Abstract
The long-term variability of marine turtle populations remains poorly understood, limiting science and management. Here we use basin-scale climate indices and regional surface temperatures to estimate loggerhead sea turtle (Caretta caretta) nesting at a variety of spatial and temporal scales. Borrowing from fisheries research, our models investigate how oceanographic processes influence juvenile recruitment and regulate population dynamics. This novel approach finds local populations in the North Pacific and Northwest Atlantic are regionally synchronized and strongly correlated to ocean conditions—such that climate models alone explain up to 88% of the observed changes over the past several decades. In addition to its performance, climate-based modeling also provides mechanistic forecasts of historical and future population changes. Hindcasts in both regions indicate climatic conditions may have been a factor in recent declines, but future forecasts are mixed. Available climatic data suggests the Pacific population will be significantly reduced by 2040, but indicates the Atlantic population may increase substantially. These results do not exonerate anthropogenic impacts, but highlight the significance of bottom-up oceanographic processes to marine organisms. Future studies should consider environmental baselines in assessments of marine turtle population variability and persistence.
Introduction
Populations from a variety of taxa and environments have long-term correlations with climate [1], [2], [3], [4], [5]. This is particularly true in marine ecosystems where the North Atlantic Oscillation (NAO) and the Pacific Decadal Oscillation (PDO) have a dramatic influence on fisheries [6], [7]. These climate indices are correlated with population dynamics [8], [9] because they reflect atmospheric circulation patterns which regulate large scale oceanographic processes and ecosystem productivity [10], [11]. But, in contrast with the El Niño Southern Oscillation index [12], the NAO and PDO operate on decadal scales, causing extended periods of high or low population abundance [5], [7]. Though the ecological effects of these climate oscillations have been described in various settings, the influence of decadal indices to long-term marine turtle population trends is largely unexplored.
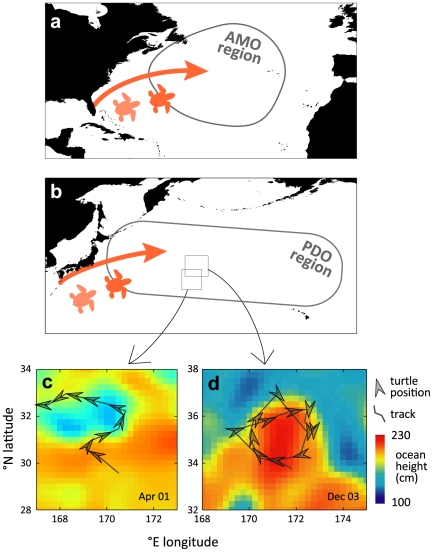
Anthropogenic pressures are considered the major driver of marine turtle populations [13], [14], [15]. A recent National Research Council (NRC) report concluded, for example, that advances in turtle population ecology will come primarily from improvements in monitoring human impacts [16]. At the same time, the NRC report noted the lack of data on juveniles to be a significant scientific challenge. After hatching loggerhead juveniles disperse to pelagic biomes (Fig 1a) thousands of kilometers from their nesting beaches where most studies occur. Although juveniles are the most numerous population segment, they are also the least accessible and least understood.
Figure 1. Pelagic habits of juvenile loggerhead turtles.
Loggerhead juveniles disperse to regions whose climatic variability is characterized (a) in the North Atlantic by the Atlantic Multidecadal Oscillation (AMO) and (b) in the North Pacific by the Pacific Decadal Oscillation (PDO). In the North Pacific, satellite-tagged juveniles forage in cyclonic (c) and anti-cyclonic (d) feature fronts where prey are abundant. Comparable satellite studies in the Northwest Atlantic population reveal similar habits [18]. Tracking maps are redrawn from a previous study [19].
Climate indices may provide insights into the dynamics of this key demographic. Genetic [17] and tracking [18], [19] studies have revealed pelagic hotspots where juvenile loggerheads congregate; foraging in oceanographic features that are productive and concentrate prey (Fig. 1c-d). Decadal indices, as they describe the variability of these hotspots, may function as proxies for juvenile recruitment. This is the case for many marine fisheries [9]. Because juvenile population dynamics are poorly understood, this may offer immediate insights. But in addition, a climatic approach holds promise for understanding population trends - of juveniles and adults - over time.
In this paper we develop models that measure climate forcing in long-term trends of loggerhead sea turtles (Caretta caretta) nesting in Japan and Florida. Our models capture climate dynamics through two mechanisms: juvenile recruitment and breeding remigration. Females do not breed annually, but when ocean conditions are sufficient for females to generate yolk [20]. Nesting activity across species and ocean basins has thus been linked to sea surface temperatures (SST) in the months preceding nesting [21], [22], [23]. But where SST anomalies explain some of the annual fluctuations in nesting, recruitment variability may be the dominant driver of the long-term dynamics. Juveniles are considered more susceptible to oceanographic variability as they have a limited ability to exploit their environs for food [2], [6], [9], [24], [25]. In the North Pacific we anticipate that juvenile recruitment is correlated with the PDO, as the index is positive when atmospheric circulation is elevated [11] in the Kuroshio Bifurcation Extension Region where juveniles congregate [19] (Fig 1b–d). In the Northwest Atlantic we expect recruitment varies with the Atlantic Multidecadal Oscillation (AMO). We use this index instead of the NAO, as the AMO is strongly correlated with thermohaline and atmospheric circulation patterns [26] as well as storm activity [27], [28] in the subtropical-temperate region where Atlantic juveniles reside [17].
Our study is the first to consider how both of these important climate dynamics impact marine turtles. In doing so, a significant aspect of our models is accounting for age to maturity. Our analysis presumes juvenile recruitment dynamics can be detected when turtles are counted as breeding adults, several decades later [29]. In fisheries, age to maturity is typically a few years and correlation lags are therefore straightforward. The longest documented fisheries lag is eight years, documented both between the AMO and striped bass (Morone saxatilis) surveys (Bob Wood, personal communication) and between the NAO and Labrador snow crab (Chionoecetes opilio) landings [30]. For Northwest Atlantic loggerheads, estimates of age at first breeding range from 30–32 years [29], [31]. We therefore fix the lag in this population at 31 years. We have no maturity estimates for North Pacific loggerhead, however. We therefore model the lag of juvenile climate dynamics over a plausible range of values, allowing the models to optimize the lag distance for the Japan regional total series.
We used general linear models to estimate annual nesting at two spatial scales relevant to conservation management [31] - local and regional nesting surveys. To examine their relative model performance, we rank the contributions from each of the climate factors. Finally, due to the mechanistic nature of the climate forcing models, we project historical and future nesting trajectories based on available climate data and under different climate change scenarios.
Results and Discussion
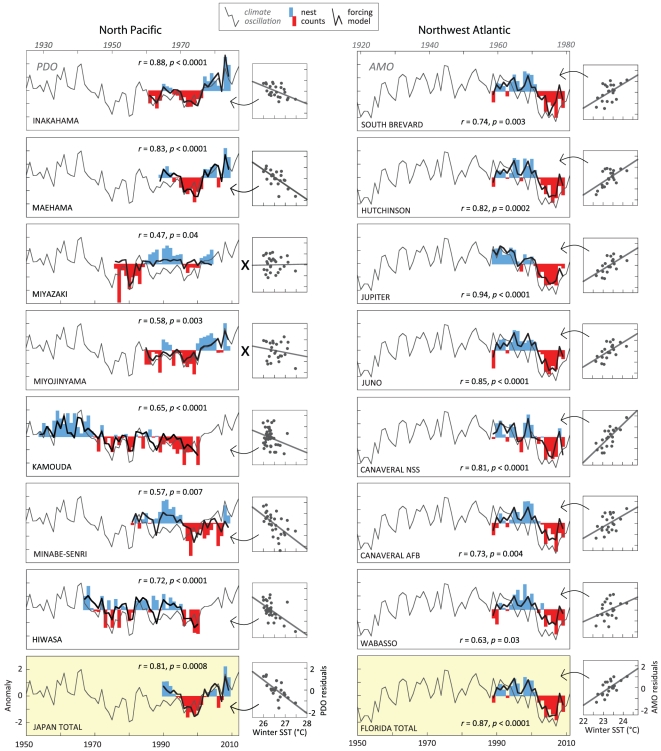
Loggerhead nesting varies synchronously within regions suggesting that common factors operating over large geographic regions are driving their numbers (Fig. 2). Surveys in Japan and Florida reveal extended periods of high and low abundance, corresponding to well-known fisheries patterns in the same ocean basins [2], [7]. Our climate forcing models account for 18–88% (ave = 0.60) of the annual variability at the local scale, and 66–77% (ave = 0.71) at the regional scale when nesting data from 1954–2009 are considered (Table S1).
Figure 2. Climate forcing of loggerhead nesting.
Nest surveys are positive (blue) and negative (red) annual anomalies plotted against the survey year on the bottom x axis. The upper x axis is the year of the decadal oscillation (grey line) represented by the PDO and the AMO lagged 25 and 31 years, respectively. Highest-ranked climate forcing model (black line) incorporates the decadal series including (←) or without (x) the winter SST series. Scatter plots display relationships of the winter SST to residuals from the decadal series models alone. Regional total series (yellow panel) accumulates nests from all locations; Japan total provided by the Sea Turtle Association of Japan, Florida total accumulates nests from 15 index nesting beach surveys. Model correlation coefficients and p-values are provided.
The models capture both annual and decadal time series patterns, including those that have raised concerns with modelers and conservationists. As an example, surveys in Japan indicated a greater than three-fold (linear scale) increase from 2007–2008. While a purely demographic model could not reproduce this trend, our climate forcing model that combines the PDO and winter SST, captures this dramatic increase (Fig. 2). Likewise, the historical decline in Kamouda, Japan from its historical peak in the late 1950s appears to have a climatic component. We note that the Kamouda series does not consistently decline from its first recorded value, but oscillates in concert with the PDO for more than four decades. The declines across Florida from 1998–2007, as well as the increase in the most recent few years, also appear tied to climatic conditions. Importantly, the models perform well locally where most turtles nest. In both ocean basins, nesting abundances at local beaches may vary by an order of magnitude. Since 2000, Inakahama and Maehama represent 36% of all North Pacific nests. Since records began in 1989, South Brevard accounts for 37% of all peninsular Florida nests. The models from these three locations explain on average two–thirds of the annual nesting variability.
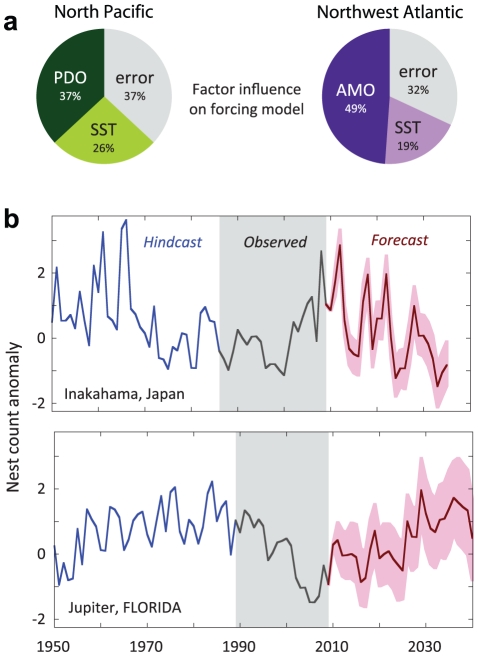
The model results also have important demographic implications. As juveniles are the most numerous population segment, we might expect factors regulating their survival could produce a signal detected later in adult surveys. Though Fig. 2 displays the model contributions from each climate factor, Fig. 3a calculates those contributions explicitly and includes the remaining model error. In both populations, the lagged decadal oscillations amount to 70% of the model performance when the results from each series are averaged. Thus, climatic factors in the hatching year are the single most important variable in our forcing models. The influence of juvenile climate factors in explaining nesting abundances may suggest neophyte breeders are a greater proportion of the breeding population than has been recognized. This is consistent with two recent tagging studies in Florida loggerheads which show that most nesting females were first-time breeders [32] and survivorship of recaptured adults was inexplicably low [33]. Though tag loss [34] influences the interpretation of these Florida studies, the research nonetheless corroborates our findings. While our aim in this analysis is a purely climatic population model, future studies might seek to incorporate anthropogenic influences, from direct [35] and indirect harvests [15], for example, or from disease [36]. Time series of such influences, however, can be elusive [37] especially at regional or basin-wide scales. Viewed in context with the present study, factors influencing juvenile recruitment appear to have a dominant role in long-term population trends, something suggested by the renowned sea turtle biologist Archie Carr almost six decades ago [38] and by fisheries biologists beginning a century ago [24].
Figure 3. Model factor contributions and population forecasts.
(a) Pie charts calculate the model contributions from each climate factor, plus error. Percentage values are the sum of squares (SS) improvements from each factor, expressed as an average weighted by the mean annual nest count. (b) Nesting anomalies during the historical (blue) and surveyed (black) period, modeled from available climate records. Future forecasts are calculated from observed, lagged oceanographic indices and with stochastic simulations of winter SST (see Methods). Forecast range (pink) and model average (red) are shown. A significant advantage of this climate-based model is the ability to estimate historical and future populations.
Age at sexual maturity is an important component in documenting the impact of climate on juvenile recruitment. However, as empirical studies are limited we have no a priori global estimate, and we might expect significant geographic variation or demographic stochasticity [39], [40]. In the Florida population, available studies [29], [31] allowed us to fix the lag at 31 years. In the Japan population, without comparable demographic studies, we optimized the lag length using the regional nesting series and fixed all subsequent lags in the Japan population to that value. The optimized lag, in this respect, is a climate-based approximation of age at first breeding. The optimal lag length was calculated to be 25 years for the Japan population (Fig. S1), six years shorter than in Florida. Though demographic information is limited, published records [41] show females breeding in Japan are considerably smaller than those in Florida. As size is positively correlated with age [29], smaller breeding turtles may be younger breeding turtles, other things being equal. In addition, over the past two decades, the number of annual nests in Japan is an order of magnitude below Florida. If this reflects overall population size, lower population densities in the North Pacific may indicate less intraspecific competition, faster growth, and perhaps earlier maturity. Future studies might compare the ocean resources available to each loggerhead population, as has been done elsewhere [42], and examine their influence on population dynamics.
Model relationships calibrated with the empirical survey data are the basis for historical and future nesting projections. Figure 3b uses available climate data to reconstruct historical nesting and available and modeled climate data to forecast future trends. We project forward the lag length, with real PDO and AMO data that have already occurred, but have not yet manifested themselves in nesting populations. Future winter SST is modeled under two scenarios (Fig. S2) based on the historical time series and the IPCC A2 projections [43]. The hindcast projections in Japan approximate the declines observed from 1960–1990 in the longest survey (Kamouda) and predict continued losses extending past 2030. In Florida, unpublished historical surveys (S.J. Epperly personal communication) are consistent with our modeled hindcasts that suggest a population increase from the 1960s through the 1980s. The future outlook in Florida is positive, and shows continued increases in nesting loggerheads to 2040, a result of the AMO signal. We do not project the climate indices beyond the lag periods, but models of the Pacific [44] indicate broad declines. In the North Pacific, projections under anthropogenic climate scenarios indicate a 34% decrease in both the area and primary production of the temperate oceanic biome, which would impact juvenile loggerheads from Japan. We do not have similar projections for the Atlantic and forecasting the oscillation indices themselves has not been possible [26].
The stewardship of marine biodiversity relies on accurate population assessments. While subsistence and commercial harvests preceded large historical declines of marine turtles [13] it is unknown how climatic and anthropogenic forces together regulate the trends of many protected species [45]. Our analysis demonstrates that changes in loggerhead nesting over at least the last several decades are strongly correlated with ocean oscillations. We observed these results in both the Atlantic and Pacific basins, using independent climate series, and when both regional and local population surveys are tested. Our models suggest that oceanographic influences to juvenile recruitment are a major factor, decades later, in breeding populations (Fig. 2) and appear robust to estimate past and future population changes (Fig. 3b).
There are immediate implications for marine turtle population ecology and management. When juvenile and adult life stages are decoupled, population size often has a weak affect on recruitment [4]. Instead, as is the case with many marine fisheries [2], [5], [6], [7], [9], [10], [11] climate forcing seems to dominate population dynamics. By contrast, turtle population assessments often rely on models of adult population growth, entirely independent of environmental factors [31]. Our observation of strong environmental forcing challenges this view. As reptiles with a significant pelagic component, marine turtles may resemble marine fisheries more than terrestrial populations. Future research that addresses the physical and ecological processes that influence pelagic juvenile turtles may provide environmental baselines of population variability. As we demonstrate here, climate models provide new insights into the historical, current, and future populations of marine turtles and provide a mechanistic modeling framework for considering anthropogenic climate change. Our results also add to the ongoing debate on top-down versus bottom-up regulation of wild populations [46], [47], [48], [49].
Materials and Methods
Data
Population counts are provided by nest surveys from the Florida Fish and Wildlife Research Institute (FWRI) and published reports, which provide further details [50], [51], [52], [53]. We used linear models of climate variables to estimate ln-transformed [49], [54] nest surveys. PDO series is the calendar year average of the monthly index values, supplied by the Joint Institute for the Study of the Atmosphere and Ocean (JISAO). AMO series is the normalized annual SST anomaly series derived from the Kaplan SST series of the North Atlantic [55], from the NOAA Earth System Research Laboratory (ESRL). SST data are the 2°×2° cell ERSSTv3b series from 1950–1981 and 1°×1° cell OIv2 series from 1981-present [56], from the National Climatic Data Center (NCDC). Japan winter SST is averaged over 8–28°N, 120–128°E [21] during the November–January before the nesting season. Florida winter SST is averaged over 22–38°N, 72–84°W in the previous December [22]. Regional temperature forecasts under the A2 scenario are from the IPCC [43]. We ranked models using the corrected Akaike Information Criterion (AICc) for small samples [57].
Climate forcing models
Nest counts series were estimated with the following general linear models:
| (1) |
| (2) |
| (3) |
| (4) |
Here Ni is the annual nesting activity predicted by the i th model. Also x(t,λ) is the oceanographic oscillation index in year t lagged by λ years and z(t) is the SST from the previous winter. The numbers β 0, β 1, β 2, β 3, are the fitted model parameters. Nesting activity is defined as the normalized value of the ln-transformed annual nest counts (Kamouda records crawls, not nests). This transformation is consistent with the observed pattern of variability of wild populations [35], [38]. We excluded annual counts <20 (2 of 595 total observations) as such extreme lows skewed the normalization procedure. Model (1) is linear and (2) is a curvilinear relationship to the oceanographic oscillations. Model (3) and (4) add winter SST as a model factor.
We lagged the effect of x on N as the variability of x influences juvenile turtles, only a portion of which breed λ years later and are observed nesting. Though we fix this value at 31 years for the Northwest Atlantic population, we have no estimates for the North Pacific population. A recent study [24] hypothesizes 30 years as the global mean time between hatching and first breeding for loggerheads. As we expect this to vary geographically we fit the above models to the observed data for the Japan Total nesting series, separately using lags from 20–40 years. We fix the subsequent local beach series models in Japan to the optimal lag derived from the regional total series.
To rank model performance, we used the corrected Akaike Information Criterion (AICc) for small populations, where:
| (5) |
where k is the number of model parameters, n is the series length and D is the mean square deviation between the data and the model [57]. In determining the optimal lag for the Japan Total series, we average the AICc values from models (1–4) for each lag length and select the lag with the lowest average AICc (Fig. S1). For the remaining models where the lag length is fixed, the highest-ranked model has the lowest AICc value of the four tested models. The model contributions from each variable are calculated from their sum of squares.
Population forecasts
We estimated historical and future nesting in each series using the fitted model relationships. We calculate hindcasts from 1950- using the selected model for each series, including historical oscillation indices and available winter SST data. Because of the lagged influence of the oceanographic indices, we already possess real climate data that has yet to manifest itself in nesting females, N. Therefore we forecast nest surveys from 2010 forward, for a period equal to the optimum lag, λ. When the models include winter SST, we forecast two scenarios for its change. The first extrapolates from the fitted linear 1950–2010 trend for each region. The second uses an ensemble forecast of multiple Atmosphere-Ocean General Circulation Models (AOGCMs) under the A2 emissions scenario from the IPPC Fourth Assessment Report [43]. The A2 scenario reflects continued global population growth with decentralized ecomonic and technological changes and forecasts more extreme warming than most emission scenarios. The ensemble A2 forecasts show both geographic regions (Fig. S2) have an approximate linear increase of 0.0275°C yr−1 during 2010–2040 [43]. To model the uncertainty in the forecast for both scenarios, we added autocorrelated noise based on the power spectrum of noise from the 1950–2010 merged ERSST series [58]. We applied this characteristic noise pattern to the projected annual trends and generated 100 simulations for each scenario. All forecasted SST series were pooled and for each calendar year the forecasted nest abundances is the model average for the ensemble of 200 simulations, essentially, deterministic models within a stochastic shell [59]. We select the upper and lower 2.5% of the simulation forecasts to serve as the upper and lower bounds of the forecast.
Supporting Information
Climate lag optimization For North Pacific (Japan) population. Residual mean square (RMS) values for each model at each lag length. Green lines are two linear models, red lines are two curvilinear models (see Methods). Black line is the average of the two best series. Blue rectangle identifies the optimum lag length according to the data, for the most numerous, the longest, and the regional total series. All three series agree on a 25 year optima.
(EPS)
Winter sea-surface temperature series. 1950–1980 data are from the ERSSTv3b series (green) and 1981–2010 are from the OIv2 series (green) provided by NOAA (see Methods). Japan series measures November–January records over 5–28°N, 120–128°E; Florida winter SST measures December records over 22–38°N, 72–84°W – regions shaded green in the inset maps. Future winter SST forecasts are projected linear trends with an added stochastic component derived from the empirical noise. The blue line is forecast according the linear trend of the 1950–2010 data, the red line is according to the Intergovernmental Panel on Climate Change (IPCC) A2 emissions scenario. Both lines are the ensemble average of 100 simulations, given the characteristic noise of the ERSST series.
(EPS)
Details on the surveys and forcing models. This table describes the nesting beach series and key statistics from the highest-ranked models. DPS is the distinct population segment, a genetic population division made for conservation and management. Optimum lags for each winning model are weighted by local nesting population size in Fig. 3a, separately for Japan and Florida. The R and P values for forcing models report on goodness-of-fit and statistical significance, respectively. One series (MacArthur, Florida) has a weak statistical correlation.
(DOC)
Acknowledgments
We are thankful for data providers to, J. Polovina, M. Snover, T. Fahy, B. Witherington, S. Epperly, I. Kelly, and B. Schroeder, and to S. Pimm, F. Parrish, B. Antonelis, M. Snover, S. Pooley, and J. Polovina for critical review.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Boyce DG, Lewis MR, Worm B. Global phytoplankton decline over the past century. Nature. 2010;466:591–596. doi: 10.1038/nature09268. [DOI] [PubMed] [Google Scholar]
- 2.Ottersen G, Planque B, Belgrano A, Post E, Reid P, et al. Ecological effects of the North Atlantic Oscillation. Oecologia. 2001;128:1–14. doi: 10.1007/s004420100655. [DOI] [PubMed] [Google Scholar]
- 3.Pimm SL. London: Chapman & Hall; 1982. Food webs. [Google Scholar]
- 4.Steele JH. A comparison of terrestrial and marine ecological esystems. Nature. 1985;313:355–358. [Google Scholar]
- 5.Stenseth NC, Mysterud A, Ottersen G, Hurrell JW, Chan K-S , et al. Ecological effects of climate fluctuations. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. [DOI] [PubMed] [Google Scholar]
- 6.Beamish RJ, Bouillon DR. Pacific salmon production trends in relation to climate. Can J Fish Aquat Sci. 1993;50:1002–1016. [Google Scholar]
- 7.Chavez FP, Ryan J, Lluch-Cota SE, Niquen CM. From anchovies to sardines and back: multidecadal change in the Pacific ocean. Science. 2003;299:217–221. doi: 10.1126/science.1075880. [DOI] [PubMed] [Google Scholar]
- 8.Dawe EG, Hendrickson LC, Colbourne EB, Drinkwater KF, Showell MA. Ocean climate effects on the relative abundance of short-finned (Illex illecebrosus) and long-finned (Loligo pealeii) squid in the northwest Atlantic Ocean. Fish Oceanogr. 2007;16:303–316. [Google Scholar]
- 9.Drinkwater KF, Belgrano A, Borja A, Conversi A, Edwards M, et al. The response of marine ecosystems to climate variability associated with the North Atlantic Oscillation. In: Hurrell JW, Kushnir Y, Ottersen G, Visbeck M, editors. The North Atlantic Oscillation Climate Significance and Environmental Impact. Washington DC: American Geophysical Union; 2003. pp. 211–233. [Google Scholar]
- 10.Hurell JW. Decadal trends in the North Atlantic Oscillation: regional temperatures and precipitation. Science. 1995;269:676–679. doi: 10.1126/science.269.5224.676. [DOI] [PubMed] [Google Scholar]
- 11.Mantua N, Hare S. The Pacific Decadal Oscillation. J Oceanogr. 2002;58:35–44. [Google Scholar]
- 12.Philander SG. San Diego, CA: Academic Press; 1990. El Niño, La Niña, and the Southern Oscillation. [DOI] [PubMed] [Google Scholar]
- 13.Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, et al. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 14.Van Houtan KS, Bass OL. Stormy oceans are associated with declines in sea turtle hatching. Curr Biol. 2007;17:R590–R591. doi: 10.1016/j.cub.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Wallace BP, Lewison RL, McDonald SL, McDonald RK, Kot CY, et al. Global patterns of marine turtle bycatch. Conserv Lett. 2010;3:131–142. [Google Scholar]
- 16.Bjorndal KA, Bowen BW, Chaloupka M, Crowder LB, Heppell SS, et al. Washington DC: National Academies Press; 2010. Assessment of sea-turtle status and trends: intergrating demography and abundance. [Google Scholar]
- 17.Bolten AB, Bjorndal KA, Martins HR, Dellinger T, Biscoito MJ, et al. Transatlantic developmental migrations of loggerhead sea turtles demonstrated by mtDNA sequence analysis. Ecol Al. 1998;8:1–7. [Google Scholar]
- 18.Mansfield K, Saba V, Keinath J, Musick J. Satellite tracking reveals a dichotomy in migration strategies among juvenile loggerhead turtles in the Northwest Atlantic. Mar Biol. 2009;156:2555–2570. [Google Scholar]
- 19.Polovina J, Uchida I, Balazs G, Howell EA, Parker D, et al. The Kuroshio Extension Bifurcation Region: a pelagic hotspot for juvenile loggerhead sea turtles. Deep-Sea Res II. 2006;53:326–339. [Google Scholar]
- 20.Rivalan P, Prévot-Julliard A–C, Choquet R, Pradel R, Jacquemin B, et al. Trade-off between current reproductive effort and delay to next reproduction in the leatherback sea turtle. Oecologia. 2005;145:564–574. doi: 10.1007/s00442-005-0159-4. [DOI] [PubMed] [Google Scholar]
- 21.Chaloupka M, Kamezaki N, Limpus C. Is climate change affecting the populations dynamics of the endangered Pacific loggerhead sea turtle? J Exp Mar Biol Ecol. 2008;356:136–143. [Google Scholar]
- 22.Saba VS, Santidrian-Tomillo P, Reina RD, Spotila JR, Musick JA, et al. The effect of the El Niño Southern Oscillation on the reproductive frequency of eastern Pacific leatherback turtles. J Appl Ecol. 2007;44:395–404. [Google Scholar]
- 23.Solow AR, Bjorndal KA, Bolten AB. Annual variation in nesting numbers of marine turtles: the effect of sea surface temperature on re-migration intervals. Ecol Lett. 2002;5:742–746. [Google Scholar]
- 24.Hjort J. Fluctuations in the great fisheries of Northern Europe viewed in light of biological research. Rapp Cons int Explor Mer. 1914;20:3–228. [Google Scholar]
- 25.Lasker R. The role of a stable ocean in larval fish survival and subsequent recruitment. In: Lasker R, editor. Marine fish larvae: morphology, ecology, and relation to fisheries. Seattle: Washington Sea Grant Program; 1981. pp. 79–87. [Google Scholar]
- 26.Knight JR, Allan RJ, Folland CK, Vellinga M, Mann ME. A signature of persistent natural thermohaline circulation cycles in observed climate. Geophys Res Lett. 2005;32:L20708. [Google Scholar]
- 27.Knight JR, Folland CK, Scaife AA. Climate impacts of the Atlantic Multidecadal Oscillation. Geophys Res Lett. 2006;33:L17706. [Google Scholar]
- 28.Nyberg J, Malmgren BA, Winter A, Jury MR, Kilbourne KH, et al. Low Atlantic hurricane activity in the 1970s and 1980s compared to the past 270 years. Nature. 2007;447:698–701. doi: 10.1038/nature05895. [DOI] [PubMed] [Google Scholar]
- 29.Snover ML. Durham, NC: Doctoral dissertation, Duke University; 2002. Growth and ontogeny of sea turtles using skeletochronology: methods, validation, and application to conservation. [Google Scholar]
- 30.Pearson A. Why storms are good news for fishermen. New Sci. 2009;200:32–35. [Google Scholar]
- 31.Conant TA, Dutton PH, Eguchi T, Epperly SP, Fahy CC, et al. Silver Spring, MD: National Marine Fisheries Service. 222 pages p; 2009. Loggerhead sea turtle (Caretta caretta) 2009 status review under the U.S. Endangered Species Act. [Google Scholar]
- 32.Thompson N, Bolten AB, Dodd M, Epperly SP, Foley A, et al. NOAA Technical Memorandum NMFS-SEFSC-575 (Turtle Expert Working Group); 2009. An assessment of the loggerhead turtle population in the western North Atlantic Ocean.131 [Google Scholar]
- 33.Sasso CR, Epperly SP, Johnson C . Annual survival of loggerhead sea turtles nesting in peninsular Florida: a cause for concern. (in review)
- 34.Rivalan P, Godfrey MH, Prévot-Julliard A–C, Girondot M, Lubow Maximum likelihood estimates of tag loss in leatherback sea turtles. J Wildl Manage. 2005;69:540–548. [Google Scholar]
- 35.Santidrián-Tomillo P, Saba VS, Piedra R, Paladino FV, Spotila JR. Effects of Illegal Harvest of Eggs on the Population Decline of Leatherback Turtles in Las Baulas Marine National Park, Costa Rica. Conserv Biol. 2008;22:1216–1224. doi: 10.1111/j.1523-1739.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 36.Van Houtan KS, Hargrove SK, Balazs GH. Land use, macroalgae, and a tumor-forming disease in marine turtles. PLoS ONE. 2010;5:e129000. doi: 10.1371/journal.pone.0012900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewison RL, Soykan CU, Franklin J. Mapping the bycatch seascape: multispecies and multi-scale spatial patterns of fisheries bycatch. Ecol Appl. 2009;19:920–930. doi: 10.1890/08-0623.1. [DOI] [PubMed] [Google Scholar]
- 38.Carr AF. Ithaca, NY: Cornell University Press; 1952. Handbook of turtles. [Google Scholar]
- 39.Nee S, Colegrave N, West SA, Grafen A. The illusion of invariant quantities in life histories. Science. 2005;309:1236–1239. doi: 10.1126/science.1114488. [DOI] [PubMed] [Google Scholar]
- 40.Stearns SC, Koella JC. The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution. 1986;40:893–913. doi: 10.1111/j.1558-5646.1986.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 41.Hatase H, Matsuzawa Y, Sato K, Bando T, Goto K. Remigration and growth of loggerhead turtles (Caretta caretta) nesting on Senri Beach in Minabe, Japan: life-history polymorphism in a sea turtle population. Mar Biol. 2004;144:807–811. [Google Scholar]
- 42.Wallace BP, Kilham SS, Paladino FV, Spotila JR. Energy budget calculations indicate resource limitation in Eastern Pacific leatherback turtles. Mar Ecol Prog Ser. 2006;318:263–270. [Google Scholar]
- 43.IPCC Cambridge, UK: Cambridge University Press; 2007. Climate Change 2007: Synthesis Report. editor. [Google Scholar]
- 44.Polovina JJ, Dunne JP, Woodworth PA, Howell EA. ICES J Mar Sci; 2011. Projected expansion of the subtropical biome and contraction of the temperate and equatorial upwelling biomes in the North Pacific under global warming. doi: 10.1093/icesjms/fsq1198. [Google Scholar]
- 45.Edwards M, Beaugrand G, Hays GC, Koslow JA, Richardson AJ. Multi-decadal oceanic ecological datasets and their application in marine policy and management. Trends Ecol Evol. 2010;25:602–610. doi: 10.1016/j.tree.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Crooks KR, Soule ME. Mesopredator release and avifaunal extinctions in a fragmented system. Nature. 1999;400:563–566. [Google Scholar]
- 47.Estes JA, Tinker MT, Williams TM, Doak DF. Killer Whale Predation on Sea Otters Linking Oceanic and Nearshore Ecosystems. Science. 1998;282:473–476. doi: 10.1126/science.282.5388.473. [DOI] [PubMed] [Google Scholar]
- 48.Terborgh J, Estes JA, editors. Washington DC: Island Press; 2010. Trophic cascades: predators, prey, and the changing dynamics of nature. [Google Scholar]
- 49.Van Houtan KS, Halley JM, van Aarde RJ, Pimm SL. Achieving success with small, translocated mammal populations. Conserv Lett. 2009;2:254–262. [Google Scholar]
- 50.Kamezaki N, Toji Y, Matsuzawa Y, editors. Osaka: Sea Turtle Association of Japan; 2002. Current status of Japanese loggerhead turtle nesting and beach environment (in Japanese). [Google Scholar]
- 51.Matsuzawa Y. Honolulu: NOAA Western Pacific Regional Fishery Management Council; 2010. Nesting beach management in Japan to conserve eggs and pre-emergent hatchlings of the north Pacific loggerhead sea turtle, 2009 season. [Google Scholar]
- 52.Takeshita H. The current status of loggerhead sea turtle rookeries in Miyazaki, Japan. In: Kinan I, editor. Proceedings of the second Western Pacific sea turtle cooperative research & management workshop. Honolulu, HI: Western Pacific Regional Fishery Management Council; 2005. pp. 27–29. [Google Scholar]
- 53.Kamezaki N, Matsuzawa Y, Abe O, Asakawa H, Fujii T, et al. In: Bolten AB, Witherington BE, editors. Loggerhead sea turtles. Washington DC: Smithsonian Books; 2003. Loggerhead turtles nesting in Japan. pp. 210–217. [Google Scholar]
- 54.Halley JM, Inchausti P. Lognormality in ecological time series. Oikos. 2002;99:518–530. [Google Scholar]
- 55.Enfield DB, Mestas-Nunez AM, Trimble PJ. The Atlantic Multidecadal Oscillation and its relationship to rainfall and river flows in the continental U.S. Geophys Res Lett. 2001;28:2077–2080. [Google Scholar]
- 56.Smith TM, Reynolds RW, Peterson TC, Lawrimore J. Improvements to NOAA's Historical Merged Land–Ocean Surface Temperature Analysis (1880–2006). J Climate. 2008;21:2283–2296. [Google Scholar]
- 57.Hurvich CM, Simonoff JS, Tsai C-L. Smoothing parameter selection in nonparametric regression using an improved Akaike information criterion. J R Stat Soc B. 1998;60:271–293. [Google Scholar]
- 58.Halley JM. Using models with long-term persistence to interpret the rapid increase of Earth's temperature. Physica A. 2009;388:2492–2502. [Google Scholar]
- 59.Clark JS. Princeton, NJ: Princeton University Press; 2007. Models for ecological data. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Climate lag optimization For North Pacific (Japan) population. Residual mean square (RMS) values for each model at each lag length. Green lines are two linear models, red lines are two curvilinear models (see Methods). Black line is the average of the two best series. Blue rectangle identifies the optimum lag length according to the data, for the most numerous, the longest, and the regional total series. All three series agree on a 25 year optima.
(EPS)
Winter sea-surface temperature series. 1950–1980 data are from the ERSSTv3b series (green) and 1981–2010 are from the OIv2 series (green) provided by NOAA (see Methods). Japan series measures November–January records over 5–28°N, 120–128°E; Florida winter SST measures December records over 22–38°N, 72–84°W – regions shaded green in the inset maps. Future winter SST forecasts are projected linear trends with an added stochastic component derived from the empirical noise. The blue line is forecast according the linear trend of the 1950–2010 data, the red line is according to the Intergovernmental Panel on Climate Change (IPCC) A2 emissions scenario. Both lines are the ensemble average of 100 simulations, given the characteristic noise of the ERSST series.
(EPS)
Details on the surveys and forcing models. This table describes the nesting beach series and key statistics from the highest-ranked models. DPS is the distinct population segment, a genetic population division made for conservation and management. Optimum lags for each winning model are weighted by local nesting population size in Fig. 3a, separately for Japan and Florida. The R and P values for forcing models report on goodness-of-fit and statistical significance, respectively. One series (MacArthur, Florida) has a weak statistical correlation.
(DOC)