Abstract
Variants in the gene encoding fibroblast growth factor 1 (FGF1) co-segregate with familial susceptibility to hypertension, and glomerular upregulation of FGF1 associates with hypertension. To investigate whether variants in other members of the FGF signaling pathway may also associate with hypertension, we genotyped 629 subjects from 207 Polish families with hypertension for 79 single nucleotide polymorphisms in eight genes of this network. Family-based analysis showed that parents transmitted the major allele of the rs16892645 polymorphism in the gene encoding FGF binding protein 1 (FGFBP1) to hypertensive offspring more frequently than expected by chance (P = 0.005). An independent cohort of 807 unrelated Polish subjects validated this association. Furthermore, compared with normotensive subjects, hypertensive subjects had approximately 1.5- and 1.4-fold higher expression of renal FGFBP1 mRNA and protein (P = 0.04 and P = 0.001), respectively. By immunohistochemistry, hypertension-related upregulation of FGFBP1 was most apparent in the glomerulus and juxtaglomerular space. Taken together, these data suggest that FGFBP1 associates with hypertension and that systematic analysis of signaling pathways can identify previously undescribed genetic associations.
Essential hypertension is a complex, multifactorial, heritable disease. The contribution of genetic factors to the development of hypertension is estimated at 30 to 50%, but the causative alleles and related molecular mechanisms remain largely elusive. However, it is becoming increasingly clear that genes underlying familial susceptibility to essential hypertension may not necessarily reside within the most obvious pathways involved in BP regulation.1 Indeed, a substantial proportion of novel alleles associated with hypertension and BP in the recent genome-wide association scans map to genes that do not belong to classical systems of BP regulation (such as the sympathetic nervous system or renal sodium handling).2,3 Fibroblast growth factor (FGF) family is an example of a novel group of molecules identified in these studies; one of the most significant associations with BP/hypertension was mapped to the FGF5 locus.2 This association has been recently confirmed in another large scale genetic study of approximately 25,000 Japanese subjects.4 A member of a different phylogenetic subfamily of FGFs (FGF1 gene: FGF1) was implicated in human hypertension in family-based linkage and association analysis.5 Indeed, a common allelic variant of a FGF1 single nucleotide polymorphism (SNP) co-segregated with familial susceptibility to essential hypertension in Polish pedigrees.5 Hypertension was also associated with up-regulation of FGF1 expression within the human kidney.5 Specifically, compared with normotensive subjects, hypertensive patients exhibited higher abundance of FGF1 in endothelial and mesangial cells of the glomeruli.5 The FGF1 paralog—FGF2 gene (FGF2)—is also expressed in the human kidney and was postulated to contribute to glomerular metabolism of nitric oxide.6,7 Both FGF1 and FGF2 are also expressed in blood vessels whereby, on binding to specific tyrosine kinase receptors (FGF receptors [FGFRs]), they regulate vascular tone and stimulate growth and proliferation of endothelial cells and apoptosis of smooth muscle cells.8–10 By virtue of these effects on glomerular hemodynamics and vascular remodeling, FGFs may potentially contribute to BP elevation.5,11 Indeed, FGF2 has recently been characterized as a major player in human pulmonary hypertension, whereas inhibition of signaling through one of the FGFRs decreased BP in pulmonary circulation.11 Collectively, these data indicate that FGF system may play an important role in hypertension. Given that biologically relevant genetic effects on BP are likely to arise from molecules signaling through the same pathway, we hypothesized that other genes in the FGF1 signaling cascade may harbor variants that are associated with hypertension.
Herein, we report a systematic genetic analysis of the FGF1 pathway using a cohort in which the original association between hypertension and FGF1 was identified.5 We also examined the most significant genetic associations in an independent population of the same origin. Finally, we used human kidney tissue from subjects with contrasting BP phenotypes to compare the expression of the molecule identified in DNA-based analysis at mRNA and protein levels.
RESULTS
Silesian Hypertension Study, Silesian Cardiovascular Study, and Silesian Renal Tissue Bank: Demographic and Clinical Characteristics
A majority of subjects (63.8%) from 207 families recruited into the Silesian Hypertension Study (SHS; discovery cohort) were hypertensive. Hypertension was also highly prevalent (72.5 and 61.3%) in 807 biologically unrelated subjects from the Silesian Cardiovascular Study (SCS; replication cohort) and 62 individuals from the Silesian Renal Tissue Bank (SRTB; the resource for gene expression studies), respectively. The detailed demographic and clinical characteristics of subjects from the three Silesian cohorts are presented in Table 1. Information on the structure of families recruited into SHS is given in the Supplementary Table 1.
Table 1.
General clinical characteristics of subjects from SHS, SCS, and SRTB
| Phenotype | SHS—Parents | SHS—Offspring | SCS | SRTB |
|---|---|---|---|---|
| n | 274 | 355 | 807 | 62 |
| Age (years) | 55.0 ± 10.5 | 38.8 ± 15.6 | 55.3 ± 11.6 | 58.3 ± 11.4 |
| Gender (M/F) | 130/144 | 183/172 | 465/342 | 35/27 |
| Body mass index (kg/m2) | 27.6 ± 4.6 | 26.2 ± 4.6 | 27.5 ± 4.2 | 27.3 ± 4.0 |
| Systolic BP (mmHg) | 139.0 ± 21.6 | 138.0 ± 18.5 | 131.5 ± 19.1 | 134.4 ± 12.1 |
| Diastolic BP (mmHg) | 88.0 ± 21.6 | 88.1 ± 11.4 | 75.1 ± 11.1 | 82.2 ± 7.6 |
| Hypertension (%) | 146 (53.3) | 254 (71.5) | 585 (72.5) | 38 (61.3) |
| Anti-hypertensive treatment (%) | 102 (37.2) | 171 (48.2) | 478 (59.2) | 36 (58.1) |
Data are means and SD or counts and percentages. SHS, Silesian Hypertension Study; SCS, Silesian Cardiovascular Study; SRTB, Silesian Renal Tissue Bank.
FGF1 Signaling Pathway Genes and Hypertension: Primary Family-Based Association Analysis in SHS
Of 79 SNPs in eight genes selected for genotyping in SHS, 8 failed to produce assays at the pregenotyping design stage (Supplementary Tables 2 and 3). Five other SNPs were excluded after genotyping because of failing quality control (genotyping rate <90% [n = 1], violation of Hardy-Weinberg equilibrium [HWE]: P < 0.01 [n = 1], and minor allele frequency [MAF] <5% [n = 3]; Supplementary Table 3).
A total of 66 SNPs that passed the quality control filters provided sufficiently dense coverage for each of the eight FGF1 pathway genes: the proportion of common genetic variance explained by the selected SNPs ranged from 67 to 100% (mean, 92.9%; mean maximum r2 = 0.939; Supplementary Table 2).
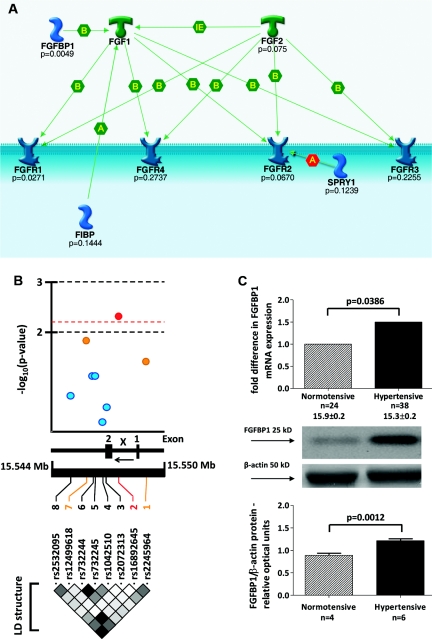
A family-based association test (FBAT) showed that alleles of two SNPs (rs2245964 and rs16892645) in the FGF binding protein 1 gene (FGFBP1) and one polymorphism (rs2956724) in FGFR1 were transmitted from parents to hypertensive offspring more frequently than expected by chance (Figure 1; Supplementary Table 4). However, only one of these associations survived the correction for multiple testing (based on calculation of false positive discovery rate); the major allele of rs16892645 in FGFBP1 was associated with increased susceptibility to hypertension in SHS (P = 0.0049, q < 0.25; Supplementary Table 4).
Figure 1.
FGFBP1 is associated with hypertension: family-based association analysis and gene expression studies. (A) The results of the primary family-based association analysis (the most significant P values) in SHS are shown at the background of molecular interactions within the pathway (image created using the GeneGo software). FGF1, fibroblast growth factor 1; FGF2, fibroblast growth factor 2; FGFBP1, fibroblast growth factor binding protein 1; FGFR1, fibroblast growth factor receptor 1; FGFR2, fibroblast growth factor receptor 2; FGFR3, fibroblast growth factor receptor 3; FGFR4, fibroblast growth factor receptor 4; FIBP, fibroblast growth factor (acidic) intracellular binding protein; SPRY1, sprouty homolog 1, antagonist of FGF signaling; B, binding; A, allosteric effect; IE, influence on expression. (B) FGFBP1 and hypertension: fine mapping analysis in SHS. (Top) −Log-transformed probability values from the family-based association analysis. (Middle) Structure of FGFBP1 gene: 1, first untranslated exon; 2, coding exon; x, intron 1; ←, direction of transcription. (Bottom) LD map (r2 coefficients–based) of FGFBP1 (black corresponds to r2 = 0.8 to 1 − maximal LD, and white corresponds to r2 = 0 to 0.2 − no or weak LD) in SHS. (C) Comparative analysis of FGFBP1 mRNA and protein expression in hypertensive and normotensive kidneys from SRTB. n, number of subjects.
FGFBP1 and Hypertension: Fine Mapping and Linkage Disequilibrium–Based Analysis in SHS
None of the five additional FGFBP1 SNPs genotyped in SHS showed a stronger association with hypertension than rs16892645 (Figure 1). Joint analysis of eight SNPs in FGFBP1 mapped the most significant association signal to its intron 1 (Figure 1). This association remained significant after applying a correction for multiple testing based on spectral decomposition of linkage disequilibrium (LD) matrices of FGFBP1 SNPs (the corrected threshold of statistical significance: P = 0.0064). In silico analysis of LD between rs16892645 and all variants within 500 kb in the population of Northern and Western European ancestry of HapMap and 1000 Genomes Project did not identify any statistically similar SNPs (r2 > 0.8) that could account for the identified association (Supplementary Figure 1).
FGFBP1 and Hypertension: Replication in SCS and Combined Association Analysis of SHS and SCS
All three FGFBP1 SNPs showing at least nominal association with hypertension in family-based analysis of SHS when genotyped in SCS were in HWE (P = 0.29983, P = 0.4581, P = 0.21 for rs12499618, rs2245964 and rs16892645, respectively). Of these, two (rs16892645 and rs12499618) were associated with hypertension in SCS (P = 0.0491 and P = 0.049). The third SNP (rs2245964) showed borderline association with hypertension in SCS (P = 0.0559). Under the additive model of inheritance and after adjustment for age, gender, and body mass index, each major allele (C) copy of rs16892645 increased the odds ratio (ORs) of hypertension by approximately 1.5 (OR = 1.48; 95% confidence interval [CI]: 1.001 to 2.19) in SCS. The direction of allelic associations of rs12499618 and rs2245964 in SCS was also consistent with that observed in SHS.
Joint analysis of SHS families and the biologically unrelated subjects from SCS strengthened the evidence for an association between rs16892645 and hypertension (P = 0.0024).
FGFBP1 and FGF1 Genetic Score and Hypertension in SCS
The previously reported association between FGF1 variant (rs152524) and hypertension in SHS families5 was replicated in SCS subjects; after adjustment for age, gender, and body mass index, each major allele (A) copy was associated with an approximately 1.3-fold increase in odds of hypertension (OR = 1.29; 95% CI: 1.01 to 1.64, P = 0.0412).
There was also a significant joint effect of rs152524 (FGF1) and rs16892645 (FGFBP1) upon hypertension in SCS; after adjustment for age, gender, and body mass index in binary logistic regression, each unit increase in allelic score (additive dosage of pro-hypertensive alleles for rs16892645 and rs152524) increased the odds of hypertension by approximately 34% (OR = 1.34; 95% CI: 1.09 to 1.65, P = 0.005).
Renal FGFBP1 Expression in Hypertension: SRTB
Quantification of mRNA from 62 human kidneys showed an approximately 1.5-fold higher expression of FGFBP1 in hypertensive patients than normotensive controls (P = 0.0386; Figure 1). The association between hypertension and renal expression of FGFBP1 transcript remained statistically significant even after adjusting for age, gender, and body mass index (P = 0.045). Western blotting confirmed that, compared with patients with normal BP, hypertensive subjects had an approximately 1.4-fold higher abundance of FGFBP1 protein in the kidney (P = 0.001; Figure 1).
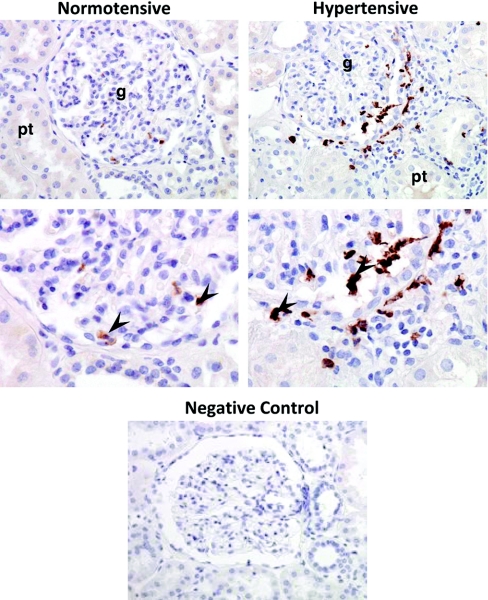
Immunohistochemistry showed that hypertension-related up-regulation of FGFBP1 was most apparent in (but not exclusive to) the glomerulus. Indeed, immunostaining for FGFBP1 was also identified in the juxtaglomerular compartment. Monocytes/macrophages were the major type of cells expressing FGFBP1 (Figure 2).
Figure 2.
FGFBP1 expression is up-regulated in the human hypertensive kidney. FGFBP1 was immunolocalized mainly to glomerulus (g) and juxtaglomerular space. Intensity of FGFBP1 immunostaining in the hypertensive kidney was higher than the normotensive control. pt, proximal tubule, arrows indicate cells that express FGFBP1 (mostly monocytes/macrophages).
Distribution of rs16892645 genotypes among the SRTB subjects did not violate HWE (P = 0.7133). There was no statistically significant difference in renal expression of FGFBP1 mRNA between nine carriers of the rare (T) variant of rs16892645 and 53 subjects homozygous for its common (C) allele either in the unadjusted (P = 0.810) or age-, gender-, body mass index–, and hypertension status–adjusted (P = 0.690) analysis.
DISCUSSION
Our family-based analysis uncovered an association between a novel locus (FGFBP1) within the FGF1 signaling pathway and hypertension. We also replicated the new (FGFBP1) and previously shown (FGF1) associations in an independent population recruited in the same region where the primary cohort was collected.5,12 More importantly, we discovered an additive effect of both FGFBP1 and FGF1 alleles on the risk of hypertension. Finally, we determined that, similar to FGF1,5 FGFBP1 is up-regulated in the hypertensive kidney and that this up-regulation is apparent mainly (but not exclusively) within the glomerulus.
FGFs have a well-established role in control of wound healing, male reproduction, and tumor angiogenesis.13 However, accumulating evidence indicates that FGFs may also play an important role in cardiovascular regulation. Indeed, data from genetic and molecular studies implicated FGF1 and two other FGFs (FGF2 and FGF5) as potential players in human essential and pulmonary hypertension.2,4,5,11 These biologic effects of FGFs are mediated through their interactions with specific FGF chaperone molecules and four types of tyrosine kinase receptors. However, to date, there has been no systematic analysis of the FGF1 pathway in relation to genetic susceptibility to hypertension. Our study is the first to show a novel association between one of the FGF1 partner molecules (FGFBP1) and hypertension. FGFBP1 encodes a carrier protein that releases FGF1 from its extracellular storage pool and shuttles it to the cell surface where FGF1 binds to its high affinity receptors.14 Through this chaperoning, FGFBP1 is thought to potentiate the biologic effects of FGFs on target cells.15 Indeed, mitogenic activity of FGF1 is enhanced by its binding to FGFBP1.14,16 This augmenting effect on FGF1-driven proliferation may be particularly relevant to hypertension because FGF1 acts as a regulator of cellular growth and apoptosis in several BP-regulating tissues,10 and by virtue of these cellular mechanisms, may contribute to hypertensive target organ damage.17
FGFBP1 is encoded by a gene located within the distal portion of the short arm of human chromosome 4. This chromosomal segment has been linked to BP in at least three scans of the human genome.18–20 Indeed, three linkage studies showed a quantitative trait locus for systolic BP, postexercise diastolic BP, and longitudinal change of diastolic BP from childhood to adulthood in close proximity to FGFBP1.18–20 Moreover, the chromosomal region that harbors FBFBP1 is syntenic to BP quantitative trait locus on rat chromosome 14 (QTL189).21 It is not clear to what extent genetic variation within FGFBP1 could explain the reported linkage signals, but our association analysis makes it a strong positional candidate.
We mapped the most significant association between hypertension and FGFBP1 to its common intronic polymorphism (rs16892645) and showed that no other currently known SNPs within 500 kb of rs16892645 are its statistically similar proxies. Although there was no difference in renal mRNA expression of FGFBP1 between carriers of contrasting rs16892645 genotypes, we cannot fully refute the hypothetical contribution of this polymorphism to FGFBP1 transcription given the power limitations of this analysis (low number of carriers of the minor allele). Alternatively other, as yet unidentified, functional variant(s) within this locus may be the actual driver(s) of this association. Future resequencing efforts combined with gene expression studies in populations with larger sample sizes will be needed to identify the genetic mediator(s) of the detected associations. Given that FGFBP1 expression remains under strict genetic regulation,22 it is likely that the causative variant will act through renal up-regulation of FGFBP1.
Under normal conditions, FGFBP1 mRNA shows a very low expression in human tissues23 and is only increased in diseases such as cancer or human nephropathies.16,22,24 Both HIV-associated nephropathy and hemolytic uremic syndrome are associated with increased expression of FGFBP1, predominantly within the tubular epithelium.16,24 Our studies showed that renal FGFBP1 is also up-regulated in hypertensive kidney, but this increased expression maps mainly to the glomerulus and juxtaglomerular compartment. Interestingly, our previous study showed that the molecule for which FGFBP1 is a carrier (FGF1) is up-regulated mainly within hypertensive glomerulus.5 Apart from this co-localization, both molecules show similar magnitude of up-regulation in the hypertensive kidney. These data clearly indicate that FGF1 signaling is activated in hypertension, and the renal mechanisms are most likely to explain how this up-regulation may translate into BP elevation. Future studies will be needed to elucidate the exact molecular mechanisms underlying the association between up-regulation of FGF1–FGFBP1 system in the kidney and human hypertension.
There are certain limitations of our analysis. We recognize that our data are based on subjects recruited exclusively in the south of Poland. This reduces a potential confounding effect of genetic heterogeneity across studies but also makes it more difficult to extrapolate the results from this analysis into other populations. We also appreciate that criteria of inclusion to SHS and SCS were not identical (hypertension in SHS and cardiovascular disease or its risk factors in SCS), which is clearly less optimal than having two populations selected for the same disease. However, we should stress that hypertension was a leading criterion of inclusion into SCS,12 and the overall demographic and clinical characteristics of 807 subjects in SCS was similar to the age-matching parental generation of SHS. We are aware that our pathway analysis did not capture other FGFs with a potential relevance to hypertension (i.e., FGF5). It is also fair to acknowledge the modest power of our study to detect genetic effects of small magnitude (allelic OR ≤ 1.3). Studies in larger cohorts of different origins will be necessary to provide further replication of our findings.
Within the interpretational limitations discussed above and together with previously published data, our results suggest that the FGF1 signaling pathway harbors common alleles and functionally relevant genes that may impact on the risk of hypertension. We should stress that the identified molecules were not previously suspected to play a role in human hypertension and they were uncovered through SNP-based association studies. Our investigation clearly shows a potential of systematic genetic analysis to illuminate novel biologic mechanisms that may underlie BP elevation.
CONCISE METHODS
Subjects
The SHS is a cohort of 207 families with essential hypertension from the Silesian region of Southern Poland.5,25,26 All hypertensive index patients (probands in the offspring generation) were identified and recruited together with available members of their families (parents and/or siblings). All 629 subjects were phenotyped using standardized questionnaires, physical examination, basic anthropometry, and fasting blood biochemistry. BP measurements were taken in line with the guidelines.5,25 Hypertension was defined as SBP and/or DBP ≥ 140/90 mmHg on at least three separate occasions and/or treatment with anti-hypertensive medication.
The SCS is a cohort of 213 Polish families and 435 singletons recruited through probands with high cardiovascular risk (history of hypertension, coronary artery disease, and/or multiple cardiovascular risk factors).12 Briefly, the phenotyping included collecting clinical information (using coded questionnaires), anthropometric measurements, and biochemical studies.12 BP was measured in triplicate using an oscillometric method according to the guidelines.27 Hypertension was defined as BP ≥140/90 mmHg measured on at least two separate occasions and/or taking anti-hypertensive medication.12 For the purpose of replication analysis, all biologically unrelated subjects (807 individuals) were selected from this cohort for genotyping.
The SRTB is an ongoing recruitment of subjects for analysis of gene expression in human hypertensive and normotensive kidney.5 In brief, Polish subjects with noninvasive renal cancer treated with elective unilateral nephrectomy were classified as hypertensive or normotensive based on the criteria used in SHS.5,25 Tissue samples from 62 individuals were collected from the healthy (unaffected by cancer) pole of the kidney and appropriately preserved immediately after surgery in RNAlater (Ambion, Austin, TX) for further transcript analysis. Renal tissue samples from 10 subjects collected into dry tubes and stored at −70°C were available for Western blotting. Additional tissue was immersion-fixed in 10% buffered formalin for immunhistochemistry.
The studies were approved by the local Bioethical Committee and adhere to the Declaration of Helsinki. All subjects have given informed written consent for participation.
DNA Analysis
Selection of Genes and SNPs for Primary Association Testing of the FGF1 Pathway in SHS
Of 31 putative FGF1-associated genes identified through HuGE Navigator web-based interface (http://hugenavigator.net/: FGFR1, ADA, ADAR, APOE, CASP5, EGF, FBN2, FGF2, IRF8, LOX, PIK3C2G, PI4KA, PSAP, TAP2, VEGFA, TGFBRAP1, ARHGEF10, IFI44, CSNK2A1, CSNK2A2, CSNK2B, FGFR4, FGFR2, FGFR3, HSPA9, MMP14, S100A13, SYT1, NRP1, FIBP, and FGFBP1), 7 filtered through the Reactome database (http://www.reactome.org/) into the FGF signaling pathway. An additional gene (SPRY1) identified as a negative regulator of the FGF cascade through The National Center for Biotechnology Information data mining was also included in the analysis. The eight genes encode critical components of FGF1 signaling including FGF1-paralog/competitive ligand (FGF2), extracellular chaperone (FGFBP1), four types of transmembrane receptors (FGFR1, FGFR2, FGFR3, and FGFR4), and specific intracellular messengers (FIBP and SPRY1). The graphical representations of the candidates along with their interactions within the FGF1 signaling pathway have been created using the GeneGo systems biology analytical program (http://www.genego.com/). The detailed characteristics of these genes are presented in Supplementary Table 2.
Each of the candidates was saturated with common (MAF >10%) tagging (under a threshold of r2 ≥ 0.8) SNPs selected from the population of Northern and Western European ancestry panel of the HapMap project (http://hapmap.ncbi.nlm.nih.gov/). In addition, all polymorphisms with MAF >10% and in silico functional annotations to regulatory regions of the selected genes (http://brainarray.mbni.med.umich.edu/Brainarray/Database/SearchSNP/snpfunc.aspx) were also included in the analysis.28 Altogether, 79 SNPs (60 tags and 19 potentially functional SNPs) were selected for genotyping (Supplementary Table 3).
Selection of SNPs for Fine Mapping in SHS and Replication Analysis in SCS
After primary association analysis, five additional SNPs (rs2532095, rs12499618, rs732244, rs732245, and rs2072313) with confirmed heterozygosity (MAF >1%) in a white population were selected for further fine mapping of the most significant locus (FGFBP1) in SHS. The SNP that showed the most significant association with hypertension (rs16892645) in SHS and the previously implicated hypertension-associated variant of FGF1 (rs152524)5 were chosen for replication genotyping in an additional cohort (SCS).
Genotyping
DNA was extracted from peripheral blood leukocytes. Genotyping in SHS was conducted by matrix-assisted laser desorption/Ionisation–time of flight mass spectrometry (MassARRAY system; Sequenom). Discrimination of genotypes was performed with the use of Typer software (version 3.4). Genotyping in SCS was conducted using in-house TaqMan SNP Genotyping Assay on ABI PRISM 7900HT Sequence Detection System (Applied Biosystems), and alleles were called using allelic discrimination software integrated with the DNA sequence analysis system.
RNA and Protein Analysis
Real-Time PCR.
FGFBP1 mRNA expression was quantified using an inventoried TaqMan gene expression assay (Applied Biosystems) and normalized to the expression of a housekeeping control gene (β-microglobulin) on the 7900HT Sequence Detection System (Applied Biosystems). Real-time PCR was carried out in a 20-μl volume using a reaction buffer containing 10 μl TaqMan Genotyping PCR Master Mix, 1 μl TaqMan probe/primer mix (both Applied Biosystems), 8 μl RNase-free water (Milli-Q system; Millipore), and 1 μl cDNA template from the reverse transcription reaction. Conditions of the reactions were as follows: 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds, followed by 60°C for 1 minute. Data were expressed as cycle threshold (Ct) and used to determine dCt values [Ct (FGFBP1) − Ct(Control)]. The fold difference in FGFBP1 expression between hypertensive and normotensive subjects was calculated according to the following formula: fold difference = 2−difference in dCt.5
Western Blotting.
Renal tissue was homogenized and diluted to equal protein concentrations (as measured by the BioRad protein assay), applied to 4 to 15% gradient acrylamide gels, and electroblotted onto nitrocellulose membranes. The membranes were incubated with the primary antibody against FGFBP1 (1:250, mouse monoclonal; R&D Systems, Minneapolis, MN). After rinsing, membranes were incubated with the horseradish peroxidase–labeled goat-anti-mouse IgG (1:10,000), and proteins were visualized by enhanced chemiluminescence (KPL, Gaithersburg, MD). The densities of specific bands were normalized to the total amount of protein loaded in each well following densitometric analysis of gels stained with β-actin (1:3000, rabbit monoclonal; Cell Signaling Technology, Boston, MA). The densities of specific bands were quantified by densitometry using the Scion Image beta (version 4.02) software.
Immunohistochemistry.
Paraffin sections were cut at 4 μm, and nonspecific immuno-labeling was blocked with 10% nonimmune goat serum. After incubation with the primary antibody against FGFBP1, the sections were rinsed with PBS and incubated with biotinylated anti-rabbit IgG for 1 hour at room temperature. This was followed by washing with PBS and incubation with the avidin-biotin complex. A positive reaction was identified after a 10-minute treatment with 3′,3′-diaminobenzadine containing 3% H2O2 and counterstaining with Mayer's hematoxylin.
Statistical Analysis
HWE was computed using the PLINK-based χ2 test in biologically unrelated individuals (all parents from SHS and all available subjects from SCS and SRTB) under the previously used threshold of P < 0.01.5
All FBATs were carried out after checking for Mendelian inconsistencies (http://www.biostat.harvard.edu/∼fbat/fbat.htm). Primary analysis of associations between each SNP and hypertension in SHS was conducted under an additive model of inheritance and null hypothesis of no linkage and no association using FBAT software. The family-based test extracts information from different types of families (nuclear families, sibships, families with more than one offspring), can handle missing parental genotypes, and is robust to population admixture and ascertainment based on phenotypes.29 To correct for multiple testing at the screening stage, we used the QVALUE software (http://www.genomics.princeton.edu/storeylab/qvalue/index.html) that calculates Storey and Tibshirani's q-values (q)30 for each individual FBAT result. Each individual estimate of corrected statistical significance (q) measures a minimum false-positive discovery rate that is generated when calling the test significant.30 A threshold of q < 0.25 (1 in 4) was used to identify findings that represent suggestive associations. At the fine mapping stage (one locus − FGFBP1), we applied Nyholt's spectral decomposition of pairwise LD matrices between SNPs.31 In brief, this correction accounts for LD-driven nonindependence of allelic segregation by calculation of the effective number of independent SNPs and the respective corrected threshold of statistical significance required to keep type I error rate at 5%.
Age-, gender-, and body mass index–adjusted logistic regression models (with hypertension as dependent variable) were constructed in PLINK32 under additive model of inheritance to replicate findings from family-based tests in biologically unrelated subjects from SCS. ORs with SEs generated in the adjusted models were used as estimates of genetic effect sizes.32 A combined analysis of association between the most significant FGFBP1 SNP and hypertension in SHS and SCS was conducted using the novel generalized estimating equations–based approach that extracts information from both unrelated individuals and different types of nuclear families in a pooled analysis.33 By integrating principal component analysis with transmission disequilibrium test strategies, this method minimizes potential stratification effects driven by heterogeneity of the combined populations.33 Zheng's test can handle the data from families with multiple siblings and account for missing parental information.33 Combining data from biologically related and unrelated subjects, this method improves power to detect genetic associations compared with typical tests used in analysis of family and unrelated samples separately.33
The combined effect of FGFBP1 (rs16892645) and FGF1 (rs152524) SNPs on hypertension was examined by calculation of their cumulative genotype scores in all available unrelated individuals from SCS. The score was calculated as the summative dosage of risk alleles (C for rs16892645 and A for rs152524) and ranged from 0 (individuals with TT genotype for rs16892645 and GG genotype for rs152524) to 4 (double homozygous for risk alleles of rs16892645 and rs152524). Association between the genetic score and hypertension was calculated using binary logistic regression with hypertension as an outcome phenotype and age, gender, and body mass index, as well as genetic score (coded as 0 through 4; whereby 0 defines none risk alleles and 4 defines four risk alleles) as independent variables.
A t test was used to compare groups in quantitative real-time PCR and Western blotting analysis. Adjusted (for age, gender, and body mass index) analysis of association between hypertension and mRNA of FGFBP1 was conducted using binary logistic regression.
Power-based association test-based34 calculations showed that, under additive model of inheritance, both the family-based study (SHS) and the case-control sample from SCS had moderate to good power (0.5 to 1.0) to detect associations of larger magnitude (allelic OR of 1.6 to 1.9) between common alleles (MAF = 0.1 to 0.4) and hypertension and modest to moderate power (0.2 to 0.6) to detect associations with smaller effect sizes (allelic OR ≤ 1.3).
DISCLOSURES
None.
Acknowledgments
This work was supported by a University of Leicester Departmental grant-in-aid (M61MF99 to M.T.) and a National Institutes of Health Fogarty International Research Collaboration award (R03 TW007165 to M.T., C.M., and E.Z.-S.). F.J.C. was supported by a L.E.W. Carty Charitable Fund establishment grant and a Helen MacPherson Smith Trust small grant. T.B. was supported by a British Heart Foundation project grant (PG/06/097 to M.T.). N.J.S. holds a British Heart Foundation Chair of Cardiology. Fellowship of W.Y.S. in the Department of Cardiovascular Sciences (University of Leicester) was supported by Royal Australasian College of Physicians Barry Young Fellowship. This study is part of the research portfolio supported by the Leicester NIHR Biomedical Research Unit in Cardiovascular Disease.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this title is available online at http://www.jasn.org/.
REFERENCES
- 1. Tomaszewski M, Zimmerli L, Charchar FJ, Dominiczak AF: Genetic information in the diagnosis and treatment of hypertension. Curr Hypertens Rep 10: 309–316, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Wellcome Trust Case Control Consortium, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Doring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O'Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O'Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvanen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dorr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Volker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Volzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB: Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 41: 666–676, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Kottgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O'Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM: Genome-wide association study of blood pressure and hypertension. Nat Genet 41: 677–687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takeuchi F, Isono M, Katsuya T, Yamamoto K, Yokota M, Sugiyama T, Nabika T, Fujioka A, Ohnaka K, Asano H, Yamori Y, Yamaguchi S, Kobayashi S, Takayanagi R, Ogihara T, Kato N: Blood pressure and hypertension are associated with 7 loci in the Japanese population. Circulation 121: 2302–2309, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Tomaszewski M, Charchar FJ, Lynch MD, Padmanabhan S, Wang WY, Miller WH, Grzeszczak W, Maric C, Zukowska-Szczechowska E, Dominiczak AF: Fibroblast growth factor 1 gene and hypertension: From the quantitative trait locus to positional analysis. Circulation 116: 1915–1924, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Strutz F, Zeisberg M, Hemmerlein B, Sattler B, Hummel K, Becker V, Muller GA: Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int 57: 1521–1538, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Kunz D, Walker G, Eberhardt W, Messmer UK, Huwiler A, Pfeilschifter J: Platelet-derived growth factor and fibroblast growth factor differentially regulate interleukin 1beta- and cAMP-induced nitric oxide synthase expression in rat renal mesangial cells. J Clin Invest 100: 2800–2809, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, Yin M, Coffin JD, Kong L, Kranias EG, Luo W, Boivin GP, Duffy JJ, Pawlowski SA, Doetschman T: Fibroblast growth factor 2 control of vascular tone. Nat Med 4: 201–207, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nabel EG, Yang ZY, Plautz G, Forough R, Zhan X, Haudenschild CC, Maciag T, Nabel GJ: Recombinant fibroblast growth factor-1 promotes intimal hyperplasia and angiogenesis in arteries in vivo. Nature 362: 844–846, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Cuevas P, Carceller F, Reimers D: Gimenez-Gallego: Fibroblast growth factor-1 inhibits medial smooth muscle cells apoptosis after balloon injury. Neurol Res 22: 185–188, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Izikki M, Guignabert C, Fadel E, Humbert M, Tu L, Zadigue P, Dartevelle P, Simonneau G, Adnot S, Maitre B, Raffestin B, Eddahibi S: Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest 119: 512–523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomaszewski M, Charchar FJ, Barnes T, Gawron-Kiszka M, Sedkowska A, Podolecka E, Kowalczyk J, Rathbone W, Kalarus Z, Grzeszczak W, Goodall AH, Samani NJ, Zukowska-Szczechowska E: A common variant in low-density lipoprotein receptor-related protein 6 gene (LRP6) is associated with LDL-cholesterol. Arterioscler Thromb Vasc Biol 29: 1316–1321, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cotton LM, O'Bryan MK, Hinton BT: Cellular signaling by fibroblast growth factors (FGFs) and their receptors (FGFRs) in male reproduction. Endocr Rev 29: 193–216, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tassi E, Al-Attar A, Aigner A, Swift MR, McDonnell K, Karavanov A, Wellstein A: Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J Biol Chem 276: 40247–40253, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Brown RH: A reinnervating microRNA. Science 326: 1494–1495, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Ray PE, Tassi E, Liu XH, Wellstein A: Role of fibroblast growth factor-binding protein in the pathogenesis of HIV-associated hemolytic uremic syndrome. Am J Physiol Regul Integr Comp Physiol 290: R105–113, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Tomita Y, Kusama Y, Seino Y, Munakata K, Kishida H, Hayakawa H: Increased accumulation of acidic fibroblast growth factor in left ventricular myocytes of patients with idiopathic cardiomyopathy. Am Heart J 134: 779–786, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Allayee H, de Bruin TW, Michelle Dominguez K, Cheng LS, Ipp E, Cantor RM, Krass KL, Keulen ET, Aouizerat BE, Lusis AJ, Rotter JI: Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension 38: 773–778, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Ingelsson E, Larson MG, Vasan RS, O'Donnell CJ, Yin X, Hirschhorn JN, Newton-Cheh C, Drake JA, Musone SL, Heard-Costa NL, Benjamin EJ, Levy D, Atwood LD, Wang TJ, Kathiresan S: Heritability, linkage, and genetic associations of exercise treadmill test responses. Circulation 115: 2917–2924, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Chen W, Li S, Srinivasan SR, Boerwinkle E, Berenson GS: Autosomal genome scan for loci linked to blood pressure levels and trends since childhood: The Bogalusa Heart Study. Hypertension 45: 954–959, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Moreno C, Dumas P, Kaldunski ML, Tonellato PJ, Greene AS, Roman RJ, Cheng Q, Wang Z, Jacob HJ, Cowley AW: Genomic map of cardiovascular phenotypes of hypertension in female Dahl S rats. Physiol Genomics 15: 243–257, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Abuharbeid S, Czubayko F, Aigner A: The fibroblast growth factor-binding protein FGF-BP. Int J Biochem Cell Biol 38: 1463–1468, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Xie B, Tassi E, Swift MR, McDonnell K, Bowden ET, Wang S, Ueda Y, Tomita Y, Riegel AT, Wellstein A: Identification of the fibroblast growth factor (FGF)-interacting domain in a secreted FGF-binding protein by phage display. J Biol Chem 281: 1137–1144, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Liu XH, Aigner A, Wellstein A, Ray PE: Up-regulation of a fibroblast growth factor binding protein in children with renal diseases. Kidney Int 59: 1717–1728, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Tomaszewski M, Brain NJ, Charchar FJ, Wang WY, Lacka B, Padmanabahn S, Clark JS, Anderson NH, Edwards HV, Zukowska-Szczechowska E, Grzeszczak W, Dominiczak AF: Essential hypertension and β2-adrenergic receptor gene: Linkage and association analysis. Hypertension 40: 286–291, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Tomaszewski M, Charchar FJ, Lacka B, Pesonen U, Wang WY, Zukowska-Szczechowska E, Grzeszczak W, Dominiczak AF: Epistatic interaction between β2-adrenergic receptor and neuropeptide Y genes influences LDL-cholesterol in hypertension. Hypertension 44: 689–694, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B: Management of Arterial Hypertension of the European Society of Hypertension, European Society of Cardiology: 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 25: 1105–1187, 2007. 17563527 [Google Scholar]
- 28. Tomaszewski M, Charchar FJ, Samani NJ: Association studies in current cardiovascular genetics: Functional variants, tags or both? J Hum Hypertens 21: 425–426, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Horvath S, Xu X, Laird NM: The family based association test method: Strategies for studying general genotype-phenotype associations. Eur J Hum Genet 9: 301–306, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Storey JD, Tibshirani R: Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100: 9440–9445, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nyholt DR: A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 74: 765–769, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC: PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang L, Pei YF, Li J, Papasian CJ, Deng HW: Univariate/multivariate genome-wide association scans using data from families and unrelated samples. PLoS One 4: e6502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lange C, DeMeo D, Silverman EK, Weiss ST, Laird NM: PBAT: Tools for family-based association studies. Am J Hum Genet 74: 367–369, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]