Abstract
In biopsies of renal allografts, arteriosclerosis is often more severe than expected based on the age of the donor, even without a history of rejection vasculitis. To determine whether preformed donor-specific antibodies (DSAs) may contribute to the severity of arteriosclerosis, we examined protocol biopsies from patients with (n = 40) or without (n = 59) DSA after excluding those with any evidence of vasculitis. Among DSA-positive patients, arteriosclerosis significantly progressed between month 3 and month 12 after transplant (mean Banff cv score 0.65 ± 0.11 to 1.12 ± 0.10, P = 0.014); in contrast, among DSA-negative patients, we did not detect a statistically significant progression during the same timeframe (mean Banff cv score 0.65 ± 0.11 to 0.81 ± 0.10, P = not significant). Available biopsies at later time points supported a rate of progression of arteriosclerosis in DSA-negative patients that was approximately one third that in DSA-positive patients. Accelerated arteriosclerosis was significantly associated with peritubular capillary leukocytic infiltration, glomerulitis, subclinical antibody-mediated rejection, and interstitial inflammation. In conclusion, these data support the hypothesis that donor-specific antibodies dramatically accelerate post-transplant progression of arteriosclerosis.
Arterial lesions are often prominent in post-transplant renal biopsies. In cell-mediated rejection, these lesions are usually clearly inflammatory in nature, with monocytic and lymphocytic infiltration of the intima (Banff v1 and v2), and myofibroblasts laying down extracellular matrix, progressively compromising the lumen. These lesions are designated as “endarteritis,” reflecting their inflammatory nature, and in later stages, when inflammation diminishes and/or disappears, as “transplant arteriopathy.”1–3
In antibody-mediated rejection (AMR), in contrast, intimal vascular lesions (v1,v2) were initially thought to be rare and are not included among the Banff criteria for AMR.4 However, it is becoming apparent that intimal vasculitic lesions may be associated with AMR5–7; therefore, these criteria may have to be re-evaluated.
Arteriosclerosis may be conspicuous, particularly in AMR, occurring in the absence of evident concurrent or prior intimal vascular inflammation. The observer is often left with the impression that the arteriosclerosis is worse than would be expected on the basis of the donor's age. Arteriosclerosis in fact is one of the criteria for diagnosis of chronic humoral rejection (CHR), described by Colvin et al.1,3,4 The CHR criteria stress that the arteriosclerosis here should consist only of intimal thickening without duplication of the elastica, distinguishing this lesion in the opinion of the authors from the fibroelastic thickening seen in hypertension.
In this study, we investigated post-transplant progression of arteriosclerosis in a series of donor-specific antibody (DSA)+ and DSA− patients with systematic 3- and 12-month screening biopsies with C4d staining and DSA quantification by Luminex. We used as a baseline for the natural progression of arteriosclerosis, as reflected by the progression of Banff cv scores with age, 91 d-0 biopsies, 80 of which were drawn from the cohorts studied. Also evaluated were 38 late biopsies, performed primarily for clinical reasons. As will be seen, there was indeed an impressive acceleration of arteriosclerosis in DSA+ patients, strongly associated with the presence of the newly described entity of subclinical AMR,8,9 including microcirculation inflammation and C4d and DSA positivity.
Acceleration of arteriosclerosis also occurred to a lesser, but significant, extent in DSA− patients. However, conversion to de novo DSA positivity in four initially DSA− patients speeded the rate of acceleration to more near the rates seen in patients that were DSA+ from the outset.
RESULTS
The observations on acceleration of arteriosclerosis are divided into two parts. The first describes the clinical and morphologic factors associated with progression. The second details the morphologic observations of the arteries themselves.
Clinical Comparisons Between DSA+ and DSA− Groups
The DSA+ and DSA− groups were entirely comparable in clinical terms, with no significant differences in terms of donor and recipient ages, frequency of hypertension, cold ischemia time, HLA mismatch, or renal function at 3 months and 1 year (Table 1).
Table 1.
Population characteristics
| Parameters | DSA Positive (n = 40) | DSA Negative (n = 59) | P |
|---|---|---|---|
| Recipient characteristics | |||
| recipient age (years, mean ± SD) | 46 ± 12 | 48 ± 12 | NS |
| deceased donor (%) | 100% | 100% | NS |
| donor age (years, mean ± SD) | 47 ± 15 | 51 ± 14 | NS |
| cold ischemia time (hours, mean ± SD) | 27 ± 7 | 29 ± 9 | NS |
| retransplantation (%) | 22 (55%) | 4 (6.7%) | <0.001 |
| Immunology | |||
| CDC positive cross-match (n, %) | 19 (47.5%) | 0 (0%) | <0.001 |
| class I or II Peak pretransplant DSA MFImax | 7479 ± 872 | — | |
| HLA A + B mismatch (mean ± SD) | 2.1 ± 1.0 | 1.9 ± 1.1 | NS |
| HLA DR mismatch (mean ± SD) | 1.3 ± 0.7 | 0.9 ± 0.7 | NS |
| Transplant outcome | |||
| GFR at 3 months (ml/min per 1.73 m2) | 54 ± 3 | 49 ± 17 | NS |
| GFR at 1 year (ml/min per 1.73 m2) | 46 ± 3 | 49 ± 3 | NS |
| proteinuria at 3 months (g/24 h) | 0.25 ± 0.04 | 0.39 ± 0.09 | NS |
| proteinuria at 1 year (g/24 h) | 0.21 ± 0.05 | 0.43 ± 0.16 | NS |
| last SCr (μmol/dl, mean ± SD) | 214 ± 33 | 139 ± 50 | <0.001 |
| follow-up (months, mean ± SD) | 42 ± 14 | 59 ± 20 | <0.05 |
| graft loss | 2 | 2 | — |
| patient death | 0 | 0 | — |
Values expressed in mean ± SEM unless otherwise specified. NS, not significant.
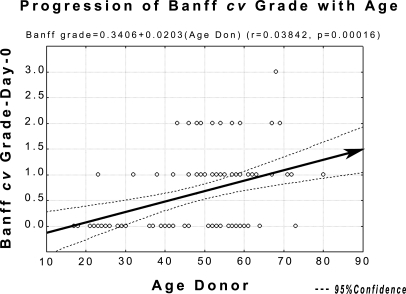
Normal Progression of Arteriosclerosis with Age.
The normal progression of arteriosclerosis with age, as gauged by the Banff cv scores, the relevant parameter in evaluating post-transplant biopsies, was estimated from 91 d-0 biopsies: 80 from the final cohort and 11 from more recent biopsies (median age, 53.0 years; range, 17 to 80 years; Figure 1). The linear regression obtained was Banff cv grade = − 0.3406 + 0.0203(AgeDonor), (r = 0.3840, P = 0.00016). The regression line and confidence intervals from this correlation served as the reference in showing acceleration of arteriosclerosis in transplant patients. (The Banff cv grades were not, in fact, normally distributed, but the Pearson correlation is the simplest method for presenting their natural progression graphically). The adequacy of this approach was shown by the fact that all of the 3-month values in the various comparisons by age, DSA type, etc., started from within the confidence limits for natural progression. Changes in grade at different intervals were analyzed by the Mann-Whitney U test.
Figure 1.
Progression of Banff cv grade at 1 year post-TX, based on 91 d-0 biopsies. The regression line here and the confidence intervals will be used in subsequent graphs showing acceleration of arteriosclerosis, using as the point of depart the mean donor age at outset.
Sampling error, always a problem when comparing successive biopsies from an individual patient, was effectively ruled out as an explanation for the overall results by the Kolmogorov-Smirnov and Lillefors tests for normality of distribution (both P < 0.01).
Acceleration of Arteriosclerosis after Transplant
The values below indicate the progression of arteriosclerosis, in terms of Banff score, between 3 and 12 months, with late biopsies discussed separately. It would have been preferable to measure progression from day 0, but 19.2% of patients lacked day-0 biopsies.
Overall Results
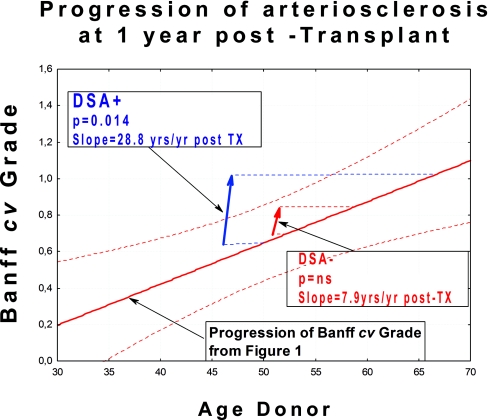
Taken as a group, the DSA+ group progressed from Banff cv grade 0.65 ± 0.11 at 3 months to grade 1.12 ± 0.10 at 1 year after transplant, (slope of increase in Banff cv score = 28.8 yr/yr after transplantation [Tx]; P = 0.014; Figure 2). The DSA− group also showed an increase from 0.65 ± 0.11 to 0.81 ± 0.10 (slope = 7.9 yr /yr after Tx), but this difference was not significant at this time point.
Figure 2.
Lesions progress more rapidly in DSA+ patients than in DSA− patients. Figure shows overall progression of Banff cv grade at 1 year post-TX. The 89 patients in this study were only analyzed completely for the first 12 months. The arrows begin at the median donor age for the cohort in question.
Clinical Factors.
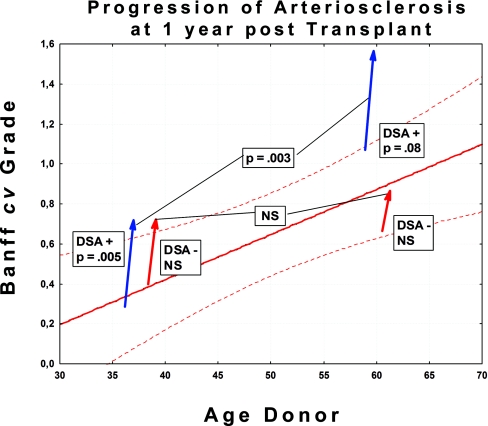
In both DSA+ and DSA− groups, the rate of progression of arteriosclerosis was unaffected by the age of the donor or recipient. Division of patients into those ≤50 and >50 years of age confirmed that the older patients had much higher baseline grades than the younger ones, as expected (Figure 3). However, there were no significant differences in rate of progression for older versus younger patients (Table 2). Hypertension also might theoretically be expected to play a role in the acceleration of arteriosclerosis. However, there were no significant differences in progression normotensive patients, including those normotensive under anti-hypertensive therapy and those who were hypertensive despite therapy (Table 2).
Figure 3.
There are no significant differences in rate of progression between patients over 50 years of age compared with those below 50 years of age. Figure shows progression of Banff cv grade at 1 year post-TX. Cohorts are divided into those ≤50 and <50 years of age. The arrows begin at the median donor age for the cohort in question.
Table 2.
Clinical factors in progression of arteriosclerosis
| Parameter | DSA-Positive |
DSA-Negative |
||||
|---|---|---|---|---|---|---|
| No. | Δcv Gradea | P | No. | Δcv Gradea | P | |
| Donor age | ||||||
| ≤50 years | 20 | 0.45 | NS | 20 | 0.30 | NS |
| > 50 years | 20 | 0.45 | 39 | 0.21 | ||
| Recipient age | ||||||
| ≤50 years | 26 | 0.46 | NS | 31 | 0.25 | NS |
| > 50 years | 14 | 0.39 | 27 | 0.11 | ||
| BP | ||||||
| normotensiveb | 20 | 0.35 | NS | 18 | 0.30 | NS |
| hypertensive | 20 | 0.49 | 37 | 0.06 | ||
| Acute cellular rejection (morphologic) | ||||||
| present | 4 | 0.13 | NS | 8 | 0.50 | NS |
| absent | 36 | 0.43 | 51 | 0.14 | ||
NS, not significant.
aΔ cv grade, increment of cv score between 3- and 12-month biopsies.
bGroup includes those normotensive on medication.
Morphologic Parameters.
All of the various Banff parameters were tested for their correlation with acceleration of arteriosclerosis (Table 3). Among the DSA+ patients, all of the parameters associated with active humoral response (C4d peritubular capillary [PTC] staining, PTC leukocytic infiltration, glomerulitis, subclinical AMR, and interstitial inflammation) or its sequelae (interstitial fibrosis/tubular atrophy) were significantly associated with acceleration. Transplant glomerulopathy also seems likely to be associated (P = 0.11), but there were too few cases to reach significance. Two other parameters with an interstitial inflammatory component, tubulitis and borderline lesion, were also associated with acceleration. Among DSA+ subjects, the presence of anti-calcineurin–related arteriolopathy showed no relation to acceleration, with nearly identical increments of arteriosclerosis in those with anti-calcineurin arteriolopathy (Δgrade = 0.39) and those without (Δgrade = 0.37, P = not significant).
Table 3.
Increment in grade of arteriosclerosis between 3 and 12 months as analyzed by morphologic parameter
| DSA+ |
DSA− |
|||||
|---|---|---|---|---|---|---|
| No. | Δ cv Grade | P | No. | Δ cv Grade | P | |
| Glomerulitis | 31 | 0.45 | 0.04 | 11 | 0.06 | NS |
| Transplant glomerulopathy | 11 | 0.59 | 0.11 | 5 | 0.08 | NS |
| Tubulitis | 10 | 0.80 | 0.01 | 11 | −0.17 | NS |
| Interstitial inflammation | 29 | 0.64 | 0.008 | 19 | −0.07 | NS |
| IF/TA | 30 | 0.61 | 0.008 | 34 | 0.18 | NS |
| PTC infiltration | 20 | 0.66 | 0.003 | — | — | — |
| C4d capillary staining | 23 | 0.63 | 0.019 | — | — | — |
| Acute cellular rejection | 4 | 0.13 | NS | 8 | 0.50 | NS |
| Borderline lesion | 14 | 0.69 | 0.03 | 7 | −0.28 | NS |
| Subclinical AMR | 23 | 0.67 | 0.006 | — | — | — |
| Anticalcineurin arteriolopathy | 8 | 0.50 | NS | 44 | 0.06 | NS |
NS, not significant.
Relationship to Renal Function.
There were no significant differences between cases whose arterial lesions advanced and those where arterial lesions remained stable or decreased either among the DSA+ or the DSA− patients at either 3 months or 1 year. Nor were there any significant differences between the serum creatinine (SCr) at the time of last follow-up; for example, among the DSA+ patients, final SCr was 206 ± 45 versus 227 ± 50 μmol/L for advancing versus stable or decreasing lesions, respectively (P = not significant).
Late Biopsies.
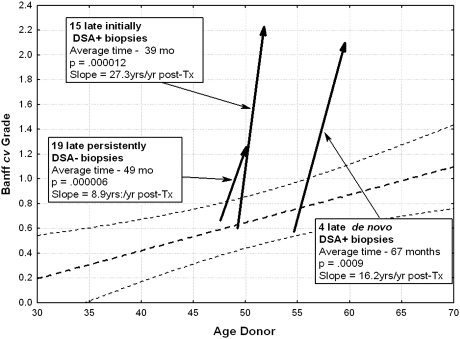
Late biopsies were performed at an average of 39 ± 4 months after transplant in 15 DSA+ patients. Mean Banff cv grade of arteriosclerosis progressed from 0.57 ± 0.17 at 3 months to 2.23 ± 0.15 (P = 0.000012; Figure 4; Table 4). This translates to a slope of increase in Banff cv score of roughly 27 yr/yr after Tx.
Figure 4.
Patients with de novo positivity had rates of progression more nearly approaching that of initially DSA+ cases than that of persistently DSA− patients. Figure shows progression of Banff cv in late biopsies. The arrows begin at the median donor age for the cohort in question.
Table 4.
Late biopsies: acceleration of arteriosclerosis according to presence of preformed and de novo DSA
| Preformed DSA | No Preformed DSA |
||
|---|---|---|---|
| De Novo DSA | Persistent Negative DSA | ||
| Donor age at transplantation | 48 ± 4 | 50 ± 6 | 49 ± 4 |
| No. patients | 15 | 4 | 19 |
| 3-month Bx | 0.57 ± 0.17 | 0.50 ± 0.29 | 0.63 ± 0.17 |
| Last Bx | 2.23 ± 0.15 | 2.25 ± 0.25 | 1.32 ± 0.15 |
| Δ cv grade | 1.67 ± 0.20 | 1.75 ± 0.25 | 0.63 ± 0.23 |
| P = 0.000012 | P = 0.0009 | P = 0.000006 | |
| Months to last Bx | 39 ± 4 | 67 ± 16 | 49 ± 4 |
| Slope of Banff cv grade | 27.3 yr/yr | 16.2 yr/yr | 8.9 yr/yr |
Late biopsies were also performed in 23 initially DSA− patients, primarily for renal insufficiency. Among them, four developed de novo DSA positivity, with an increase of 1.75 ± 0.75 over 50 ± 6 months (P = 0.0009). The 19 patients who remained DSA− had a slower, but significant (P = 0.00006) increase in grade over 49 ± 4 months. The rate of progression of arteriosclerosis in DSA− patients was thus about one third that in DSA+ patients (Table 4).
Morphologic Observations
Arteriosclerotic lesions will be described first in DSA-positive patients, in which progression is faster and the evolution of lesions more evident. Then follow comments on the progression of lesions in initially DSA− patients.
DSA+ Biopsies.
At 3 months, the arterial endothelium was more prominent and basophilic than in normal aging arteries (Figure 5, A and B). Variable numbers of round cells, staining as myofibroblasts with α-smooth muscle actin (SMA), were interposed between the internal elastica and the endothelium (Figure 5A), with modest increase in extracellular matrix (ECM). None of the cases stained immunohistochemically showed any CD3+ or CD20+ cells at 3 months or subsequently. In arteries with prior intimal lesions, the same round cells were identified, but here they were largely intermixed with preexisting collagen fibers (Figure 5B).
Figure 5.
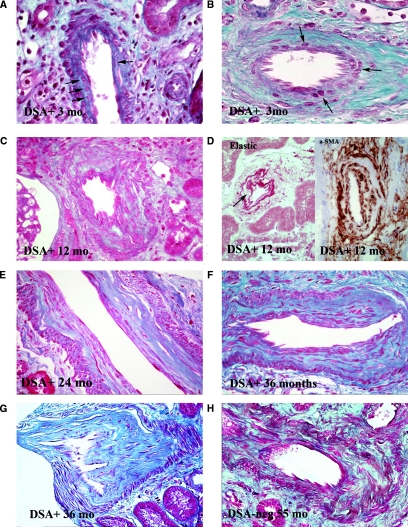
Morphologic features of accelerated arteriosclerosis. (A) Artery without preexisting arteriosclerosis, 3 months after TX. Several cells (arrows) interposed between internal elastica and endothelim, with little associated extracellular matrix. Such cells stain positively for α-SMA. Masson trichrome stain (MT), ×550. (B) Artery with prior arteriosclerosis, 3 months after TX. Several cells lying immediately beneath the endothelium. The amount of extracellular matrix they have elaborated is not possible to determine. MT, ×500. (C) Artery at 1 year after TX. The intima is markedly thickened and is quite cellular throughout with abundant relatively mature collagen fibers. MT, ×400. (D) Arteries at 1 year after TX. The left panel shows an artery stained for elastic fibers, showing well-formed layer adjacent to endothelium with prominent cells (arrow) just beneath in the thickened intima. Orcein stain, ×550. Right panel shows artery stained for α-SMA showing hypercellular intima with abundant positivity. Arrow shows the gray-staining internal elastica. Everything within represents expanded intima. Magnification, ×600. (E) Artery at 2 years after TX. The outer portions of the intima are composed of dense collagen, but the inner portion remains hypercellular, although collagen appears to be relatively mature. Patient had active ongoing SAMR. MS, ×350. (F) Artery at 3 years after TX. Distinction between relatively hypercellular inner intimal zone and dense collagen in outer intima remains. Patient had active SAMR. Magnification, ×350 (G) Artery at 3 years. Dense collagenous intima shows only modest cellularity. Patient currently had only minimal evidence of AMR (minimal glomerulitis graded as ± with no PTC infiltration). MT, ×350. (H) Artery in DSA− patient at 55 months. The intima is mildly hypercellular, the elongated cells lying between bands of mature collagen. MT,×350.
At 12 months, numerous myofibroblasts were identifiable in the intima, and there was now substantial ECM staining as collagen (Figure 5C), with recognizable elastic fibers admixed on special stain (Figure 5D). α-SMA positivity was maintained, particularly in the subendothelial zones (Figure 5D). In smaller arteries, the entire intimal layer had a cellular quality, but in larger arteries, it was often possible to identify older condensed, acellular collagen adjacent to the internal elastica, whereas the layer adjacent to the lumen, representing the newly laid down collagen, was relatively cellular.
Late Biopsies in DSA+ Patients.
Fifteen late biopsies, taken at 24 to 84 months, were available. The appearance of the arteries seemed to depend on the patient's AMR status. Most patients had ongoing AMR, with glomerulitis and PTC leukocytic infiltration. In these cases, the distinction between a dense collagenous, relatively acellular outer intimal zone, and a hypercellular inner zone adjacent to the lumen was maintained (Figure 5, E and F). The picture was one of simple arteriosclerosis. Only the somewhat hypercellular subintimal zone suggesting that the lesion had taken origin as part of an AMR.
DSA− Biopsies.
These biopsies overall showed a mild increase of grade of arteriosclerosis between 3 and 12 months, but this difference was not significant, and examination showed only occasional mild intimal hypercellularity.
Late Biopsies in Initially DSA− Patients.
Of the 23 late biopsies in this group, taken at 18 to 90 months, 19 were in patients who were persistently DSA−. In these patients, there was mild intimal hypercellularity, the cells sitting between bands of mature collagen fibers (Figure 5G). However, neither the degree of hypercellularity nor the tendency to stratification into outer dense collagenous and inner cellular intimal zones was as impressive as in DSA+ patients. Three of the four patients with de novo DSA positivity showed arteries similar to those described above for DSA+ patients.
Bland Arteriosclerosis in DSA+ Patients.
Arteries in three patients, two initially DSA+ and one de novo DSA+, showed similar bland appearances. The intima, although massively thickened, was now bland, with condensed collagen and elastic fibers, and relatively acellular. Such arteries were indistinguishable from those of banal arteriosclerosis. All three such DSA+ cases had a similar history of AMR or chronic AMR (CAMR) documented on prior biopsies, but with no glomerulitis or PTC infiltration to suggest active AMR on the present biopsy.
DISCUSSION
The central observation in this study is that arteriosclerosis is accelerated after transplant in both DSA+ and DSA− patients. It progresses much more rapidly in DSA+ patients than in DSA− patients, roughly three times faster in our series. The importance of DSA positivity was manifest in four initially DSA− patients who later developed de novo DSA positivity and had a rate of progression intermediate between initially DSA+ and persistently DSA− patients (Table 4).
We were able to eliminate certain factors, among them donor age, recipient age, and the presence or absence of hypertension, and anti-calcineurin arteriolopathy, as having any major influence on the rate of progression of lesions, in either DSA+ or DSA− patients (Table 2). This rate seemed relatively constant across all age ranges, although obviously the baseline on which the new lesions were superimposed increased with increasing age (Figure 3).
Because the majority of late biopsies were indication biopsies, the late lesions seen might possibly not be representative of the population as a whole. However, the 3- and 12-month protocol biopsies for the entire population already showed the same pattern of acceleration. Furthermore, the estimated slopes of change in Banff cv scores at 12 months and for late biopsies were remarkably similar (for initially DSA+ patients, 28.8 and 27.3 yr/yr after Tx, respectively).
Role of DSA Positivity
Among DSA+ patients, acceleration of arteriosclerosis was strongly associated with the features of AMR, including C4d positivity, PTC leukocytic infiltration, glomerulitis, and interstitial inflammation (Table 3). In fact, all patients who showed acceleration of arteriosclerosis, either at 1 year or on late (24 to 84 months) biopsies had subclinical AMR (SAMR) at 3 months, 12 months, or both.
The evidence thus points clearly to DSAs as a major factor in accelerated progression of arteriosclerosis. There is abundant literature laying out the pathophysiologic underpinnings for such a progression. It has been shown that ligation of anti-class I antibodies to class I molecules on the vascular endothelium and smooth muscle cells induces release of a variety of cytokines and stimulates proliferation of myofibroblasts.10–13 Similar reactions occur with anti-class II antibodies, and evidence would suggest that these antibodies correlate better with both morphologic and functional changes than do class I antibodies.13–17
Other Possible Mechanisms in the Acceleration of Arteriosclerosis
Persistently DSA− biopsies had increases in grade of arteriosclerosis, slower and not yet significant at 12 months, but highly significant for late biopsies (P = 0.000006). The reasons for this increase are not clear. There is a tantalizing association with morphologic acute cellular rejection (ACR) at 1 year (Table 3), but this association does not reach significance, and only interstitial fibrosis is significantly associated with progression at any point. In addition to possible T cell–mediated processes, the possibility must be kept in mind that antibodies other than standard class I and class II antibodies, for example, MHC class I–related chain A antibodies,18,19 might be playing a lesser role in progression of arteriosclerosis.
Implications for the Diagnosis of CAMR
Arteriosclerosis is one of the criteria for the diagnosis of CAMR.1,4 As a means of separating arteriosclerosis related to transplantation from pretransplant lesions, the criteria require the presence of intimal thickening only, without duplication of the elastica, which the authors considered to be a mark of pretransplant lesions. However, the criterion of lack of elastic fibers as a means of distinguishing pre- from post-transplant intimal lesions breaks down fairly rapidly, such that by 1 year, mature elastic fibers and relatively condensed collagen fibers are seen. That these fibers are new and not preexisting is shown by the fact that in most instances the inner intima is relatively hypercellular, indicating its recent origin. Thus, beyond the first few months, the criterion of absence of elastic fibers is no longer a valid distinguishing characteristic in the diagnosis of CAMR. By contrast, a relatively hypercellular intima, particularly the inner intimal zone, is a very good sign of ongoing AMR (Figure 5, C, E, and F). In our series, it was invariably accompanied by PTC leukocytic infiltration and glomerulitis.
There remain, however, a few quiescent cases in which there has been progression in degree of arteriosclerosis over that on prior biopsies but which present an entirely bland, relatively acellular intima indistinguishable from banal arteriosclerosis of aging (Figure 5G; Supplemental Figure S3). In the three such cases we had, accompanying signs of AMR were minimal or absent, thus not qualifying as CAMR. However, it will take greater experience to see if this distinction holds.
Relationship of Arteriosclerosis to Vasculitic (v1 and v2) Lesions
According to the current Banff criteria, vasculitic v1 and v2 lesions are considered related to ACR, as are v3 medial necrotizing lesions.20,21 For AMR only, the severe medial v3 lesions are recognized in the criteria.21 However, the notion that v1 and v2 lesions are the unique province of cell-mediated rejection is coming under challenge. In a 2007 study of biopsies for clinical rejection in confirmed AMR, we found v1 and v2 in 33.3 and 23.8% of patients at first biopsy, respectively.22 Furthermore, we found that v1–v2 was closely correlated with signs of AMR (glomerulitis, PTC) and that the tubulitis expected with ACR was totally absent in 12/25 cases. We suggested on the basis of these findings that intimal vasculitis could be a manifestation of AMR rather than simply concomitant ACR.5 This notion has been furthered and strengthened in a microarray analysis of isolated v-lesions that showed similar levels for IFN-γ effects and macrophage burden but lower T cell transcripts, with three of nine patients with isolated lesions being DSA+.7 A recent Japanese study6 has also suggested that DSA may be implicated in intimal vasculitis. With the passage of time and improvement in desensitization techniques and immunosuppressive regimens, overt vasculitic lesions have become quite uncommon in protocol biopsies in stable patients,14,23 both in DSA+ and DSA− patients.
Although, in this study, we eliminated cases with any recognizable vasculitis, including endothelial “sticking,” we obviously cannot rule out the total absence of inflammatory cells at any point. What we can affirm is that arterial lesions largely indistinguishable from banal arteriosclerosis can occur with no or minimal recognizable intimal inflammation in both DSA+ and DSA− patients. It is known that a battery of inflammatory cytokines are released after transplant in DSA+ patients, inciting endothelial and myofibroblast proliferation.1,2,13 A similar cascade is unleashed in cell-mediated rejection.2,3
Perhaps it is simplest to consider both the DSA+ and DSA− cases of accelerated arteriosclerosis as representing a muted, largely subleucocytic inflammatory and proliferative response forming a continuum with more overt vasculitic lesions. DSAs seem to push this acceleration most effectively, particularly in patients manifesting SAMR, but it seems likely that cell-mediated reactions may do the same, although at a slower rate.
Thus, these low-grade subleucocytic arterial proliferative lesions lead more slowly, but just as inexorably, to the same luminal narrowing and occlusion seen in the postvasculitic lesions we label “transplant arteriopathy.” The logical conclusion to be drawn from these parallels is that the accelerated arteriosclerosis we see here is in fact a form of transplant arteriopathy, but one that may not be recognized as such unless it betrays its origins by a hypercellular intimal zone, masquerading instead as banal arteriosclerosis.
CONCISE METHODS
Study Design
This study included, from January 2002 to March 2007, 44 consecutive kidney transplant recipients derived from our transplant program of patients with preformed donor specific anti-HLA antibodies (DSA). This population of DSA+ patients was compared with a control group of patients without preformed DSA (n = 62). All patients received a single ABO-compatible deceased donor kidney transplant. All patients underwent screening graft biopsies at 3 months and 1 year after transplant, defined as a biopsy performed in patients in a steady state, without any context of acute graft failure or recent immunological event, with contemporaneous measured GFRs. Patients with early graft loss (<3 months after transplant) or death were excluded from the study.
Four DSA+ patients and three DSA− patients were excluded because of arterial endothelial “sticking” or vasculitis (v1) at 3- or 12-month biopsy, leaving 40 DSA+ patients and 59 DSA− patients in the final cohort. A total of 91 d-0 biopsies, 80 from the the final cohort and 11 from more recent biopsies, were available for study of baseline arteriosclerosis. Finally, 15 late biopsies in the DSA+ group and 23 late biopsies in the DSA− group were performed at a mean of 39 ± 4 and 44 ± 5 months, respectively.
The clinical data gathered on each patient included age and sex of donors and recipients, BP, necessity for antihypertensive medication, SCr, and proteinuria at 3 months, 1 year, and as of the end of follow-up.
Pretransplant cross-match techniques and results are detailed in an earlier study.25 All patients received the same basic immunosuppressive regimen, with DSA+ patients receiving additional high dose-IVIg and anti-CD20 rituximab, together with plasmapheresis. In addition to standard ELISA assays for antibody detection, retrospective analysis of DSA class I and class II antibodies was performed by Luminex SA techniques on peak, day 0, 3-month, and 1-year sera, with antibody specificity and mean intensity of fluorescence recorded, as detailed in that communication.25
Histology and Immunohistochemistry
Renal biopsies were fixed in acetic acid-formol-absolute alcohol fixative and stained by standard methods for routine microscopy. The biopsies were reviewed by two renal pathologists (D.N. and G.H.), blinded to clinical information. In DSA+ patients, additional immunohistochemical C4d staining was performed retrospectively on paraffin sections using human polyclonal antibody (Biomedica). All DSA+ patients with C4d− biopsies by peroxidase were also analyzed by a two-step indirect immunofluorescence method with a monoclonal antibody specific for C4d on frozen tissue (Quidel, Santa Clara, CA), confirming C4d negativity. C4d staining was graded by the 2007 Banff classification update, varying from 0 to 3 (negative, minimal, focal, diffuse) depending on the percentage of PTCs having a linear staining pattern.21
Histologic changes were graded according to the Banff 97 classification.20 Diagnosis of acute cellular or humoral rejection was based on Banff criteria in conjunction with allograft dysfunction. SAMR8 was defined as the presence on a screening biopsy of glomerulitis, peritubular capillary leukocytic infiltration, positive C4d, and the presence of positive class I or II DSA by Luminex SA (>300 mean intensity of fluorescence) in contemporaneous serum.
Arteriosclerosis was graded using the Banff 1997 scoring criteria.20 In that system, cv0 = no chronic vascular changes. The scores, cv1–cv3, reflect the degree of narrowing of the luminal area in the worst-involved artery in the biopsy as follows: cv1, narrowing up to 25%: cv2, 26 to 50%; cv3, >50% narrowing of luminal area. The progression of arteriosclerosis, as gauged by the Banff cv score, was estimated from 91 d-0 biopsies. The equation for the resulting linear regression was Banff cv score = −0.3406 + 0.0203 × AgeDonor. From this, the slope of the acceleration of the Banff score can be calculated: slope = (increase in Banff cv score)/0.0203[(months after Tx − 3)/12]. The constant 0.0203 is derived from the linear regression formula. The time after transplant is adjusted for the fact that the first measures are taken at 3 months after Tx. The units are as follows: years of change in Banff score divided by years after transplant. (In addition to linear regression, other models for fit of data were tested: quadratic, cubic, quartic, and exponential. The resulting lines of fit were all very similar, so the linear regression, the simplest conceptually, was retained for calculation of change of slope in acceleration.)
Periodic acid-Schiff, silver, and elastic stains were used for appreciation of the internal elastica and elastic fibers in the thickened intima. In addition, immunohistochemical stains were used on selected cases for α-SMA, CD3, and CD20.
Statistical Analysis
Continuous variables were analyzed by t test or Mann-Whitney U test according to the normality of their distribution. Because most variables did not have a normal distribution, values are expressed as the mean ± SEM, unless otherwise specified. Categoric variables were analyzed by χ2 tests. Statistical significance was set at P ≤ 0.05, but because of often-small sample sizes, values of 0.05 < P < 0.10 are cited as such. The statistical program used was Statsoft version 6 (Statsoft, Tulsa, OK).
DISCLOSURES
None.
Acknowledgments
This work was presented in part as an abstract for the 2009 meeting of the American Society of Nephrology.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Colvin RB: Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol 18: 1046–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Colvin RB: Renal transplant pathology. In: Heptinstall's Pathology of the Kidney, 6th ed., edited by Jennette JC, Olson JL, Schwartz MM, Silva FG. Philadelphia, Lippincott, Williams & Wilkins, 2007, pp 1347–1490 [Google Scholar]
- 3. Cornell LD, Smith RN, Colvin RB: Kidney transplantation: Mechanisms of rejection and acceptance. Annu Rev Pathol 3: 189–220, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K: Antibody-mediated rejection criteria: An addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 3: 708–714, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Lefaucheur C, Nochy D, Hill GS, Glotz: Are transplant endarteritis (v1, v2) part of the spectrum of antibody-mediated rejection? [Abstract] 9th Banff Conference on Allograft Pathology, La Coruña, Spain, 2007 [Google Scholar]
- 6. Shimizu T, Ishida H, Shirakawa H, Omoto K, Tsunoyama K, Tokumoto T, Tanabe K: Clinicopathological analysis of acute vascular rejection cases after renal transplantation. Clin Transplant 24[Suppl 22]: 22–26, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Sis B, Mengel M, Eineke L, Hidalgo J. FPHP: Microarray analysis of isolated v-lesion in kidney transplant biopsies for cause. [Abstract] American Transplant Congress, San Diego, 2010 [Google Scholar]
- 8. Haas M, Montgomery RA, Segev DL, Rahman MH, Racusen LC, Bagnasco SM, Simpkins CE, Warren DS, Lepley D, Zachary AA, Kraus ES: Subclinical acute antibody-mediated rejection in positive crossmatch renal allografts. Am J Transplant 7: 576–585, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Gloor JM, Cosio FG, Rea DJ, Wadei HM, Winters JL, Moore SB, Degoey SR, Lager DJ, Grande JP, Stegall MD: Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant 6: 1841–1847, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Bian H, Reed EF: Anti-HLA class I antibodies transduce signals in endothelial cells resulting in FGF receptor translocation, down-regulation of ICAM-1 and cell proliferation. Transplant Proc 33: 311, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Bian H, Reed EF: Alloantibody-mediated class I signal transduction in endothelial cells and smooth muscle cells: Enhancement by IFN-gamma and TNF-alpha. J Immunol 163: 1010–1018, 1999 [PubMed] [Google Scholar]
- 12. Bieri M, Oroszlan M, Farkas A, Ligeti N, Bieri J, Mohacsi P: Anti-HLA I antibodies induce VEGF production by endothelial cells, which increases proliferation and paracellular permeability. Int J Biochem Cell Biol 41: 2422–2430, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Hill GS, Nochy D, Loupy A: Accelerated arteriosclerosis: A form of transplant arteriopathy. Curr Opin Organ Transplant 15: 11–15, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Loupy A, Suberbielle-Boissel C, Zuber J, Anglicheau D, Timsit MO, Martinez F, Thervet E, Bruneval P, Charron D, Hill GS, Nochy D, Legendre C: Combined posttransplant prophylactic IVIg/anti-CD 20/plasmapheresis in kidney recipients with preformed donor-specific antibodies: A pilot study. Transplantation 89: 1403–1410, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Le Bas-Bernardet S, Hourmant M, Valentin N, Paitier C, Giral-Classe M, Curry S, Follea G, Soulillou JP, Bignon JD: Identification of the antibodies involved in B-cell crossmatch positivity in renal transplantation. Transplantation 75: 477–482, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Campos EF, Tedesco-Silva H, Machado PG, Franco M, Medina-Pestana JO, Gerbase-DeLima M: Post-transplant anti-HLA class II antibodies as risk factor for late kidney allograft failure. Am J Transplant 6: 2316–2320, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Issa N, Cosio FG, Gloor JM, Sethi S, Dean PG, Moore SB, DeGoey S, Stegall MD: Transplant glomerulopathy: risk and prognosis related to anti-human leukocyte antigen class II antibody levels. Transplantation 86: 681–685, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Sumitran-Holgersson S: Relevance of MICA and other non-HLA antibodies in clinical transplantation. Curr Opin Immunol 20: 607–613, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Stastny P, Zou Y, Fan Y, Qin Z, Lavingia B: The emerging issue of MICA antibodies: Antibodies to MICA and other antigens of endothelial cells. Contrib Nephrol 162: 99–106, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Lefaucheur C, Nochy D, Hill GS, Suberbielle-Boissel C, Antoine C, Charron D, Glotz D: Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant 7: 832–841, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, Hill GS, Glotz D, Suberbielle-Boissel C: Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant 9: 1099–1107, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, Gautreau C, Charron D, Glotz D: Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant 8: 324–331, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, Martinez F, Thervet E, Mejean A, Charron D, van Huyen JP, Bruneval P, Legendre C, Nochy D: Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant 9: 2561–2570, 2009 [DOI] [PubMed] [Google Scholar]