differed markedly in 18-week-old animals, before the onset of renal injury. However, by 30 weeks of age, total serum IgG levels were not significantly different (Figure 1).
Figure 1.
Serum total IgG levels, measured by ELISA, are increased in 18 wk old SHR-A3 rats compared to SHR-B2.
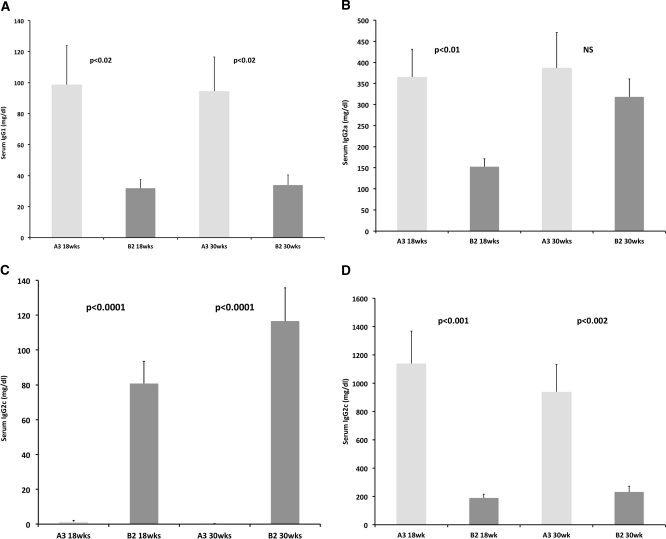
Assessment of IgG subclass levels by ELISA was performed to determine whether the difference in 18-week-old animals was attributable to any IgG subclass. Large statistically significant differences across rat lines in serum IgG subclass levels were observed for IgG1, IgG2a, IgG2b, and IgG2c at 18 weeks of age (Figure 2). These differences were sustained at 30 weeks of age for all subclasses except IgG2a, for which no difference was detected at 30 weeks of age between SHR-A3 and SHR-B2 (P = 0.48). Serum levels of IgG2b were generally undetectable in SHR-A3 at both 18 and 30 weeks of age.
Figure 2.
Serum IgG subclass levels in SHR-A3 and SHR-B2 show persistent differences (IgG1, IgG2b, IgG2c) at 18 and 30wks of age. In SHR-A3, no IgG2b can be detected by ELISA.
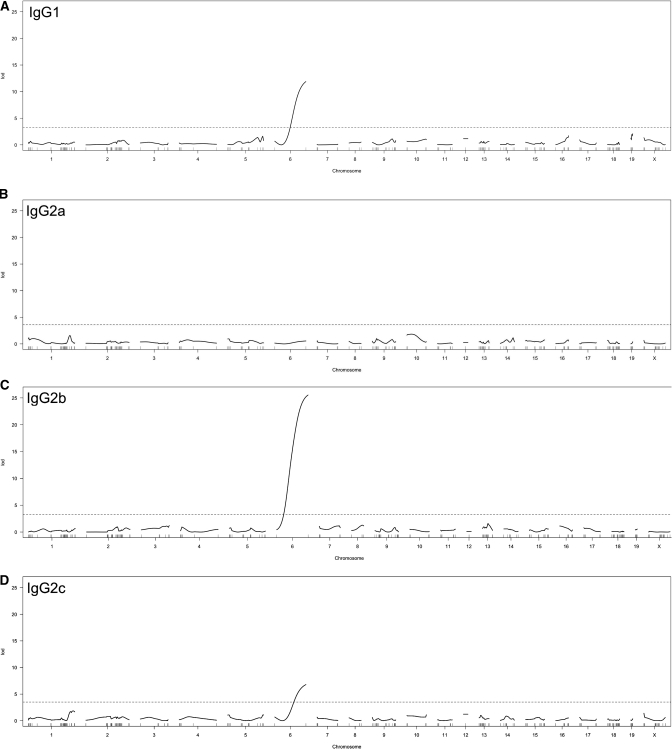
Heritability of IgG subclass levels was assessed using trait variance in F1 and F2 animals (25 weeks of age). Heritability of each subclass (IgG1, IgG2a, IgG2b, and IgG2c) was estimated at 95.5, 59.4, 93.4, and 92.7%, respectively. Because of evidence of heritable factors influencing serum IgG subclass levels, we used genetic mapping to determine whether we could identify a genomic quantitative trait locus (QTL) influencing the level of each serum IgG subclass. For each of the 25-week-old F2 progeny of an SHR-A3 × SHR-B2 intercross, we measured serum IgG subclass levels and determined single nucleotide polymorphism (SNP) genotypes at genomic regions where these two closely related lines were not identical by descent. No significant QTL could be mapped for IgG2a (Figure 3). For each of the other three subclasses, a major QTL was identified that, in every case, mapped to the chromosome 6 haplotype block that contains the Ig heavy chain gene (IgH) from which the IgG isotype subclasses are transcribed. Chromosome 6 is 98% genetically identical by descent between SHR-A3 and SHR-B2. There are two haplotype blocks of nonidentical alleles. The one we have mapped is in the distal part of the chromosome and is tagged by four SNPs (Supplemental Table 1). This block extends over ∼7 Mb and, in addition to the IgH locus, contains 20 rat RefSeq genes. This suggests that genome sequence variation in or near the IgH region influences serum IgG subclass levels.
Figure 3.
Major IgG subclass quantitative trait locus peaks are detected for IgG1, IgG2b and IgG2c each center on the rat IgH locus on chromosome 6 (P<0.00001 for each). The genome-wide LOD scores for IgG subclass levels in the F2 progeny of an SHR-A3 x SHR-B2 cross are plotted. Broken lines indicate LOD threshold (P<0.05) determined by permutation. No LOD peaks achieved statistical significance for IgG2a.
The haplotype block was initially marked by adjacent mapping SNPs that were ∼5 Mb from the IgH gene. Because there were no informative SNPs to indicate the presence of sequence difference in the IgH gene segments encoding the Fc region of IgG, we performed resequencing of the IgH locus focusing on regions encoding the Fc exons of the IgG subclasses to determine whether sequence variation could be detected. GenBank cDNA sequences were used to identify genomic regions encoding the IgH gene. These regions were then amplified from genomic DNA of each parental line and submitted for sequence analysis. The results show that SHR-A3 contains a large amount of sequence variation in this region that includes a high degree of nonsynonymous (Table 1 and Supplemental Table 2) and other nonprotein sequence altering variations (GenBank accession numbers HQ640950-3 and HQ693704-7). In contrast, the genomic sequence in SHR-B2 was much more similar to the rat genome reference sequence (derived from inbred Brown-Norway rats) and to the GenBank IgG subclass sequences obtained largely from PVG rats.5 SHR-A3 appears to have fixed an IgH locus that is highly diverged from that observed in other rat strains (SHR-B2, PVG, and Brown-Norway strain [BN]) and that contains extensive alterations in the predicted amino-acid composition of IgG Fc regions.
Table 1.
Nonsynonymous variation in rat IgG subclass Fc region
| Immunoglobulin Subclass | GenBank Accession Number | Rat Genome (BN) versus GenBank Sequence (PVG) | SHR-A3 versus GenBank Sequence (PVG) | SHR-B2 versus GenBank Sequence (PVG) |
|---|---|---|---|---|
| IgG-1 | M28670 | 0 | 3 | 3 |
| IgG-2a | M28669 | 0 | 7 | 1 |
| IgG-2b | M28671 | 0 | 19 | 2 |
| IgG-2c | X07189 | 0 | 3 | 4 |
| Kabat Position | GenBank (PVG) | Rat Genome (BN) | SHR-A3 | SHR-B2 |
| IgG-1 | ||||
| 195 | Pro | Pro | Pro | Ser |
| 196a | Thr | Thr | Ala | Ala |
| 202 | Val | Val | Ile | Val |
| 359 | Thr | Thr | Ile | Ile |
| IgG-2a | ||||
| 195 | Ser | Ser | Pro | Pro |
| 202 | Val | Val | Ile | Val |
| 234 | Gly | Gly | Val | Gly |
| 235 | Ser | Ser | Pro | Ser |
| 236 | Glu | Glu | Asp | Glu |
| 237 | Val | Val | Ile | Val |
| IgG-2b | ||||
| 162 | Ala | Ala | Pro | Ala |
| 188 | Val | |||
| 189 | Pro | |||
| 215 | Val | Val | Ile | Val |
| 221 | Ile | Ile | Ile | Asn |
| 221a | Gly | Gly | Glu | Gly |
| 221b | His | His | Pro | His |
| 223 | Pro | |||
| 224 | Cys | Cys | Ile | Cys |
| 231 | Val | Val | Ala | Val |
| 361 | Gln | Gln | Lys | Gln |
| 362 | Thr | Thr | Ala | Thr |
| 364 | Ser | Ser | Ile | Ser |
| 369 | Thr | Thr | Ile | Thr |
| 370 | Ser | Ser | Thr | Ser |
| 371 | Gly | Gly | Asp | Gly |
| 376 | Asp | Asp | Asn | Asp |
| 387 | His | His | Arg | Arg |
| 422 | Pro | Pro | Ser | Pro |
| 435 | His | His | Arg | His |
| Kabat Position | GenBank (AO/LOU) | Rat Genome (BN) | SHR-A3 | SHR-B2 |
| IgG-2c | ||||
| 160 | Ser | Ser | Ser | Tyr |
| 210 | Asn | Asn | Asn | Asp |
| 250 | Ile | Ile | Thr | Thr |
| 282 | Val | Val | Val | Ile |
| 351 | Ile | Ile | Phe | Ile |
| 375 | Ala | Ala | Pro | Ala |
Rat genome sequence is derived from the Brown-Norway strain, cDNA sequences are from the PVG strain and an AO/LOU hybrid rat cell line.
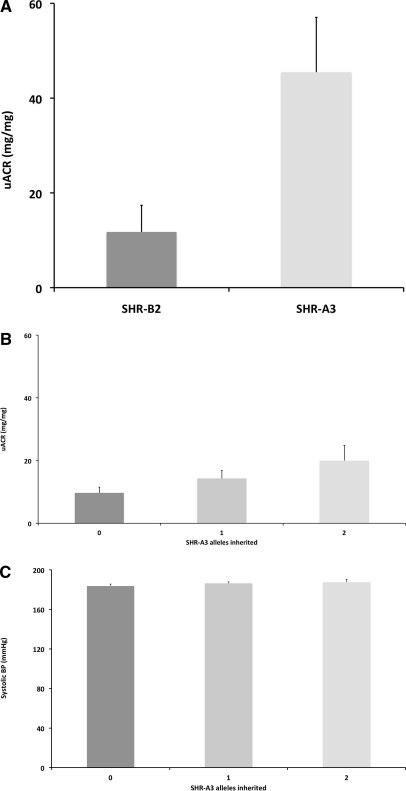
To assess whether genetic variation at this important immunological locus was associated with hypertensive renal injury, a trait by which SHR-A3 and SHR-B2 contrast notably,13 we examined whether urinary albumin excretion, a biomarker of renal injury, was related to the inheritance of SHR-A3 alleles in the haplotype block containing the IgH locus. There was a statistically significant relationship between inheritance of zero, one, or two SHR-A3 alleles at the IgH locus and the level of albuminuria (P < 0.05, determined by linear regression analysis). This correlation predicted a doubling of urinary albumin-creatinine ratio from 9.4 to 18.7 mg/mg in F2 animals inheriting two SHR-A3 alleles compared with F2 animals inheriting only SHR-B2 alleles. Figure 4 shows the mean albumin excretion in the F2 progeny by allelic state at the locus. Alleles at this locus were not associated with statistically significant effects on BP. However, BP may have an important direct effect on albuminuria in these animals, contributing to the portion of albuminuria that is not accounted for by the effects of the chromosome 6 locus on this trait.
Figure 4.
IgH variation is associated with albuminuria, but not blood pressure. (A) Measurement of urinary albumin to creatinine ratio (uACR) in SHR-A3 (n=27) and SHR-B2 (n=19) at 24 to 25 weeks of age demonstrates the emergence of marked albuminuria in SHR-A3 at this age. (B) Inheritance of SHR-A3 IgH locus alleles are associated with increased albuminuria in individual 25 week old F2 SHR-A3 × SHR-B2 rats (P<0.05, n = 49, 90 and 55 F2 rats for zero, one and two SHR-A3 alleles inherited.) (C) Inheritance of SHR-A3 alleles at the IgH locus is not associated with changes in systolic BP measured by telemetry. No significant differences were observed.
DISCUSSION
Genes involved in immunity, protection from infection, and regulation of the inflammatory response may contain variation capable of modulating disease susceptibility. This can reflect pressure for the retention of adaptive variation in these genes. The Fc-γ receptor, which transduces signals initiated by the Fc region of IgG, possesses a copy number polymorphism that contributes to risk of anti-glomerular basement membrane disease.1 Other known elements of the immune signaling pathway contribute to proteinuric disease. For example, IgA nephropathy is attributable to IgA deposition in the glomerulus, immunotactoid glomerulopathy involves damage to glomeruli resulting from deposition of microtubular IgG and complement C3, and heavy chain deposition disease involves IgG deposits in the glomeruli. Although the causes of these diseases are diverse, they have in common a primary pathogenesis involving immunoglobulins or their proximal signaling molecules: complement and Fc receptors.
There are several examples of human IgG allotypes that have been associated with differences in susceptibility to immune and infectious disease.14–20 Such adaptive effects can also confer disadvantages. This is exemplified in the recent observation in humans that the APOL1 gene contains variation that, although conferring resistance to trypanosome infection, creates an increased risk of renal proteinuric disease.21 Our observations in this study demonstrate the existence of extensive, previously unrecognized variation in the rat IgH locus that is associated with altered protein sequence and persistent heritable differences in serum levels of rat IgG subclasses. Our findings also indicate that variation in the IgH locus is positionally associated with a two-fold increase in proteinuria in the context of hypertension. This effect is unrelated to BP because no BP effect of SHR-A3 alleles at this locus was observed (Figure 4). The trait of albuminuria in SHR-A3 is likely to be polygenic and may include effects arising from BP differences in individual F2 animals as well as from other loci containing sequence variation capable of modifying albumin excretion. Thus, we have identified only one element of a complex trait.
We examined the rate of synonymous and nonsynonymous variation across the regions of the IgH locus that we resequenced, comparing variation in the SHR-A3 line with the rat reference genomic sequence (Table 1). We observed 32 nonsynonymous variants and six synonymous variants in SHR-A3. The majority of the nonsynonymous variation was localized to the IgG2a and IgG2b exon sequences (seven and 19 nonsynonymous variants, respectively). Sequence variation in the SHR-A3 IgH locus may have been subject to positive selection, implying adaptive benefit to the sequence change. However, assessment of the evolution of Ig genes, in which gene conversion can be an important cause of sequence variation, requires a more detailed study of the mechanism of divergence of gene sequence than is possible here.22–24
The Fc-γ receptor, to which serum IgG binds to modulate inflammatory signaling, has been shown to contain sequence variation in the WKY rat that amplifies proteinuria in autoimmune glomerular nephritis.1 Because our SHR lines are both derived from the same progenitor stock as WKY, we examined whether the WKY Fc-γ receptor haplotype is present in our SHR lines and confirmed that both lines contain the Fc-γ receptor haplotype previously identified in the WKY rat (data not shown).
After identifying substantial changes in serum IgG subclass levels, we considered the possible origin of such differences. Allelic variation in the transcription factors IRF-1 and STAT6 has been shown to affect serum IgE levels,25,26 and IgE levels can also be affected by transacting variation in the Fcer1a receptor,27 necessitating consideration of the possibility that expression of the IgG isotype subclasses in SHR rat lines is regulated in trans. Another possibility was that sequence variation within or nearby the IgH locus generates cis-acting effects on expression of IgG. Finally the possibility should be considered that nonsynonymous variation in the coding sequence might affect ELISA antigen-antibody interactions, leading to the observed divergence in serum IgG subclass levels. Our use of genetic mapping showed strong single quantitative trait locus effects on abundance of three of the four IgG subclasses. In every case, the controlling locus mapped in cis to the IgH locus. The lack of an identifiable QTL for IgG2a may reflect the loss of differences in IgG2a levels across parental lines from 18 to 30 weeks of age. At 18 weeks of age, the differences in albuminuria between the parental lines are small; thus we waited until 25 weeks of age to phenotype F2 animals and perhaps thereby failed to detect an age-dependent serum IgG2a subclass QTL.
We have not been able to completely resolve the issue of whether heritable effects on serum IgG subclass arise from differences in antiserum specificity for the variant subclass proteins or from difference in level of expression, or both. In the case of IgG2b, which contains the greatest extent of protein dissimilarity (94% identity at the amino-acid level) as well as the greatest difference in serum level measured by ELISA, we have developed some evidence that antiserum specificity plays a role in the observed difference. Thus, although IgG2b is undetectable in SHR-A3 serum using the nondenaturing conditions of ELISA, protein denaturation during gel electrophoresis and subsequent blotting allowed us to detect IgG2b protein in SHR-A3 at a level 10 to 20% of that in similarly-treated SHR-B2 serum. Similarly, in IgG2c, in which protein identity is greater but serum levels are highly divergent by ELISA, denaturation of proteins resulted in the detection of similar levels of antigen across the two lines. Thus, the differences observed in serum IgG subclass levels in ELISA may be influenced, at least in part, by differences in antigenic recognition resulting from amino-acid substitution.
There has been considerable work to assign specific functional attributes to IgG subclasses and to account for these attributes at the level of conserved amino-acid sequences. For example, the rat IgG2b subclass is the only subclass that binds to Fc receptors on human monocytes.6 The residues P-E-L-L-G-G-P located at positions 234 to 238 (numbering system of Kabat et al.28) appear to play an important role in this binding,29 and among rat IgG subclasses, only IgG2b preserves this motif. In SHR-A3 and SHR-B2, these residues are fully retained. However, the adjacent hinge region upstream of this motif may also be required for providing flexibility that facilitates Fc receptor interaction. The hinge region of IgG2b in SHR-A3 is highly divergent from SHR-B2 and from the rat reference sequence, losing one of the cysteine residues participating in stabilization and flexibility of the hinge region. It may therefore be constrained in its ability to modulate Fc receptor signaling. In the CH1 region, V-P residues at positions 188 to 190 are present in all rat IgG isotypes except IgG2b. These residues have been proposed to create a loop in the CH1 region5 that is absent in IgG2b from the rat reference sequence and from SHR-B2, but in SHR-A3 the V-P motif is present and may permit the retention of this loop. Functional implications of this change are uncertain. The principal complement activation motif (amino acids 318 to 322, T-F-R-C-K in IgG1) that is present in the distal CH2 region of each of the four rat subclasses is unaffected by sequence variation in our rat lines. IgG serum half-life is determined by interactions with the neonatal Fc receptor, FcRn. This receptor prevents catabolism of IgG by diverting it from lysosomal pathways and returning it to the serum.30 The CH3 histidine residue at position 435 that is mutated to arginine in IgG2b of SHR-A3 is involved in this interaction.31 The loss of this residue in SHR-A3 IgG2b can be predicted to reduce interaction with FcRn, thereby facilitating IgG2b clearance and reducing serum half-life, perhaps contributing the deficiency in SHR-A3 IgG2b that we observed. Indeed, because the same receptor is essential for maternal-offspring IgG delivery, fetal and neonatal SHR-A3 animals can be predicted to be deficient in maternal IgG2b. In addition to these potential effects that are restricted to Fc-specific functions, there is growing evidence that IgG variable-region binding properties can be influenced by sequence of the Fc region, and this may also drive selection for novel Fc sequence variation.32–36 Given the extent of amino-acid substitution in the SHR-A3 IgH locus, it is likely that important functional attributes uniquely associate with the IgH haplotype present in SHR-A3.
Our studies indicate that IgH locus variation can be one variable influencing susceptibility to proteinuria in these hypertensive rats. BP differences among individual F2 animals may also contribute the variation in albumin excretion. Urinary albumin loss is a complex phenotype. Ongoing studies in our SHR lines seek to determine whether BP alleles segregate in this intercross, whether such alleles are associated with albuminuria, whether albuminuria loci can be mapped at the level of whole genome QTL analysis, and what relationship exists between albuminuria QTL and the regulation of BP.
There is increasing interest in the possible role of inflammation in the genesis and progression of renal injury, including hypertensive renal injury. Although both lines share a variant Fc-γ receptor implicated in autoimmune mechanisms of glomerular injury, the injury-prone line SHR-A3 also contains a highly divergent coding sequence across the Fc region of IgG that is implicated in Fc-γ binding. Variation in this locus in SHR-A3 is associated with a doubling of urinary albuminuria, indicating that IgG may amplify proteinuria in this hypertensive rat line. Human GWAS studies have not implicated the IgH locus in risk of proteinuria. However, it has been pointed out that none of the 18 IgG allotypes in humans is represented in the HapMap variation map that provides the genotyping markers for human GWAS studies.37 Thus, our studies indicate that the allotype-creating IgG Fc variation in humans should be examined for a relationship to susceptibility to proteinuric disease.
CONCISE METHODS
Animals
Studies were performed on male rats of the injury-prone spontaneously hypertensive-A3 (SHR-A3, SHRSP/Bbb) line that have been maintained in our facility for 15 years. We also used male animals from the SHR-B2 line that were bred in our facility from stocks originating from colonies held at Kinki University School of Medicine in Japan. The animals were housed under controlled conditions in an Association for the Assessment and Accreditation of Laboratory Animal Care-approved animal facility and provided a standard rodent chow diet and drinking water ad libitum. No dietary sodium loading was used. BP was measured by implanted telemetry. SHR-A3 (males) and SHR-B2 (females) parental lines were crossed to generate the F1 progeny. This progeny was further crossed to generate a freely segregating F2 progeny. Only male parental, F1, and F2 animals were used in the studies performed. All of the animal use was prospectively reviewed and approved by the authors' University's Animal Welfare Committee.
Serum IgG Levels
We measured total serum IgG and subclass IgG levels by ELISA with commercial assay reagents (Bethyl Labs, Montgomery, TX). According to the vendor, these ELISAs used antisera that were raised against intact, affinity-purified rat IgG antigen and the class-specific antisera were then affinity purified by binding to subclass-specific IgG coupled to agarose beads. The peroxidase-coupled ELISA detection antibodies provided in these assays were used in Western blots to examine antiserum interaction with IgG that had been separated by gel electrophoresis under denaturing conditions. Serum total IgG and serum IgG subclass levels were measured in parental animals at 18 and 30 weeks of age. Serum IgG subclass levels were measured in F1 and F2 animals at 25 weeks of age.
Albuminuria
We measured albuminuria as an urinary albumin:creatinine ratio. Urinary-creatinine concentrations were determined by HPLC in spot urine samples collected from parental, F1, and F2 animals at 25 weeks of age by puncture of the urinary bladder. Urine samples contaminated with red blood cells were discarded. Urine albumin was measured by an ELISA specific for rat serum albumin (Bethyl Labs, Montgomery, TX).
SNP Genotyping, Heritability Estimation, Mapping, and Resequencing
See supplemental methods.
Statistical Analysis
Genome-wide significance of QTL was determined in R/QTL by permutation testing (n = 1000). Association of the inheritance of SHR-A3 and SHR-B2 alleles with traits in the intercross progeny was examined by linear regression of the number of alleles inherited on the trait values.
DISCLOSURES
None.
Acknowledgments
This project was funded in part by National Institutes of Health Grants R01 DK069632 and R01 DK081866 (to P.A.D.) and American Heart Association Grant 09GRNT2240045 (to P.A.D.)
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, Hodges MD, Bhangal G, Patel SG, Sheehan-Rooney K, Duda M, Cook PR, Evans DJ, Domin J, Flint J, Boyle JJ, Pusey CD, Cook HT: Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature 439: 851–855, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Salmon JE, Millard S, Schachter LA, Arnett FC, Ginzler EM, Gourley MF, Ramsey-Goldman R, Peterson MG, Kimberly RP: Fc gamma RIIA alleles are heritable risk factors for lupus nephritis in African Americans. J Clin Invest 97: 1348–1354, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hughes-Jones NC, Gorick BD, Howard JC: The mechanism of synergistic complement-mediated lysis of rat red cells by monoclonal IgG antibodies. Eur J Immunol 13: 635–641, 1983 [DOI] [PubMed] [Google Scholar]
- 4. Medgyesi GA, Fust G, Gergely J, Bazin H: Classes and subclasses of rat immunoglobulins: Interaction with the complement system and with staphylococcal protein A. Immunochemistry 15: 125–129, 1978 [DOI] [PubMed] [Google Scholar]
- 5. Bruggemann M: Evolution of the rat immunoglobulin gamma heavy-chain gene family. Gene 74: 473–482, 1988 [DOI] [PubMed] [Google Scholar]
- 6. Woof JM, Partridge LJ, Jefferis R, Burton DR: Localisation of the monocyte-binding region on human immunoglobulin G. Mol Immunol 23: 319–330, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Okamoto K, Yamori Y, Nagaoka A: Establishment of the stroke-prone spontaneously hypertensive rat. Circ. Res. 34/35: I-143–I-153, 1974 [Google Scholar]
- 8. Okamoto K, Yamori Y, Nosaka S, Ooshima A, Hazama F: Studies on hypertension in spontaneously hypertensive rats. Clin Sci Mol Med 45[Suppl 1]: 11s–14s, 1973 [DOI] [PubMed] [Google Scholar]
- 9. Reynolds J, Cook PR, Ryan JJ, Norsworthy PJ, Glazier AM, Duda MA, Evans DJ, Aitman TJ, Pusey CD: Segregation of experimental autoimmune glomerulonephritis as a complex genetic trait and exclusion of Col4a3 as a candidate gene. Exp Nephrol 10: 402–407, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Kvakan H, Luft FC, Muller DN: Role of the immune system in hypertensive target organ damage. Trends Cardiovasc Med 19: 242–246, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC: Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verlohren S, Muller DN, Luft FC, Dechend R: Immunology in hypertension, preeclampsia, and target-organ damage. Hypertension 54: 439–443, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Dmitrieva RI, Hinojos CA, Boerwinkle E, Braun MC, Fornage M, Doris PA: Hepatocyte nuclear factor 1 and hypertensive nephropathy. Hypertension 51: 1583–1589, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Migot-Nabias F, Noukpo JM, Guitard E, Doritchamou J, Garcia A, Dugoujon JM: Imbalanced distribution of GM immunoglobulin allotypes according to the clinical presentation of Plasmodium falciparum malaria in Beninese children. J Infect Dis 198: 1892–1895, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Namboodiri AM, Budkowska A, Nietert PJ, Pandey JP: Fc gamma receptor-like hepatitis C virus core protein binds differentially to IgG of discordant Fc (GM) genotypes. Mol Immunol 44: 3805–3808, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Hanlon TP, Rider LG, Schiffenbauer A, Targoff IN, Malley K, Pandey JP, Miller FW: Immunoglobulin gene polymorphisms are susceptibility factors in clinical and autoantibody subgroups of the idiopathic inflammatory myopathies. Arthritis Rheum 58: 3239–3246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pandey JP, Luo Y, Elston RC, Wu Y, Philp FH, Astemborski J, Thomas DL, Netski DM: Immunoglobulin allotypes influence IgG antibody responses to hepatitis C virus envelope proteins E1 and E2. Hum Immunol 69: 158–164, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pandey JP, Namboodiri AM, Luo Y, Wu Y, Elston RC, Thomas DL, Rosen HR, Goedert JJ: Genetic markers of IgG influence the outcome of infection with hepatitis C virus. J Infect Dis 198: 1334–1336, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pandey JP, Nasr A, Rocca KM, Troy-Blomberg M, Elghazali G: Significant differences in GM allotype frequencies between two sympatric tribes with markedly differential susceptibility to malaria. Parasite Immunol 29: 267–269, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Pandey JP, Page GP, Silver RM, LeRoy EC, Bona CA: Anti-fibrillin-1 autoantibodies in systemic sclerosis are GM and KM allotype-restricted. Exp Clin Immunogenet 18: 123–129, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morgado MG, Cam P, Gris-Liebe C, Cazenave PA, Jouvin-Marche E: Further evidence that BALB/c and C57BL/6 gamma 2a genes originate from two distinct isotypes. EMBO J 8: 3245–3251, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ollo R, Rougeon F: Gene conversion and polymorphism: Generation of mouse immunoglobulin gamma 2a chain alleles by differential gene conversion by gamma 2b chain gene. Cell 32: 515–523, 1983 [DOI] [PubMed] [Google Scholar]
- 24. Schreier PH, Bothwell AL, Mueller-Hill B, Baltimore D: Multiple differences between the nucleic acid sequences of the IgG2aa and IgG2ab alleles of the mouse. Proc Natl Acad Sci U.S.A. 78: 4495–4499, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schedel M, Frei R, Bieli C, Cameron L, Adamski J, Lauener R, Kabesch M: An IgE-associated polymorphism in STAT6 alters NF-kappaB binding, STAT6 promoter activity, and mRNA expression. J Allergy Clin Immunol 124: 583–589, e1–e6, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Schedel M, Pinto LA, Schaub B, Rosenstiel P, Cherkasov D, Cameron L, Klopp N, Illig T, Vogelberg C, Weiland SK, von Mutius E, Lohoff M, Kabesch M: IRF-1 gene variations influence IgE regulation and atopy. Am J Respir Crit Care Med 177: 613–621, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Weidinger S, Gieger C, Rodriguez E, Baurecht H, Mempel M, Klopp N, Gohlke H, Wagenpfeil S, Ollert M, Ring J, Behrendt H, Heinrich J, Novak N, Bieber T, Kramer U, Berdel D, von Berg A, Bauer CP, Herbarth O, Koletzko S, Prokisch H, Mehta D, Meitinger T, Depner M, von Mutius E, Liang L, Moffatt M, Cookson W, Kabesch M, Wichmann HE, Illig T: Genome-wide scan on total serum IgE levels identifies FCER1A as novel susceptibility locus. PLoS Genet 4: e1000166, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C: Sequences of Proteins of Immunological Interest, Darby, PA, Diane Books Publishing Company, 1992 [Google Scholar]
- 29. Burton DR: Immunoglobulin G: Functional sites. Mol Immunol 22: 161–206, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Simister NE, Jacobowitz Israel E, Ahouse JC, Story CM: New functions of the MHC class I-related Fc receptor, FcRn. Biochem Soc Trans 25: 481–486, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Weng Z, Gulukota K, Vaughn DE, Bjorkman PJ, DeLisi C: Computational determination of the structure of rat Fc bound to the neonatal Fc receptor. J Mol Biol 282: 217–225, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Dam TK, Torres M, Brewer CF, Casadevall A: Isothermal titration calorimetry reveals differential binding thermodynamics of variable region-identical antibodies differing in constant region for a univalent ligand. J Biol Chem 283: 31366–31370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janda A, Casadevall A: Circular dichroism reveals evidence of coupling between immunoglobulin constant and variable region secondary structure. Mol Immunol 47: 1421–1425, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Torres M, Casadevall A: The immunoglobulin constant region contributes to affinity and specificity. Trends Immunol 29: 91–97, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Torres M, Fernandez-Fuentes N, Fiser A, Casadevall A: Exchanging murine and human immunoglobulin constant chains affects the kinetics and thermodynamics of antigen binding and chimeric antibody autoreactivity. PLoS One 2: e1310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Torres M, Fernandez-Fuentes N, Fiser A, Casadevall A: The immunoglobulin heavy chain constant region affects kinetic and thermodynamic parameters of antibody variable region interactions with antigen. J Biol Chem 282: 13917–13927, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Pandey JP: Candidate gene approach's missing link. Science 329: 1148, 2010 [DOI] [PubMed] [Google Scholar]