Abstract
Residual renal function and the integrity of the peritoneal membrane contribute to morbidity and mortality among patients treated with peritoneal dialysis. Glucose and its degradation products likely contribute to the deterioration of the remnant kidney and damage to the peritoneum. Benfotiamine decreases glucose-induced tissue damage, suggesting the potential for benefit in peritoneal dialysis. Here, in a model of peritoneal dialysis in uremic rats, treatment with benfotiamine decreased peritoneal fibrosis, markers of inflammation, and neovascularization, resulting in improved characteristics of peritoneal transport. Furthermore, rats treated with benfotiamine exhibited lower expression of advanced glycation endproducts and their receptor in the peritoneum and the kidney, reduced glomerular and tubulointerstitial damage, and less albuminuria. Increased activity of transketolase in tissue and blood contributed to the protective effects of benfotiamine. In primary human peritoneal mesothelial cells, the addition of benfotiamine led to enhanced transketolase activity and decreased expression of advanced glycation endproducts and their receptor. Taken together, these data suggest that benfotiamine protects the peritoneal membrane and remnant kidney in a rat model of peritoneal dialysis and uremia.
Peritoneal dialysis (PD) is a well accepted alternative to hemodialysis in the treatment of end-stage renal disease. Long-term PD is limited because of structural and functional changes of the peritoneal membrane because of a loss of ultrafiltration, induced by PD fluids (PDF) containing high glucose concentrations and glucose degradation products (GDP).1 Glucose and GDP have not only been shown to be the culprit of peritoneal damage but are also suspected to be causally involved in remnant kidney damage. This is of particular importance because residual renal function (RRF) is highly associated with mortality and morbidity in PD patients.2 A recent animal experiment demonstrated that renal toxicity is mediated by GDP.3 This has been confirmed by two prospective randomized clinical studies—the Balnet trial and recently the DIUREST study—demonstrating a better preservation of RRF in PD patients using low-GDP PDF.4,5 Until today therapeutic options preventing glucose and GDP-induced toxicity are certainly needed. Hitherto, although promising, experimental trials did not find their way into clinical practice.
Benfotiamine (BF; S-benzoyl-thiaminemonophosphate) prevented high-glucose-induced tissue damage by an activation of the enzyme transketolase (TK)6 and pleiotropic direct antioxidative effects in diabetes.7 Until now there are no data available concerning BF in PD or in uremia.
The purpose of our experimental study was to address whether treatment with BF prevents local peritoneal and systemic kidney damage in an animal model of PD therapy.
RESULTS
Animals
Animal Characteristics.
Sprague-Dawley rats receiving peritoneal injections revealed a significantly lower body weight compared with rats without peritoneal injections. The serum creatinine and urea of SNX (SNX) rats were significantly higher than the levels of the Sham-operated animals. BP was significantly higher in SNX rats, and hemoglobin values were significantly decreased in SNX rats (Table 1).
Table 1.
Animal characteristics
| Characteristic | Sham (n = 12) | SNX (n = 12) | SNX+BF (n = 12) | SNX IP (n = 12) | SNX+PD (n = 12) | SNX+PD+BF (n = 12) |
|---|---|---|---|---|---|---|
| Body weight (g) | 526 ± 13.5 | 489 ± 17.9 | 483 ± 18.7 | 447 ± 16.8 | 441 ± 9.16 | 454 ± 5.63 |
| Hemoglobin (g/dl) | 12.9 ± 0.67 | 8.38 ± 0.70b | 8.67 ± 1.06b | 8.30 ± 1.53 | 8.63 ± 0.79 | 8.64 ± 1.39 |
| Serum creatinine (mg/dl) | 0.24 ± 0.02 | 0.70 ± 0.10a | 0.66 ± 0.05a | 0.74 ± 0.03 | 0.73 ± 0.06 | 0.67 ± 0.06 |
| Serum urea (mg/dl) | 41.3 ± 3.50 | 90.1 ± 11.2a | 85.6 ± 9.74a | 87.6 ± 13.0 | 85.6 ± 7.53 | 84.2 ± 10.7 |
| BP (mmHg) | 69.6 ± 5.57 | 85.5 ± 6.64c | 83.6 ± 3.48c | 86.6 ± 5.08 | 84.9 ± 4.23 | 85.3 ± 4.82 |
IP, intraperitoneal.
aP < 0.001 versus Sham;
bP < 0.01 versus Sham;
cP < 0.05 versus Sham.
TK Activity, Reactive Oxygen Species, and Protein Fluorescence in Serum and Urine.
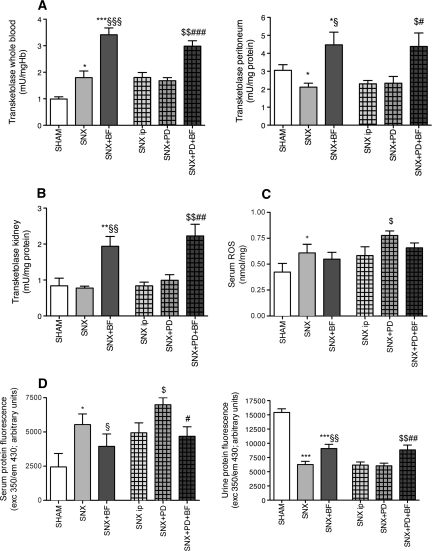
The diversion of metabolites toward the pentose-phosphate pathway via the enzyme TK is dependent on its cofactor BF.6 Blood, peritoneal, and renal TK activity of SNX rats treated with BF was significantly higher compared with the untreated SNX rats (Figure 1, A and B). Glucose and uremia are known to induce the generation of reactive oxygen species (ROS) and protein fluorescence.8,9 SNX rats had higher levels of serum ROS and serum protein fluorescence compared with Sham rats. SNX+BF rats demonstrated lower serum ROS and serum protein fluorescence compared with SNX rats without BF substitution. Compared with Sham and SNX animals treated with BF, SNX rats revealed significantly lower urinary protein fluorescence (Figure 1, C and D).
Figure 1.
Increased TK activity, decreased serum ROS and protein fluorescence, and increased urine proteine fluorescence in animals with BF treatment. Increased blood TK activity was observable in uremic rats receiving BF for 12 weeks (A). Treatment with BF resulted in a higher tissue activity of TK in the peritoneum and in the kidney (A and B). Uremic rats developed higher levels of serum ROS compared with Sham rats. PD treatment accentuates the generation of ROS in uremic rats (C). Serum protein fluorescence as a marker for systemic protein glycation was increased in uremic animals and was escalated when PD treatment was performed but was ameliorated when BF was substituted, presumably because rats treated with BF excreted a higher amount of glycated protein via the urine (D). All results represent mean ± SEM of 12 animals per group. ***P < 0.001 versus Sham, *P < 0.05 versus Sham, §§§P < 0.001 versus SNX, §§P < 0.01 versus SNX, §P < 0.05 versus SNX, $$P < 0.01 versus SNX intraperitoneal, $P < 0.05 versus SNX intraperitoneal, ###P < 0.001 versus SNX+PD, ##P < 0.01 versus SNX+PD, #P < 0.05 versus SNX+PD.
GDP and Advanced Glycation Endproducts and Their Receptor in the Peritoneum.
Peritoneal biopsies of PD patients revealed an enhanced expression of advanced glycation endproducts (AGE) in the peritoneal membrane and a consecutive upregulation of the receptor for AGE (RAGE) during long-term PD treatment.10 In the study presented here, SNX rats treated with BF revealed a lower peritoneal content of methylglyoxal-derived AGE (MG-AGE), Nε-carboxymethyllysine (CML), and RAGE compared with SNX rats not treated with BF. SNX rats receiving PDF instillation had the highest expression of MG-AGE, CML, and RAGE. SNX rats receiving PDF instillation and BF treatment revealed significantly lower MG-AGE, CML, and RAGE expression (Figure 2, A through C).
Figure 2.
Decreased peritoneal expression of AGE and RAGE in rats treated with BF. The peritoneal expression of MG-AGE and CML was highest in animals treated with PD. BF substitution reduced the expression in uremic animals and uremic animals with PD treatment (A and B). Representative photographs of the uremic groups are given in panel B. RAGE was most expressed in the peritoneal membrane of uremic rats treated with PD. A supplementation of BF abrogated the upregulation of RAGE in the peritoneum during long-term PD (C). All results represent mean ± SEM of 12 animals per group; light microscopy of CML magnification = 400×. ***P < 0.001 versus Sham, *P < 0.05 versus Sham, §§P < 0.01 versus SNX, §P < 0.05 versus SNX, $$$P < 0.001 versus SNX intraperitoneal, $$P < 0.01 versus SNX intraperitoneal, $P < 0.05 versus SNX intraperitoneal, ##P < 0.01 versus SNX+PD, #P < 0.05 versus SNX+PD.
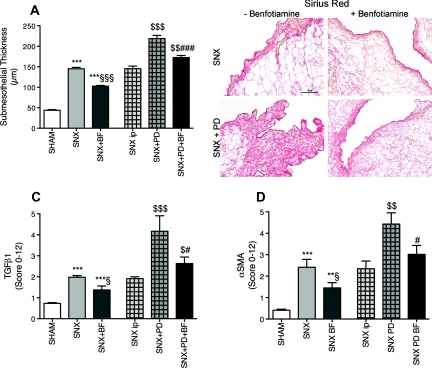
Peritoneal Fibrosis and Epithelial-to-Mesenchymal Transition.
Ongoing PD and uremia per se result in progressive fibrosis and thickening of the peritoneal membrane1 that is mediated by TGF (TGFβ1).10,11 Additionally an epithelial-to-mesenchymal transition (EMT) of human peritoneal mesothelial cells (HPMC) that is indicated by α-smooth muscle actin (αSMA) is discussed.12 Peritoneal thickness, staining for TGFβ1, and αSMA were significantly increased in SNX rats compared with Sham rats. SNX animals treated with BF revealed significantly decreased peritoneal thickness and lower TGFβ1 and αSMA expression compared with SNX rats without BF. SNX rats with PD treatment revealed the highest expression of αSMA and TGFβ1 and the highest increase in the thickening of the peritoneal membrane. PD- and BF-treated SNX rats showed significantly decreased thickening of the peritoneal membrane and staining for TGFβ1 and αSMA compared with PD-treated SNX rats not receiving BF (Figure 3, A through D).
Figure 3.
Reduced peritoneal thickening, fibrosis and EMT in rats with BF treatment. In comparison to Sham rats, uremic rats demonstrated a thickening of the peritoneal membrane that is most enhanced when PD treatment was performed. A substitution of BF reduced the thickening of the membrane (A), apparent in the representative photographs of the peritoneal Sirius Red staining (B). As a marker of ongoing fibrosis, TGFβ1 was evaluated and displayed similar results—an enhancement because of uremia and a reduction when BF was given (C). Similar results were obtainable for the staining for αSMA as a marker for EMT (D). All results represent mean ± SEM of 12 animals per group; light microscopy of Sirius Red magnification = 400×. ***P < 0.001 versus Sham, **P < 0.01 versus Sham, §§§P < 0.001 versus SNX, §P < 0.05 versus SNX, $$$P < 0.001 versus SNX intraperitoneal, $$P < 0.01 versus SNX intraperitoneal, ###P < 0.001 versus SNX+PD, #P < 0.05 versus SNX+PD.
Peritoneal Neovascularization.
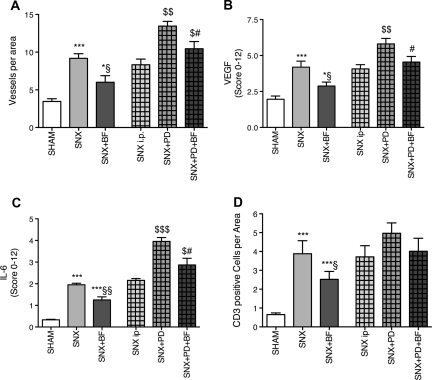
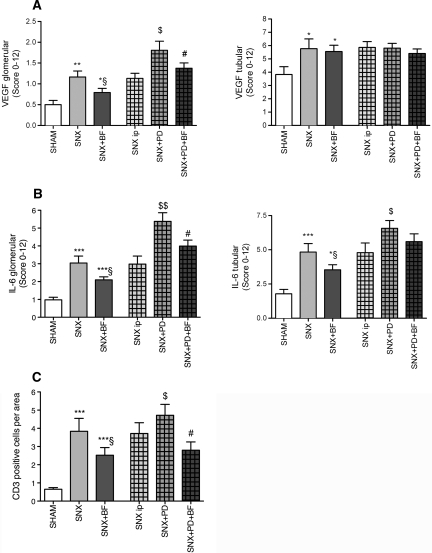
A contributing factor leading to insufficient ultrafiltration of the peritoneal membrane is an increasing number of vessels mediated by vascular endothelial growth factor (VEGF), as shown by De Vriese and co-workers.13 SNX rats demonstrated significantly more vessels per area and significantly increased staining for VEGF compared with control rats. The most pronounced staining for VEGF was observed in PD-treated rats. A substitution of BF was associated with significantly fewer vessels per area and decreased staining for VEGF (Figure 4, A and B, Supplementary Figure 1).
Figure 4.
Diminished peritoneal neovascularization in animals treated with BF. SNX rats developed a significantly increased number of peritoneal vessels compared with Sham rats. BF treatment attenuated peritoneal neovascularization that was most pronounced in PD treatment (A). To confirm these results, staining for VEGF was performed and resulted in similar observations (B). For determination of inflammatory peritoneal formation, staining for IL-6 was performed and CD3-positive leukocytes were counted. Analog results demonstrated an increase of peritoneal inflammation because of uremia that was most present in uremic animals treated with PD and was less apparent when BF was substituted (C and D). All results represent mean ± SEM of 12 animals per group. ***P < 0.001 versus Sham, *P < 0.05 versus Sham, §§P < 0.01 versus SNX, §P < 0.05 versus SNX, $$$P < 0.001 versus SNX intraperitoneal, $$P < 0.01 versus SNX intraperitoneal, $P < 0.05 versus SNX intraperitoneal, #P < 0.05 versus SNX+PD.
Peritoneal Inflammation.
To elucidate the inflammatory status of the peritoneal membrane, CD3-positive leukocytes were counted and the expression of IL-6 was analyzed. SNX rats showed significantly elevated peritoneal staining for IL-6 and a higher amount of CD3-positive cells compared with Sham-operated rats, whereas BF substitution in SNX rats was associated with a significantly reduced staining for IL-6 and CD3-positive cells. SNX rats treated with PD showed the highest expression of IL-6. IL-6 staining was significantly decreased in PD-treated rats when BF was substituted (Figure 4, C and D).
Peritoneal Equilibration Test.
The quality of PD treatment depends on adequate ultrafiltration. An appropriate method to determine the ultrafiltration capacity of the patient's peritoneal membrane is the peritoneal equilibration test.14 The quotient of dialysate and plasma concentration (D/P) of toxic uremic substances allows for determining the transport status of a peritoneal membrane. During the course of PD treatment, most patients convert to a faster transport type with a loss of ultrafiltration capacity because of changes of the peritoneal membrane as described above.15 D/P creatinine showed no significant differences, whereas D/P urea was highest in the SNX rats treated with PDF, demonstrating a faster transport type compared with SNX rats with control injection. SNX rats undergoing PDF instillation treated with BF showed a significantly slower transport type (Figure 5A). D/P sodium as a marker for aquaporin function of the peritoneal membrane did not significantly differ between the groups (data not shown).
Figure 5.
Slower transport type and decreased albuminuria in animals with BF treatment. In the peritoneal equilibration test, the peritoneal transport types of the animals differed. Uremic animals provided a faster transport type compared with Sham rats. Uremic animals with PD treatment showed the fastest transport status, whereas animals treated with PD and BF were comparable to control-injection animals (A). To obtain chronic kidney damage, albuminuria was assessed. Uremic animals treated with BF revealed less albuminuria than untreated uremic rats. Treatment with BF reduced albuminuria in uremic rats with PD treatment compared with SNX rats treated with PD only (B). All results represent mean ± SEM of 12 animals per group. ***P < 0.001 versus Sham, **P < 0.01 versus Sham, §P < 0.05 versus SNX, $P < 0.05 versus SNX intraperitoneal, #P < 0.05 versus SNX+PD.
Albuminuria.
Albuminuria was assessed as an appropriate functional marker of kidney damage. SNX rats developed significantly increased albuminuria compared with Sham rats. Albuminuria was significantly decreased in SNX animals treated with BF compared with SNX rats without BF. The highest levels of albuminuria were seen in SNX rats treated with PDF. SNX rats undergoing PD and BF treatment showed significantly less albuminuria than SNX rats undergoing PD treatment without BF supplementation (Figure 5B).
Histologic Findings of the Kidney.
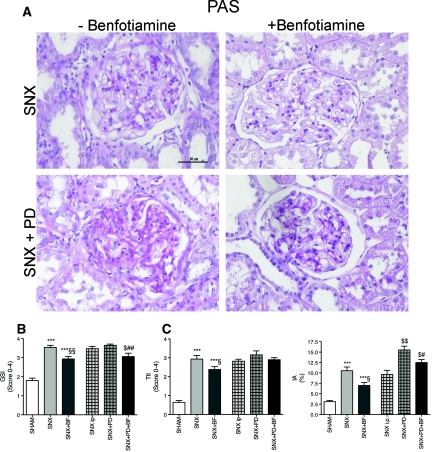
Newer insights in the pathology of PD treatment indicated a systemic toxicity of PDF. Therefore, we investigated histomorphology of the kidneys using appropriate semiquantitative scores for glomerular and tubular kidney injury.3 SNX rats revealed a significantly higher glomerular sclerosis index (GSI), tubulointerstitial injury index (TII), and interstitial area of the tubulointerstitium (IA) compared with Sham rats. SNX rats treated with BF showed significantly lower GSI, TII, and IA than untreated SNX rats. SNX animals receiving PD treatment revealed the highest GSI, TII, and IA. SNX rats treated with PDF and BF revealed significantly lower GSI and IA compared with PD-treated SNX rats without BF substitution (Figure 6, A through D).
Figure 6.
Increased morphologic kidney damage in animals not receiving BF. To obtain substantial damage of the remnant kidney in the SNX model, different established scores describing glomerular and tubular changes were estimated using PAS stained cross sections (A). SNX rats revealed an increased GSI (B), an elevated TII (C), and a larger tubulointerstitial area (D) compared with Sham animals. A treatment with BF alleviated glomerular sclerosis and tubular injury. PD treatment aggravated glomerular and tubular damage but was attenuated because of BF treatment (B through D). All results represent mean ± SEM of 12 animals per group; light microscopy of PAS staining magnification = 400×. ***P < 0.001 versus Sham, *P < 0.05 versus Sham, §§P < 0.01 versus SNX, §P < 0.05 versus SNX, $P < 0.05 versus SNX intraperitoneal, ##P < 0.01 versus SNX+PD, #P < 0.05 versus SNX+PD.
AGE and RAGE in the Kidney.
Consistent with the results of the analysis of the peritoneal membrane, SNX rats revealed significantly higher glomerular and tubular CML and RAGE expression compared with Sham-operated rats. SNX rats treated with BF revealed significantly lower glomerular expression of CML and RAGE compared with untreated SNX rats. Animals treated with PD solutions revealed the highest glomerular and tubular expression of CML and RAGE. PD- and BF-treated SNX rats showed significantly less glomerular CML and RAGE expression than SNX rats treated with PDF but not BF (Figure 7, A and B).
Figure 7.
Decreased glomerular expression of AGE and RAGE in BF treated rats. One possible mechanism of ongoing kidney damage during PD treatment is mediated via the interaction of AGE and RAGE. To clarify this, we performed glomerular and tubular quantification of the expression of CML and RAGE. The tubular expression of CML and RAGE (A and B) was increased in uremic rats in comparison to Sham animals without any difference between the treatment groups, whereas the glomerular staining for CML and RAGE reflected decreased expression of both when BF was substituted (A and B). All results represent mean ± SEM of 12 animals per group. ***P < 0.001 versus Sham, **P < 0.01 versus Sham, *P < 0.05 versus Sham, §P < 0.05 versus SNX, $$P < 0.01 versus SNX intraperitoneal, $P < 0.05 versus SNX intraperitoneal, #P < 0.05 versus SNX+PD.
Renal Fibrosis.
To confirm the morphologic markers of kidney injury, we performed staining for Sirius Red (Figure 8A) and TGFβ1 (Figure 8B). SNX rats showed significantly increased tubular and glomerular staining for Sirius Red (data not shown) and TGFβ1 compared with Sham rats. The glomerular expression of TGFβ1 was significantly increased in SNX rats treated with PDF compared with SNX rats with intraperitoneal puncture alone. PD-treated SNX rats receiving BF revealed significantly less glomerular staining intensity for TGFβ1 compared with the SNX rats with PDF injection but without BF treatment (Figure 8B).
Figure 8.
Pronounced fibrosis in rats not receiving BF. Tubulointerstitial fibrosis is the common phenotype of every kidney disease. The SNX model results in an ongoing tubulointerstitial fibrosis, representatively displayed in panel A (Sirius Red staining of the tubulointerstitium of the uremic groups). As a marker of proceeding fibrosis, the glomerular and tubular expression of TGFβ1 was evaluated (B). In comparison to Sham animals, SNX rats showed enhanced glomerular and tubular expression of TGFβ1 that was alleviated when BF was given. Similar results were obtained in the comparison of uremic rats treated with PD versus SNX rats treated with PD and BF (B). All results represent mean ± SEM of 12 animals per group; light microscopy of Sirius Red magnification = 400×. ***P < 0.001 versus Sham, *P < 0.05 versus Sham, §P < 0.05 versus SNX, $$P < 0.01 versus SNX intraperitoneal, $P < 0.05 versus SNX intraperitoneal, #P < 0.05 versus SNX+PD.
Renal VEGF.
There is some evidence for an altered renal vascularization in uremia and during PD that is associated with an increase of VEGF.3,16 SNX rats showed significantly increased tubular and glomerular staining for VEGF compared with Sham rats. The glomerular expression of VEGF was significantly increased in SNX rats treated with PDF compared with SNX rats with intraperitoneal puncture alone. PD-treated SNX rats receiving BF revealed significantly less glomerular staining intensity for VEGF compared with the SNX rats with PDF injection but without BF treatment (Figure 9A).
Figure 9.
Accentuated inflammation in rats without BF treatment. There is some evidence for a disturbed vascularization of the harmed kidney associated with upregulation of VEGF. Staining for VEGF revealed an increased glomerular expression of VEGF in SNX rats compared with control rats. BF substitution resulted in lowered glomerular VEGF expression whereas tubular expression of VEGF was not influenced by any treatment (A). Staining for IL-6 and CD3-positive cell count was performed as markers of inflammation. Both showed similar results—an enhancement of glomerular and tubular IL-6 expression accompanied by a higher number of CD3-positive cells per area in uremic animals compared with Sham animals, with the highest occurrence in uremic animals treated with PD. BF was able to reduce IL-6 expression and CD3-positive leukocyte count (B and C). All results represent mean ± SEM of 12 animals per group. ***P < 0.001 versus Sham, **P < 0.01 versus Sham, *P < 0.05 versus Sham, §P < 0.05 versus SNX, $$P < 0.01 versus SNX intraperitoneal, $P < 0.05 versus SNX intraperitoneal, #P < 0.05 versus SNX+PD.
Inflammatory Status of the Kidney.
In addition to renal fibrosis, SNX rats revealed a higher tubular and glomerular expression of IL-6 compared with Sham rats. Glomerular IL-6 was significantly increased in SNX rats treated with PDF compared with SNX rats with intraperitoneal puncture. PD-treated SNX rats receiving BF revealed significantly lower glomerular IL-6 expression compared with the SNX rats with PDF injection but without BF treatment (Figure 9B). Tubular CD3-positive cell count was significantly elevated in SNX rats compared with Sham rats. SNX rats treated with BF tended to have fewer CD3-positive cells in the kidney (Figure 9C).
Cell Culture
Cell Viability.
In HPMC treated with low- and high-glucose PDF, cytotoxicity was slightly but not significantly increased compared with control cells (control: 4.30 ± 0.57% versus PDF1.5: 4.45 ± 0.91% versus PDF3.9: 6.14 ± 1.30%, NS).
TK.
To analyze the expression of TK in cultured primary HPMC, we incubated cells with low- and high-glucose PDF with and without BF. In line with the animal experiments, TK expression was significantly higher in the control+BF and PDF1.5+BF group in comparison to the respective groups without BF. In the high-glucose group, no difference was observable when incubated with or without BF (Supplementary Figure 3A).
Marker of Oxidative Stress.
Next, the expression of the oxidative stress marker endothelial nitric oxide synthase (eNOS) was determined. When HPMC were incubated with low- and high-glucose-containing PDF, eNOS expression increased, whereas the addition of BF resulted in lower eNOS (Supplementary Figure 3B).
RAGE.
We then examined the effect of BF on RAGE regulation. In accordance with the animal experiments, a significant reduction of RAGE could be found in the PDF groups when incubated with BF (Supplementary Figure 3C).
Marker of Inflammation.
To determine inflammation, ELISA of HPMC supernatant was carried out. IL-6 release was at least partially reduced after treatment with high-glucose-containing PDF after incubation with BF (Supplementary Figure 3D).
DISCUSSION
We could demonstrate protective local and systemic effects of BF in an animal model of PD and in cultured primary HPMC. In PD, high glucose and GDP are the main contributors for peritoneal and systemic damage caused by PDF; hence, preventive strategies are of particular interest. BF has been shown to prevent diabetic complications in vivo and in vitro. We examined an animal model and cell culture study simulating PD-related peritoneal damage induced by uremia and PDF exposure. The expression of markers indicating peritoneal fibrosis, neovascularization, and inflammation were markedly elevated in SNX rats compared with Sham rats; this was even more accentuated when PD was performed. These protective properties of BF could be confirmed in our HPMC culture experiments.
The salient finding of the study is that BF substitution is not only able to reduce peritoneal AGE accumulation, fibrosis, neovascularization, and inflammation in uremic rats with and without PD treatment, but it also preserves the remnant kidney.
Our model combining uremia and PD exposure, inducing peritoneal membrane damage with consecutive impairment of the peritoneal function, is applicable for studying the pathogenesis of peritoneal damage as it mimics uremic patients on long-term PD. An indwelling PD catheter and a PD model based on daily injections cause alterations of the peritoneal membrane.17 It seems that a model utilizing injections twice daily might lead to less artificial alteration and inflammatory response of the peritoneal membrane.17,18 Hence, different mechanisms underlying peritoneal damage are operative in our model combining uremia and PD. There is growing evidence for an alteration of the peritoneal membrane due to uremia per se. This has been shown in human peritoneal biopsies by Williams et al.1 and was later on confirmed by Honda and colleagues.19 Our data are in line with previous studies of uremic rats, revealing uremia-induced increased thickening, accentuated fibrosis, and functional changes of the peritoneal membrane.11,20,21
However, glucose-induced damage mechanisms play a pivotal role in PD. BF has been shown to reduce diabetes-induced tissue damage in vivo and in vitro.6,22–29 Until now there have been no data available dealing with uremia or PD. We chose to use BF rather than thiamine for these studies because it has a higher bioavailability than thiamine.30
BF is thought to act through at least five different mechanisms. Activation of the hexosamine pathway with subsequent decrease in the accumulation of deleterious glucose metabolites seems to be involved. Normalization of protein kinase C activity along with prevention of nuclear factor-κB activation has been found in retinas. A reduced accumulation of AGE has been postulated.6 Correction of imbalances in the polyol pathway by decreasing aldose reductase activity25 and direct antioxidant properties of BF have been reported.7 We chose to use high-glucose-containing biocompatible PDF with a low amount of GDP concerning the mechanistic understanding of BF as an activator of the pentose phosphate pathway enzyme TK.6 Consecutively, a high-glucose driven formation of AGE is thought to be decreased by TK, but an effect of BF on preformed GDP is doubtful.
As a crucial pathway leading to peritoneal damage by pronounced neovascularization, fibrosis, and inflammation, the role of AGE31 and the interaction with its receptor RAGE have been identified.9 Our study confirms the important role of the AGE-RAGE interaction and demonstrates a significant reduction of AGE accumulation and downregulation of RAGE because of BF substitution. These animal findings have been confirmed by our cell culture experiments with primary HPMC. We chose primary HPMC rather than a cell line to resemble the in vivo situation. Our results are in line with a previously reported increased accumulation of AGE in the human peritoneal membrane during PD treatment and an upregulation of RAGE.10 Treatment with BF produced a downregulation of RAGE, presumably because of a lack of ligands, as our analysis of ROS and AGE-specific fluorescence suggests.
The potential pleiotropic antioxidative effects of BF seem to be reasonable particularly for an altered neovascularization because it was reported that not only the AGE-RAGE interaction31 but especially increased oxidative stress leads to a pronounced neovascularization during PD treatment.32 Our study supports the antioxidative and antiangiogenetic effects of BF through a decreased peritoneal number of vessels per area and a reduced staining for VEGF, presumably the culprit initiator of peritoneal neovascularization.13 Within the setting that uremia is associated with increased oxidative stress,8 it is of special interest that uremic rats treated with BF revealed decreased kidney damage.
Apart from these antioxidative properties, what might be other possible explanations for the preventive effects of BF in the uremic milieu? It has been known for decades that uremic patients present a reduced TK activity33–35 as a consequence of lowered vitamin B1 levels.30 This phenomenon is pronounced in PD patients, whereas TK activity is gradable by application of thiamin not only in the PD patient, but possibly also in the renal failure patient.36,37
In addition to the decreased morphologic signs of peritoneal damage, the salient finding of the study is the protective role of BF for the remnant kidney in a model of PD. This is of particular relevance because large clinical studies demonstrated a higher morbidity and mortality associated with a decline of RRF in PD patients.5,38 It has been demonstrated that GDP enter the systemic circulation39 and lead to deterioration of RRF. Recent animal models demonstrate that PD treatment might induce kidney damage.3,40 Therefore, treatment options focusing on the prevention of glucose and GDP-induced toxicity are of great interest.
One has to keep in mind that the rats used were nondiabetic rats, so we can assume that substitution of BF may ameliorate the development and progression of chronic kidney damage per se. Hitherto, BF has been shown to prevent diabetic complications. The study presented here expands its potential indications at least to PD patients and possibly also to renal failure patients. Whether BF will meet the condition as a new promising and preventive agent has to be established in further studies.
CONCISE METHODS
Animals
Experimental Design.
Twelve-week-old male Sprague-Dawley rats (150 to 200 g; Charles River Laboratories, Sulzfeld, Germany) were maintained in single cages under conditions of constant temperature and humidity according to the guidelines of the Institute of Laboratory Animal Science of the University of Heidelberg. After 1 week of adaptation, the rats were subjected to two-step surgical SNX or a corresponding Sham operation as described before.41 In brief, rats were anesthetized with 0.02 ml of xylazine (Rompun 2%; Bayer Co., Leverkusen, Germany) and 0.2 ml of ketamine (Ketanest 10%; WDT, Garbsen, Germany). In a first operation, the right kidney was decapsulated (Sham operation) with or without subsequent nephrectomy. After 1 week, the cortex of the left kidney was subtotally resected. An amount of cortex corresponding to two-thirds of the weight of the right kidney was removed. Six groups were studied. A first group of rats received a decapsulation of both kidneys and was then kept unmanipulated to obtain baseline histologic and molecular data (Sham). The second group was implemented to obtain uremia-associated histologic and molecular data (SNX). The third group received rat chow containing BF (Wörwag Pharma, Böblingen, Germany) at a dose of 80 mg per kilogram body weight per day (SNX+BF). In a fourth group, SNX rats received an intraperitoneal injection without instilling solution as a control for puncture trauma (SNX intraperitoneal). In the fifth and sixth groups, SNX rats received an intraperitoneal injection of 10 ml of Gambrosol Trio 10 containing 3.9% glucose (Gambro Corporate Research, Hechingen, Germany) at 37°C under sterile conditions twice daily without BF treatment (SNX+PD) or with BF treatment, respectively (SNX+PD+BF). The experiment was terminated for all rats after 12 weeks.
Measurement of BP and Preparation of Renal and Peritoneal Tissue.
Systolic BP was measured in the anesthetized rats before determination via an abdominal aorta catheter. Retrograde aortic perfusion was performed as described earlier.3 For the morphologic and immunohistochemical investigations, the residual kidneys were divided, fixed in paraformaldehyde (PFA; 6%, pH 7.6), embedded in paraffin, and cut into 4-μm-thick sections. Visceral peritoneal tissue samples were fixed in 6% PFA (pH 7.6) and embedded in paraffin. Tissue sections (4 μm thick) were stained with hematoxylin and eosin, periodic acid–Schiff (PAS), and Sirius Red staining.
Immunohistochemical Staining of the Peritoneum and the Kidney.
For immunohistochemistry, tissue sections were deparaffinized, rehydrated, and incubated in Tris-buffered saline (TBS). The following antibodies were used: anti-MG-AGE, anti-CML (BioLogo, Kronshagen, Germany), anti-RAGE, anti-TGFβ1, anti-VEGF, anti-PECAM CD31 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-αSMA (Sigma Aldrich, St Louis, MO), anti-IL-6 (Bio Trend, Köln, Germany), and anti-CD3 (Dako, Carpineria, CA). The sections were incubated with the primary antibody overnight at 4°C and thereafter stained with the secondary antibody, alkaline-phosphatase-conjugated streptavidin, fast red, and peroxidase-conjugated histofine simple stain, diaminobenzidine. Replacement of the primary antibodies with TBS served as a negative control.
Indices of Peritoneal Damage.
Semiquantitative analysis of MG-AGE, CML, RAGE, TGFβ1, VEGF, and IL-6 was carried out using a scoring system that comprises a qualitative score of 0 to 3 (intensity of the staining: 0 = no staining, 1 = low intensity, 2 = medium intensity, 3 = high intensity) and a quantitative score of 0 to 4 (0 = normal, 1 = 1% to 25% of tissue affected, 2 = 26% to 50% affected, 3 = 51% to 75% affected; 4 = 76% to 100% affected). The two scores were multiplied, with a maximum possible score of 12; 50 areas per cross section were analyzed. The maximal thickness of the submesothelial compact zone was measured in sections oriented perpendicular to the serosal surface. CD3-positive T cells and vessel number (using PAS staining and confirmed by PECAM CD31 staining) per area on a 121-point grid (Leitz, Wetzlar, Germany) were counted.
Indices of Renal Damage.
All semiquantitative analyses (morphology, immunohistochemistry, and immunofluorescence) were performed in a blinded manner. Sections (4 μm) were stained with PAS, counterstained with hematoxylin and eosin, and examined via light microscopy (Laborlux K; Leitz, Wetzlar, Germany; magnification 200× to 400×). GSI and TII were quantitatively evaluated using el Nahas's scoring system.42 GSI was graded as follows: grade 0 = normal, grade 1 = 1% to 25% of glomerular area affected, grade 2 = 26% to 50% affected, grade 3 = 51% to 75% affected, and grade 4 = 76% to 100% affected.43 TII (on the basis of the parameters of basement membrane thickening, cell infiltration, tubular dilation, atrophy, or sloughing) was also graded on a 0 to 4 scale. The number of glomerular cells was obtained by counting 50 glomeruli per cross section, which were cut along the equatorial plane. IA was quantitated using the 121-point grid (Leica, Wetzlar, Germany) devised by Kaneto et al.44 and Ishidoya et al.45 Immunohistochemical analysis for CML, RAGE, TGFβ1, VEGF, and IL-6 was carried out using the scoring system that was described above. Fifty areas per cross section were analyzed; tubulointerstitium and glomeruli were viewed separately. For CD3, 50 tubulointerstitial areas per cross section were analyzed and positive cells were counted.
TK Activity.
TK activity was determined by coupling the formation of glyceraldehyde-3-phosphate from ribose-5-phosphate and xylulose-5-phosphate to the oxidation of NADH using triosephosphate isomerase and glycero-3-phosphate dehydrogenase, as described.46 In brief, 20 μl of tissue cytosolic extract was added to 200 μl of assay mixture containing 14.8 mM ribose-5-phosphate, 253 μM NADH, 185 U/ml triosephosphate isomerase, and 6.0 U/ml GDH in 250 mM TBS, pH 7.8. The optical density was measured at 340 nm immediately and then every 10 minutes for 2 hours. The activity was calculated from the linear decrease in the optical density from 10 and 80 minutes, converting this to micromoles of NADH oxidized per minute using the extinction coefficient for NADH, equivalent to micromoles of glyceraldehyde-3-phosphate formed per minute by TK activity.
Total Protein Fluorescence in Sera and Urine.
Total protein fluorescence relating to oxidative and glycation fluorescence of sera and urine samples was performed using a spectrofluorometer (LS 50 B; Perkin Elmer, Überlingen, Germany). For measuring fluorescence, intensity excitation wavelength was set at 350 nm and emission wavelength at 430 nm. Samples were diluted accordingly.
Ancillary Measurements and Analysis of ROS in Sera.
Hemoglobin, urea, sodium, potassium, glucose, and creatinine were measured with autoanalyzers (ADVIA 2400 and ADVIA 2120, Siemens, Eschborn, Germany). ROS were quantitated by measuring total protein carbonyl content using the Cayman chemical protein carbonyl assay kit's 2,4-dinitrophenylhydrazine reaction with protein carbonyl (Cayman Chemical Company, Ann Arbor, MI).
Peritoneal Equilibration Test.
A modified mini-peritoneal equilibration test was performed. In brief, 20 ml of PDF were injected. After 1 hour, the intra-abdominal PDF was aspirated and creatinine, urea, and sodium were measured with autoanalyzers (ADVIA 2400, Siemens, Eschborn, Germany). D/P was built, and the transport type was estimated according to La Milia and co-workers.47
Measurement of Albumin in Urine.
Urinary albumin was quantitated using the ELISA technique with a rat anti-albumin antibody (ICN Biomedicals, Eschwege, Germany) using the peroxidase system as described by Magnotti et al.48
Cell Culture
Cultivation and Characterization.
Primary HPMC were isolated, cultured, and characterized as described elsewhere.49 In brief, culture medium M199 was supplemented with penicillin (100 IU/ml), streptomycin (0.1 mg/ml), insulin/transferrin (5 μg/ml), hydrocortisone (0.4 μg/ml), and 10% (vol/vol) FCS. Cell culture flasks were coated with collagen I (BD Biosciences Heidelberg, Germany), and HPMC from first to third passage were used. For characterization, isolated cells of each omental sample stained positive for cytokeratin 18 and vimentin and negative for factor VIII and fibroblast-specific protein 1 (Supplementary Figure 2).
Incubation of HPMC with PDF and BF.
HPMC were serum deprived in M199 containing 0.1% FCS for 24 hours. The cells were then incubated for 48 hours with Gambrosol Trio 10 (Gambro Corporate Research, Hechingen, Germany) and with equal volume (1:1) of serum-deprived medium,50,51 in particular with control buffer (= control), PDF containing a low glucose content of 1.5% (= PDF1.5), PDF containing a high glucose content of 3.9% (= PDF3.9), and treated with ± BF (50 μM) (Wörwag Pharma, Böblingen, Germany), respectively. All experiments were repeated at least 3 times with omenta from different patients.
Cell Viability.
Release of lactate dehydrogenase (LDH) after incubation with PDF was measured in cell culture supernatants that were collected and assayed immediately for LDH using a cytotoxicity detection kit (Roche Applied Science, Mannheim, Germany). Measurements were performed in triplicates of at least three independent experiments according to the manufacturer's instructions. We carried out a background control (providing information about the LDH activity in the assay medium), a low control (spontaneous LDH release), and a high control with 1% Triton X-100 (maximum LDH release). Cytotoxicity is given in percent.
Immunofluorescence.
For immunofluorescence analysis, HPMC were grown on type I collagen-coated glass coverslips (Sigma-Aldrich, St Louis, MO) either fixed with methanol or 3% PBS-PFA and incubated with primary antibodies, anti-cytokeratin 18 (Chemicon International, Hofheim, Germany), anti-vimentin (Zytomed Systems, Berlin, Germany), anti-fibroblast-specific protein 1 (Dako Cytomation, Denmark), anti-factorVIII (F15), anti-RAGE (N16), anti-TK (N19) (goat polyclonal IgG, Santa Cruz Biotechnology, Santa Cruz, CA), anti-eNOS (rabbit polyclonal, ABR, Golden, CO), and appropriate fluorescently labeled secondary antibodies. For nuclear staining, fixed cells were stained with Hoechst 33342 (Invitrogen, Karlsruhe, Germany). A negative control was performed using PBS instead of primary antibody. Images were taken using a Nikon DS-Qi1Mc quantitative black and white charge-coupled device camera attached to a Nikon Eclipse 80i upright microscope (Nikon, Düsseldorf, Germany). The same contrast and intensity settings were applied to samples stained with identical antibodies.
ELISA.
IL-6 ELISA was performed referring to the manufacturers' instructions (human IL-6 ELISA kit, Bioscience, Inc., San Diego, CA). In brief, supernatants of incubated cells were taken and diluted accordingly. Measurement was carried out at 450 nm using a Multiscan FC ELISA reader (Thermo Scientific, Langenselbold, Germany).
Statistical Analysis
All values are expressed as mean ± SEM. Mann–Whitney and Kruskal–Wallis tests were used as appropriate to test statistical significance. Significance level was set at P < 0.05. Statistical analysis was performed using PC-Statistik (version 5.0; Hoffmann, Giessen, Germany) and GraphPad Prism (version 5.0; San Diego, CA).
DISCLOSURES
V.S. received research grants from Gambro Corporate Research (Hechingen, Germany) and Wörwag Pharma (Böblingen, Germany).
Acknowledgments
We thank Heike Ziebart and Peter Rieger (Department of Pathology, University of Heidelberg, Heidelberg, Germany) for their excellent technical assistance and Ulrike Haug and Caren Schmidt (Gambro Research, Hechingen, Germany) for support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, Mackenzie RK, Williams GT: Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13: 470–479, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bargman JM, Thorpe KE, Churchill DN: Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Muller-Krebs S, Kihm LP, Zeier B, Gross ML, Deppisch R, Wieslander A, Henle T, Penndorf I, Oh J, Reiser J, Nawroth PP, Zeier M, Schwenger V: Renal toxicity mediated by glucose degradation products in a rat model of advanced renal failure. Eur J Clin Invest 38: 296–305, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Kim SG, Kim S, Hwang YH, Kim K, Oh JE, Chung W, Oh KH, Kim HJ, Ahn C: Could solutions low in glucose degradation products preserve residual renal function in incident peritoneal dialysis patients? A 1-year multicenter prospective randomized controlled trial (Balnet Study). Perit Dial Int 28[Suppl 3]: S117–S122, 2008 [PubMed] [Google Scholar]
- 5. Haag-Weber M, Kramer R, Haake R, Islam MS, Prischl F, Haug U, Nabut JL, Deppisch R: Low-GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: A prospective randomized study. Nephrol Dial Transplant 25: 2288–2296, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M: Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 9: 294–299, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Schmid U, Stopper H, Heidland A, Schupp N: Benfotiamine exhibits direct antioxidative capacity and prevents induction of DNA damage in vitro. Diabetes Metab Res Rev 24: 371–377, 2008 [DOI] [PubMed] [Google Scholar]
- 8. D'Apolito M, Du X, Zong H, Catucci A, Maiuri L, Trivisano T, Pettoello-Mantovani M, Campanozzi A, Raia V, Pessin JE, Brownlee M, Giardino I: Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J Clin Invest 120: 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwenger V, Morath C, Salava A, Amann K, Seregin Y, Deppisch R, Ritz E, Bierhaus A, Nawroth PP, Zeier M: Damage to the peritoneal membrane by glucose degradation products is mediated by the receptor for advanced glycation end-products. J Am Soc Nephrol 17: 199–207, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Kihm LP, Wibisono D, Muller-Krebs S, Pfisterer F, Morath C, Gross ML, Morcos M, Seregin Y, Bierhaus A, Nawroth PP, Zeier M, Schwenger V: RAGE expression in the human peritoneal membrane. Nephrol Dial Transplant 23: 3302–3306, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Guo H, Leung JC, Lam MF, Chan LY, Tsang AW, Lan HY, Lai KN: Smad7 transgene attenuates peritoneal fibrosis in uremic rats treated with peritoneal dialysis. J Am Soc Nephrol 18: 2689–2703, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Yanez-Mo M, Lara-Pezzi E, Selgas R, Ramirez-Huesca M, Dominguez-Jimenez C, Jimenez-Heffernan JA, Aguilera A, Sanchez-Tomero JA, Bajo MA, Alvarez V, Castro MA, del Peso G, Cirujeda A, Gamallo C, Sanchez-Madrid F, Lopez-Cabrera M: Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med 348: 403–413, 2003 [DOI] [PubMed] [Google Scholar]
- 13. De Vriese AS, Tilton RG, Stephan CC, Lameire NH: Vascular endothelial growth factor is essential for hyperglycemia-induced structural and functional alterations of the peritoneal membrane. J Am Soc Nephrol 12: 1734–1741, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Twardowski ZJ: Clinical value of standardized equilibration tests in CAPD patients. Blood Purif 7: 95–108, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG: Meta-analysis: Peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol 17: 2591–2598, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Pawlak K, Pawlak D, Mysliwiec M: Impaired renal function and duration of dialysis therapy are associated with oxidative stress and proatherogenic cytokine levels in patients with end-stage renal disease. Clin Biochem 40: 81–85, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Flessner MF, Credit K, Henderson K, Vanpelt HM, Potter R, He Z, Henegar J, Robert B: Peritoneal changes after exposure to sterile solutions by catheter. J Am Soc Nephrol 18: 2294–2302, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Flessner MF, Credit K, Richardson K, Potter R, Li X, He Z, Hoskins G, Henegar J: Peritoneal inflammation after twenty-week exposure to dialysis solution: Effect of solution versus catheter-foreign body reaction. Perit Dial Int 30: 284–293 [DOI] [PubMed] [Google Scholar]
- 19. Honda K, Hamada C, Nakayama M, Miyazaki M, Sherif AM, Harada T, Hirano H: Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: A quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol 3: 720–728, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Combet S, Ferrier ML, Van Landschoot M, Stoenoiu M, Moulin P, Miyata T, Lameire N, Devuyst O: Chronic uremia induces permeability changes, increased nitric oxide synthase expression, and structural modifications in the peritoneum. J Am Soc Nephrol 12: 2146–2157, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Vrtovsnik F, Coester AM, Lopes-Barreto D, de Waart DR, Van der Wal AC, Struijk DG, Krediet RT, Zweers MM: Induction of chronic kidney failure in a long-term peritoneal exposure model in the rat: Effects on functional and structural peritoneal alterations. Perit Dial Int 30: 558–569 [DOI] [PubMed] [Google Scholar]
- 22. Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ: Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes 52: 2110–2120, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Balakumar P, Sharma R, Singh M: Benfotiamine attenuates nicotine and uric acid-induced vascular endothelial dysfunction in the rat. Pharmacol Res 58: 356–363, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Balakumar P, Chakkarwar VA, Singh M: Ameliorative effect of combination of benfotiamine and fenofibrate in diabetes-induced vascular endothelial dysfunction and nephropathy in the rat. Mol Cell Biochem 320: 149–162, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Berrone E, Beltramo E, Solimine C, Ape AU, Porta M: Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem 281: 9307–9313, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Ceylan-Isik AF, Wu S, Li Q, Li SY, Ren J: High-dose benfotiamine rescues cardiomyocyte contractile dysfunction in streptozotocin-induced diabetes mellitus. J Appl Physiol 100: 150–156, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Gadau S, Emanueli C, Van Linthout S, Graiani G, Todaro M, Meloni M, Campesi I, Invernici G, Spillmann F, Ward K, Madeddu P: Benfotiamine accelerates the healing of ischaemic diabetic limbs in mice through protein kinase B/Akt-mediated potentiation of angiogenesis and inhibition of apoptosis. Diabetologia 49: 405–420, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Pomero F, Molinar Min A, La Selva M, Allione A, Molinatti GM, Porta M: Benfotiamine is similar to thiamine in correcting endothelial cell defects induced by high glucose. Acta Diabetol 38: 135–138, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Sanchez-Ramirez GM, Caram-Salas NL, Rocha-Gonzalez HI, Vidal-Cantu GC, Medina-Santillan R, Reyes-Garcia G, Granados-Soto V: Benfotiamine relieves inflammatory and neuropathic pain in rats. Eur J Pharmacol 530: 48–53, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Bitsch R, Wolf M, Moller J, Heuzeroth L, Gruneklee D: Bioavailability assessment of the lipophilic benfotiamine as compared to a water-soluble thiamin derivative. Ann Nutr Metab 35: 292–296, 1991 [DOI] [PubMed] [Google Scholar]
- 31. De Vriese AS, Flyvbjerg A, Mortier S, Tilton RG, Lameire NH: Inhibition of the interaction of AGE-RAGE prevents hyperglycemia-induced fibrosis of the peritoneal membrane. J Am Soc Nephrol 14: 2109–2118, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Noh H, Kim JS, Han KH, Lee GT, Song JS, Chung SH, Jeon JS, Ha H, Lee HB: Oxidative stress during peritoneal dialysis: Implications in functional and structural changes in the membrane. Kidney Int 69: 2022–2028, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Lange K, Lonergan ET, Semar M, Sterzel RB: Transketolase inhibition as a mechanism in uremic neuropathy. Trans Assoc Am Physicians 84: 172–181, 1971 [PubMed] [Google Scholar]
- 34. Lonergan ET, Semar M, Sterzel RB, Treser G, Needle MA, Voyles L, Lange K: Erythrocyte transketolase activity in dialyzed patients. A reversible metabolic lesion of uremia. N Engl J Med 284: 1399–1403, 1971 [DOI] [PubMed] [Google Scholar]
- 35. Sterzel RB, Semar M, Lonergan ET, Lange K: Effect of hemodialysis on the inhibition of nervous tissue transketolase and on uremic neuropathy. Trans Am Soc Artif Intern Organs 17: 77–79, 1971 [PubMed] [Google Scholar]
- 36. Blumberg A, Hanck A, Sander G: Vitamin nutrition in patients on continuous ambulatory peritoneal dialysis (CAPD). Clin Nephrol 20: 244–250, 1983 [PubMed] [Google Scholar]
- 37. Boeschoten EW, Schrijver J, Krediet RT, Schreurs WH, Arisz L: Deficiencies of vitamins in CAPD patients: The effect of supplementation. Nephrol Dial Transplant 3: 187–193, 1988 [PubMed] [Google Scholar]
- 38. Fan SL, Pile T, Punzalan S, Raftery MJ, Yaqoob MM: Randomized controlled study of biocompatible peritoneal dialysis solutions: Effect on residual renal function. Kidney Int 73: 200–206, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Zeier M, Schwenger V, Deppisch R, Haug U, Weigel K, Bahner U, Wanner C, Schneider H, Henle T, Ritz E: Glucose degradation products in PD fluids: Do they disappear from the peritoneal cavity and enter the systemic circulation? Kidney Int 63: 298–305, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Breborowicz A, Pawlaczyk K, Polubinska A, Gorna K, Wieslander A, Carlsson O, Tam P, Wu G: Effect of peritoneal dialysis on renal morphology and function. Nephrol Dial Transplant 21: 3539–3544, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Dikow R, Kihm LP, Zeier M, Kapitza J, Tornig J, Amann K, Tiefenbacher C, Ritz E: Increased infarct size in uremic rats: Reduced ischemia tolerance? J Am Soc Nephrol 15: 1530–1536, 2004 [DOI] [PubMed] [Google Scholar]
- 42. el Nahas AM, Bassett AH, Cope GH, Le Carpentier JE: Role of growth hormone in the development of experimental renal scarring. Kidney Int 40: 29–34, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Raij L, Azar S, Keane W: Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984 [DOI] [PubMed] [Google Scholar]
- 44. Kaneto H, Morrissey J, McCracken R, Reyes A, Klahr S: Enalapril reduces collagen type IV synthesis and expansion of the interstitium in the obstructed rat kidney. Kidney Int 45: 1637–1647, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Ishidoya S, Morrissey J, McCracken R, Reyes A, Klahr S: Angiotensin II receptor antagonist ameliorates renal tubulointerstitial fibrosis caused by unilateral ureteral obstruction. Kidney Int 47: 1285–1294, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Thornalley PJ, Jahan I, Ng R: Suppression of the accumulation of triosephosphates and increased formation of methylglyoxal in human red blood cells during hyperglycaemia by thiamine in vitro. J Biochem 129: 543–549, 2001 [DOI] [PubMed] [Google Scholar]
- 47. La Milia V, Di Filippo S, Crepaldi M, Del Vecchio L, Dell'Oro C, Andrulli S, Locatelli F: Mini-peritoneal equilibration test: A simple and fast method to assess free water and small solute transport across the peritoneal membrane. Kidney Int 68: 840–846, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Magnotti RA, Jr, Stephens GW, Rogers RK, Pesce AJ: Microplate measurement of urinary albumin and creatinine. Clin Chem 35: 1371–1375, 1989 [PubMed] [Google Scholar]
- 49. Stylianou E, Jenner LA, Davies M, Coles GA, Williams JD: Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int 37: 1563–1570, 1990 [DOI] [PubMed] [Google Scholar]
- 50. Boulanger E, Wautier MP, Gane P, Mariette C, Devuyst O, Wautier JL: The triggering of human peritoneal mesothelial cell apoptosis and oncosis by glucose and glycoxydation products. Nephrol Dial Transplant 19: 2208–2216, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Wieslander AP, Deppisch R, Svensson E, Forsback G, Speidel R, Rippe B: In vitro biocompatibility of a heat-sterilized, low-toxic, and less acidic fluid for peritoneal dialysis. Perit Dial Int 15: 158–164, 1995 [PubMed] [Google Scholar]