Abstract
Classically, sympathetic and parasympathetic systems act in opposition to maintain the physiological homeostasis. In this article, we report that both systems work together to restrain systemic inflammation in life-threatening conditions such as sepsis. This study indicates that vagus nerve and cholinergic agonists activate the sympathetic noradrenergic splenic nerve to control systemic inflammation. Unlike adrenalectomy, splenectomy and splenic neurectomy prevent the anti-inflammatory potential of both the vagus nerve and cholinergic agonists, and abrogate their potential to induce splenic and plasma norepinephrine. Splenic nerve stimulation mimics vagal and cholinergic induction of norepinephrine and re-establishes neuromodulation in α7 nicotinic acetylcholine receptor (α7nAChR)-deficient animals. Thus, vagus nerve and cholinergic agonists inhibit systemic inflammation by activating the noradrenergic splenic nerve via the α7nAChR nicotinic receptors. α7nAChR represents a unique molecular link between the parasympathetic and sympathetic system to control inflammation.
The coordination of the immune system is essential for physiological homeostasis and critical for the proper response to infection, trauma, or injury. Immune cytokines are critical to fight infections. But at the same time, the unregulated production of these cytokines is one of the principal causes of morbidity and mortality (1–3). TNF is a pleiotropic cytokine involved in many of the physiological responses to infection, trauma, and cancer. And yet, TNF also contributes to inflammatory disorders, autoimmune diseases, and sepsis (4, 5). Inflammatory cytokines are extensively studied at the cellular and molecular levels, but the physiological mechanisms regulating their production in vivo remain relatively unknown. Among them, the autonomic nervous system connects the immune system with the other organs and orchestrates the immune responses according to the physiological needs (6, 7). Classically, sympathetic and parasympathetic systems act in opposition to maintain physiological homeostasis. Although the sympathetic system has been studied for years, only recently was demonstrated the anti-inflammatory potential of the parasympathetic system (8–10). The vagus nerve is the longest and most characteristic nerve of the parasympathetic system innervating most of the peripheral organs. The vagus nerve is involved in most of the physiological mechanisms including the respiratory rate, heartbeat, hormone secretion, intestinal peristalsis, and digestion (11). However, only recent studies indicate that the vagus nerve can also modulate immune responses in life-threatening conditions such as trauma, hemorrhage, systemic infection, or sepsis (12, 13). Electrical or pharmacological stimulation of the vagus nerve restrains the production of inflammatory cytokines in experimental ischemia and reperfusion (13–15), hemorrhage and resuscitation (13), pancreatitis (16), colitis (17), endotoxemia (8, 18), and sepsis (12, 19). Despite its clinical implications, the physiological mechanism contributing to the anti-inflammatory potential of the vagus nerve remains unknown.
The vagus nerve can restrain serum TNF levels, and prevents septic shock and organ damage (18). Because acetylcholine is the principal neurotransmitter of the vagus nerve, preliminary studies analyzed the potential of cholinergic agonists to prevent TNF production in immune cells (11, 20, 21). Acetylcholine and nicotine inhibit NF-κB and cytokine production through the α7 nicotinic acetylcholine receptor (α7nAChR) located in macrophages and splenocytes (12, 18, 22, 23). From a pharmacologic perspective, nicotine is a more stable and selective cholinergic agonist at inhibiting cytokine production in immune cells (8, 12, 21, 22). Treatment with nicotine attenuates systemic inflammation and improves survival in experimental sepsis (12, 20). Current strategies are directed to identify specific α7nAChR agonists to avoid the collateral toxicity of nonspecific nicotinic agonists (20). Both vagus nerve and α7nAChR agonists control systemic inflammation in sepsis by targeting the JAK2-STAT3 pathway (24– 27). Together, these results suggested a “direct” link between the vagus nerve and macrophages, where acetylcholine released by the vagus nerve targets macrophages to inhibit cytokine production (9, 10, 21). However, our studies indicate that the spleen is a major source of inflammatory cytokines involved in the initiation of systemic inflammation (18). This vagal inhibition of cytokine production in the spleen was surprising because the vagus nerve terminates in the celiac-mesenteric ganglia and does not innervate the spleen (28–30). Thus, the physiological mechanisms mediating, in vivo, the anti-inflammatory potential of the vagus nerve or cholinergic agonists is unknown. In this article, we analyze the physiological inhibition of cytokine production in the spleen by the vagus nerve and cholinergic agonists. The implications of the α7nAChR were analyzed by using α7nAChR The Journal of Immunology knockout and wild-type (WT) littermate mice. Our results indicate that the vagus nerve induces plasma and splenic norepinephrine by activating the sympathetic splenic nerve via the α7nAChR.
Materials and Methods
Animal experiments
All animal procedures were approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School. Adult male α7nAChR knockout and WT mice (B6, 129S7-Chrna 7tm1Bay/J) litter-mates (C57BL/6J) 8- to 12 wk-old and adult male Sprague Dawley rats 8-to 12 wk-old were purchased from The Jackson Laboratory and Charles River Laboratories, respectively. The study includes both mice and rats to confirm the results in different species and to take advantage of the knockout mice and the size of the rats for surgical procedures. Animals were randomly distributed to ensure same sex, age, and sample size in different groups for experimental treatment. The investigators analyzing the collecting samples were blinded to the experimental treatment. Animals were genotyped by PCR assay using genomic DNA from mouse tails and the Extract-N-Amp Tissue PCR kit as previously described (7). Animals were maintained on 12-h light/dark cycle, with free access to food and water. Xylazine (20 mg/kg; Lloyd Laboratories, Shenandoah, IA) and ketamine (50 mg/kg; Bioniche Pharma USA, Lake Forest, IL) was used for anesthesia. Postoperatively, sterile saline and analgesic Buprenorphin (20 µg/kg; Hospira, Lake Forest, IL) was given. Reserpine (5 mg/kg; Sigma Chemical, St. Louis, MO) was given 24 h before experiment. Endotoxemia was performed as we described previously (12). In brief, endotoxin (Escherichia coli LPS 0111:B4; Sigma) was dissolved in sterile, pyrogen-free PBS and sonicated for 30 min immediately before use. Animals received an LD50 dose of LPS (6 mg/kg). Blood was collected after 90 min for cytokine analyses. Choline from Sigma Chemical was diluted in PBS. Splenectomy was performed as we described previously (18). In brief, mice underwent anesthesia. Afterward, the spleen was identified following a midline laparotomy incision and removed after appropriate blood vessel ligation. Sham animals underwent laparotomy without splenectomy. Surgical cervical vagotomy was made as we described previously (18). In brief, a ventral cervical midline incision was used to expose the right cervical vagus trunks, which were ligated with 4–0 silk sutures and divided. In sham-operated animals, the right vagus nerve was exposed and isolated from surrounding tissue but not transected. Animals were vago-tomized 48 h before experimental sepsis. Vagus nerve stimulation (VNS) was made as we described previously (18). In brief, a small incision was made to explore and identify the right cervical vagus nerve, and a platinum electrode was then placed across. The platinum electrode was attached to the Stimulation Device (STM 150) controlled by the AcqKnowledge software (Biopac Systems). Vagus stimulation was applied for either 15 or 20 min at 5 V. Sham operation was made on control animals with exception of stimulation. Bilateral adrenalectomy was made through an abdominal midline incision under aseptic surgical conditions. Appropriate measures were taken to avoid bleeding. Control group underwent sham surgery. Splenic nerve stimulation (SNS) was performed via an abdominal flank incision. The spleen was retracted upward to have the splenic vessels exposed. Splenic artery was explored and prepared after removal of perivascular abdominal adipose tissue. Platinum electrode was placed across the splenic artery that travels along with the periarterial splenic nerve fibers. For splenic neurectomy, abdominal incision was made and splenic vessels were explored. After isolation of the splenic vessels, tiny fibers of splenic nerve were observed and divided with sharp forceps under aseptic conditions.
Catecholamine determination
In rats, collection of blood was carried out through an i.v. catheter that had been introduced into the right jugular vein of the rats at 48 h before experiment. The mice were first euthanized with carbon dioxide, and blood was then harvested via cardiac puncture. Blood for catecholamine determination was collected in EDTA tubes (BD, Franklin Lakes, NJ) to avoid clotting and then immersed in ice for 30 min. The blood samples were centrifuged at 3000 rpm for 5 min, and the supernatant plasma was collected and transferred to regular uncoated tubes. The samples were stored at −80°C until analysis using the catecholamine ELISA (Rocky Mountain Diagnostics, Colorado Springs, CO).
Cytokine analyses
Blood was collected at the indicated time points, allowed to clot for 2 h at room temperature, and centrifuged for 15 min at 2000 × g. TNF concentration was analyzed by TNF ELISA kit from (eBioscience, San Diego, CA) following manufacturer’s instructions. TNF was analyzed in serum at 90 min after LPS challenge.
Statistical analyses
All data in the figures and text are expressed as mean ± SD. Statistical analyses were performed using the Mann–Whitney U test to compare values between two experimental groups. The one-way ANOVA was used for multiple pairwise comparisons with the Bonferroni’s adjustment for multiple hypothesis testing. Normality and homogeneity of variance were analyzed. ANOVA was used to compare all treatments and specific pair-wise comparisons as stated in the experiments. Statistical analyses of survival were determined using the log rank test. The p values <0.05 were considered statistically significant.
Results
Efferent vagus nerve induces plasma norepinephrine via the α7nAChR
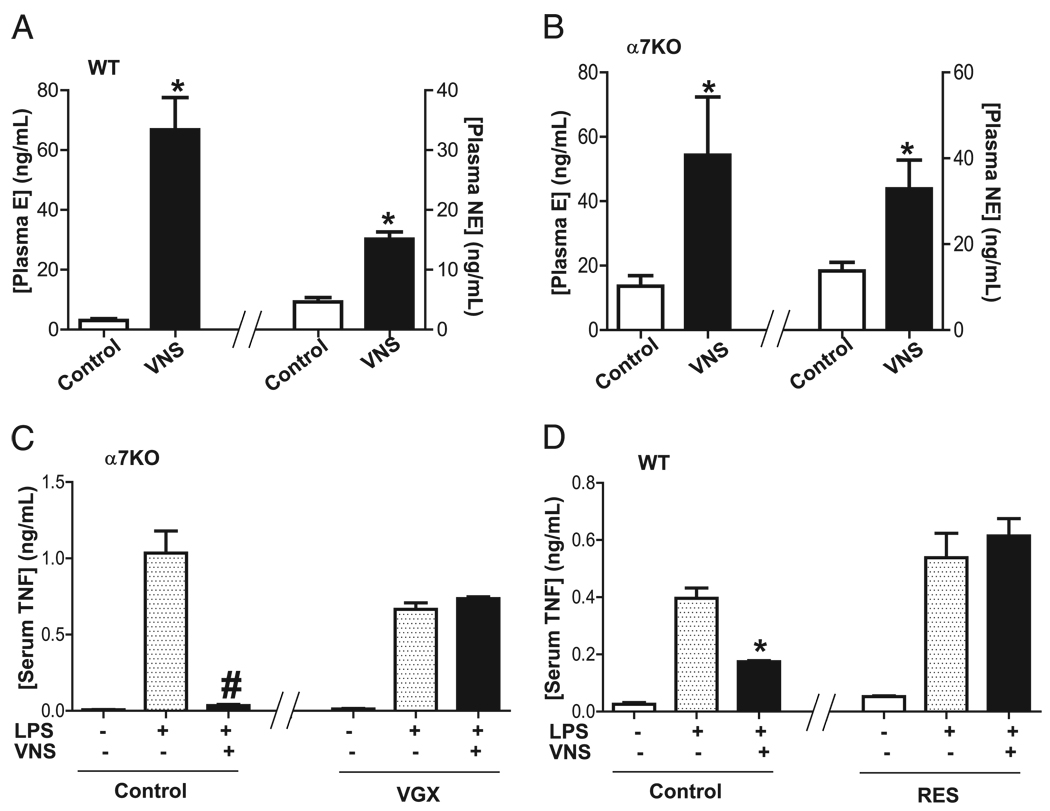
We first analyzed whether the vagus nerve modulates plasma catecholamines. VNS induces the production of both epinephrine and norepinephrine (Fig. 1A). Because previous studies indicate that the vagus nerve failed to prevent systemic inflammation in α7nAChR knockout mice (22), we analyzed whether that effect was due to an inability to produce catecholamines in those animals. Surprisingly, VNS also induces both catecholamines in α7nAChR knockout mice similar to that found in WT mice (Fig. 1B). Because stimulation of intact vagus nerve activates both afferent signaling toward the brain and efferent signals toward peripheral organs, we reasoned that our results may include unspecific afferent signals spreading through the CNS. To analyze this hypothesis, we compared stimulation of intact (nonsectioned) vagus nerves in sham mice with the stimulation of the efferent distal trunk of previously sectioned vagus nerve in vagotomized mice. Stimulation of “intact” vagus nerve attenuates serum TNF in the α7nAChR knockout mice. Unlike “intact” vagus nerve, stimulation of efferent vagus nerve fails to attenuate serum TNF levels in α7nAChR knockout mice (Fig. 1C). These results indicate that VNS can trigger an afferent signal inducing a broad activation of the CNS and sympathetic catecholamines. In contrast, vagal stimulation of previously sectioned nerves triggers specific efferent signals toward the peripheral immune organs that is specifically mediated by the α7nAChR.
FIGURE 1.
Efferent vagus nerve induces norepinephrine. A, C57BL/6J WT or (B) α7nAChR knockout (α7KO) mice underwent sham surgery (control) or VNS. Plasma epinephrine (E) and norepinephrine (NE) were analyzed at 20 min after stimulation. *p < 0.01 versus control (n = 3; Mann–Whitney U test). C, α7nAChR knockout (α7KO) mice underwent sham surgery (control) or cervical vagotomy (VGX). Twenty-four hours later, nonsectioned intact nerve (control) or the distal trunk of sectioned vagus nerve (VGX) was stimulated for 10 min before LPS. #p < 0.01 versus LPS (n = 4; one-way ANOVA with Bonferroni’s corrections). D, Adult C57BL/6J WT mice were treated with reserpine (RES; 5 mg/kg) to inhibit the release of catecholamines. Serum TNF levels were analyzed 90 min later. *p < 0.01 versus LPS (n = 4; one-way ANOVA with Bonferroni’s corrections).
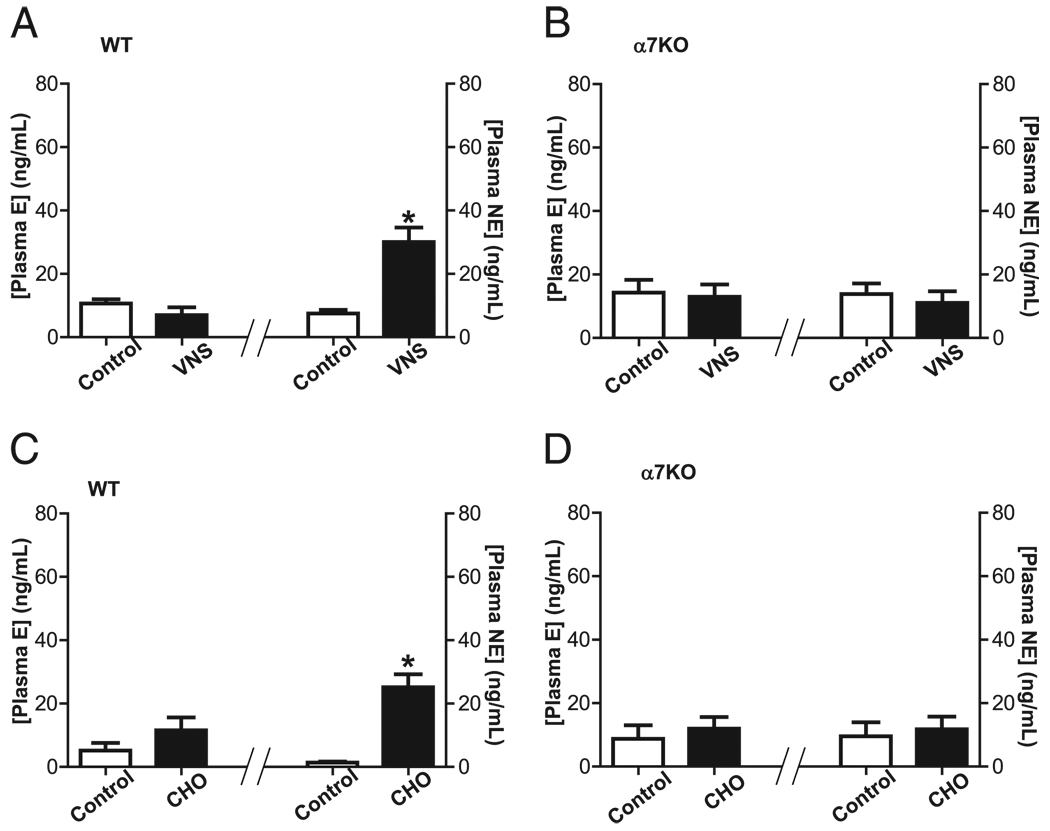
To analyze the implications of catecholamines in controlling systemic inflammation, we treated C57BL/6J mice with reserpine, a well-characterized inhibitor of catecholamine production (31, 32). Then, animals were vagotomized to specifically activate the efferent signal by electrical stimulation of the distal nerve tip. Stimulation of efferent vagus nerve inhibited serum TNF levels in control mice but not in those treated with reserpine (Fig. 1D). These results were further confirmed by studying the potential of the efferent vagus nerve to induce plasma catecholamines. Stimulation of efferent vagus nerve in WT mice specifically induces plasma norepinephrine only, without affecting epinephrine (Fig. 2A). The role of the α7nAChR in the vagal induction of norepinephrine was analyzed in α7nAChR knockout littermate mice. This mechanism is mediated by the α7nAChR because stimulation of efferent vagus nerve fails to induce norepinephrine in the α7nAChR knockout mice (Fig. 2B). These results suggest that efferent vagus nerve induces plasma norepinephrine via the α7nAChR. To further confirm this hypothesis, we used well-characterized α7nAChR agonists to induce a selective activation of this receptor. Previous studies indicate that choline is a selective α7nAChR agonist able to attenuate inflammation in WT but not in α7nAChR knockout animals (25–27). Similar to efferent vagus nerve, choline specifically induces plasma norepinephrine only, without significantly affecting epinephrine in mice (Fig. 2C). Again, this mechanism is specifically mediated by the α7nAChR because choline fails to induce norepinephrine in the α7nAChR knockout littermate mice (Fig. 2D). Together, these results indicate that efferent vagus nerve and α7nAChR agonists induce plasma norepinephrine via the α7nAChR.
FIGURE 2.
α7nAChR mediates vagal induction of norepinephrine. A and C, Adult male C57BL/6J WT littermate or (B, D) α7nAChR knockout (α7KO) mice underwent vagotomy 24 h before stimulation. Animals received (A, B) VNS or (C, D) selective α7nAChR agonist, choline (CHO; 15 mg/kg i.p.). Plasma levels of epinephrine (E) and norepinephrine (NE) were analyzed at 20 min after stimulation. *p < 0.01 versus control (n = 3; Mann–Whitney U test)
Vagus nerve and α7nAChR agonists control systemic inflammation through the splenic nerve
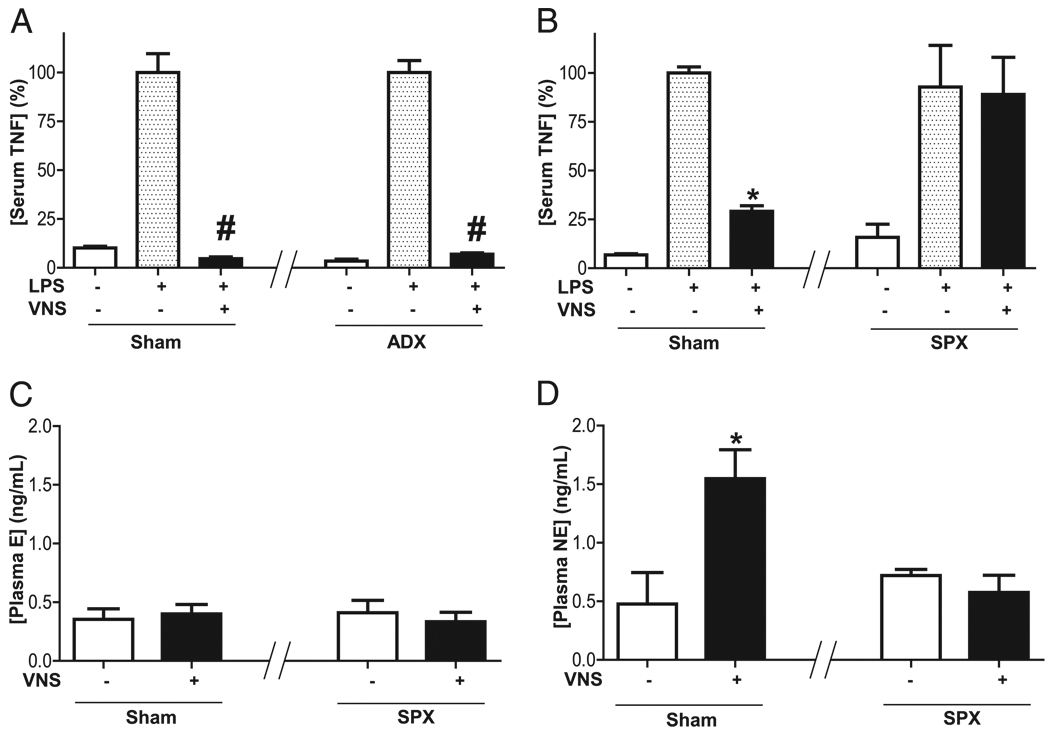
The implication of the adrenal gland as the source of plasma norepinephrine contributing to the anti-inflammatory potential of the efferent vagus nerve was analyzed in adrenalectomized rats. These experiments were performed in adult male Sprague Dawley rats to facilitate the surgical procedures. Stimulation of efferent vagus nerve attenuates serum TNF levels in both sham and adrenalectomized rats (Fig. 3A). These results indicate that the adrenal glands are not required for the anti-inflammatory potential of the vagus nerve. Unlike adrenalectomy, splenectomy prevents the anti-inflammatory potential of the vagus nerve. VNS attenuates serum TNF in control but not in splenectomized rats (Fig. 3B). Because these results suggest that the spleen is required for the anti-inflammatory mechanism of the vagus nerve, we analyzed whether the splenectomy affects the vagal induction of plasma norepinephrine. Stimulation of efferent vagus nerve fails to induce plasma epinephrine in either sham or splenectomized rats (Fig. 3C). However, stimulation of efferent vagus nerve induces plasma norepinephrine in control but not in splenectomized rats (Fig. 3D). These results indicate that efferent vagus nerve induces plasma norepinephrine through a mechanism mediated by the spleen. Because previous studies reported that the spleen is a major source of serum TNF contributing to the pathogenesis of sepsis (18), our results suggest that the vagus nerve induces splenic norepinephrine to control cytokine production and modulate systemic inflammation in sepsis.
FIGURE 3.
Splenectomy abrogates vagal induction of plasma norepinephrine. Adult male Sprague Dawley rats underwent sham surgery (sham), (A) adrenalectomy (ADX), or (B–D) splenectomy (SPX) 5 d before endotoxemia (LPS). Cervical vagotomy was performed 24 h before endotoxemia (LPS) and VNS. A and B, Serum TNF levels were analyzed at 90 min after LPS. Plasma levels of (C) epinephrine (E) and (D) norepinephrine (NE) were analyzed at 15 min after stimulation. #p < 0.01 versus LPS (n = 3; one-way ANOVA with Bonferroni’s corrections), *p < 0.01 versus control (n = 3; Mann–Whitney U test).
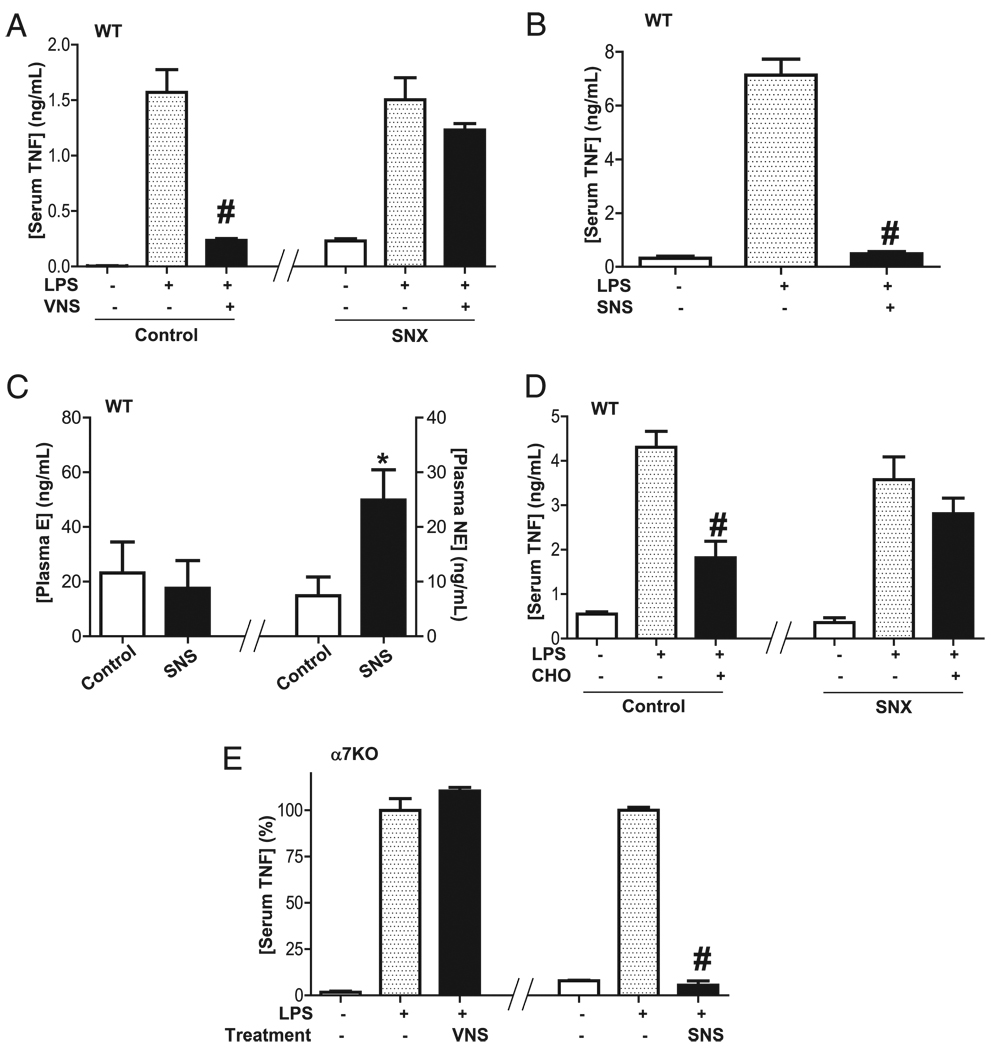
Because previous studies failed to establish a direct vagal innervation of the spleen (32), and the spleen is mainly innervated by catecholaminergic fibers originated in the mesenteric ganglia and traveling along the splenic nerve (33, 34), we analyzed whether the splenic nerve contributes to the anti-inflammatory potential of the vagus nerve. Stimulation of efferent vagus nerve attenuates serum TNF levels in sham but not in neurectomized mice (Fig. 4A), suggesting that the vagus nerve requires the splenic nerve to control systemic inflammation in sepsis. To confirm this hypothesis, we analyzed whether stimulation of the splenic nerve mimics the anti-inflammatory potential of the vagus nerve. Stimulation of the splenic nerve inhibits serum TNF during endotoxemia (Fig. 4B). Similar to that described for the vagus nerve, stimulation of the splenic nerve specifically induced plasma norepinephrine without affecting the production of epinephrine (Fig. 4C). Because these results indicate that the splenic nerve mediates the anti-inflammatory potential of the vagus nerve, we analyzed whether the α7nAChR agonists also require the splenic nerve to restrain serum TNF levels. The anti-inflammatory potential of the α7nAChR agonist was analyzed in sham and neurectomized mice. α7nAChR agonist choline attenuated serum TNF levels in sham mice, but not after splenic neurectomy (Fig. 4D), indicating that the α7nAChR also requires the splenic nerve to control systemic inflammation. These results suggest that the α7nAChR is a postsynaptic receptor required to activate the splenic nerve by either the vagus nerve or α7nAChR agonists. Thus, we reasoned that SNS can mimic α7nAChR activation, and it may re-establish neuromodulation in α7nAChR-deficient animals. VNS in the α7nAChR knockout mice fails to attenuate serum TNF levels, but SNS re-establishes neuromodulation in these animals by inhibiting serum TNF levels during endotoxemia (Fig. 4E). These results suggest that stimulation of the splenic nerve mimics the anti-inflammatory potential of VNS or it can bypass deficiencies on the α7nAChR. These results indicate that the vagus nerve activates the splenic nerve to release norepinephrine in the spleen (Fig. 5).
FIGURE 4.
Vagus nerve and α7nAChR agonists control systemic inflammation through the splenic nerve. A, C57BL/6J WT mice underwent vagotomy (VGX) and sham (control) or splenic neurectomy (SNX) 24 h before endotoxemia (LPS) and cervical vagal stimulation (VNS). Serum TNF levels were analyzed at 90 min after endotoxemia. B and C, C57BL/6J WT mice underwent SNS, and (B) serum TNF (C) or plasma epinephrine (E) and norepinephrine (NE) were analyzed by ELISA. D, C57BL/6J WT mice underwent sham or splenic neurectomy (SNX) 24 h before treatments. Selective α7nAChR agonist choline (CHO; 15 mg/kg i.p.) was administered 30 min before LPS (6 mg/kg i.p.). E, SNS re-establishes neuromodulation in α7nAChR knockout mice. Knockout mice underwent vagotomy 24 h before endotoxemia (LPS). Animals received VNS or SNS. #p < 0.01 versus LPS (n = 3; one-way ANOVA with Bonferroni’s corrections), *p < 0.01 versus control (n = 4; Mann–Whitney U test
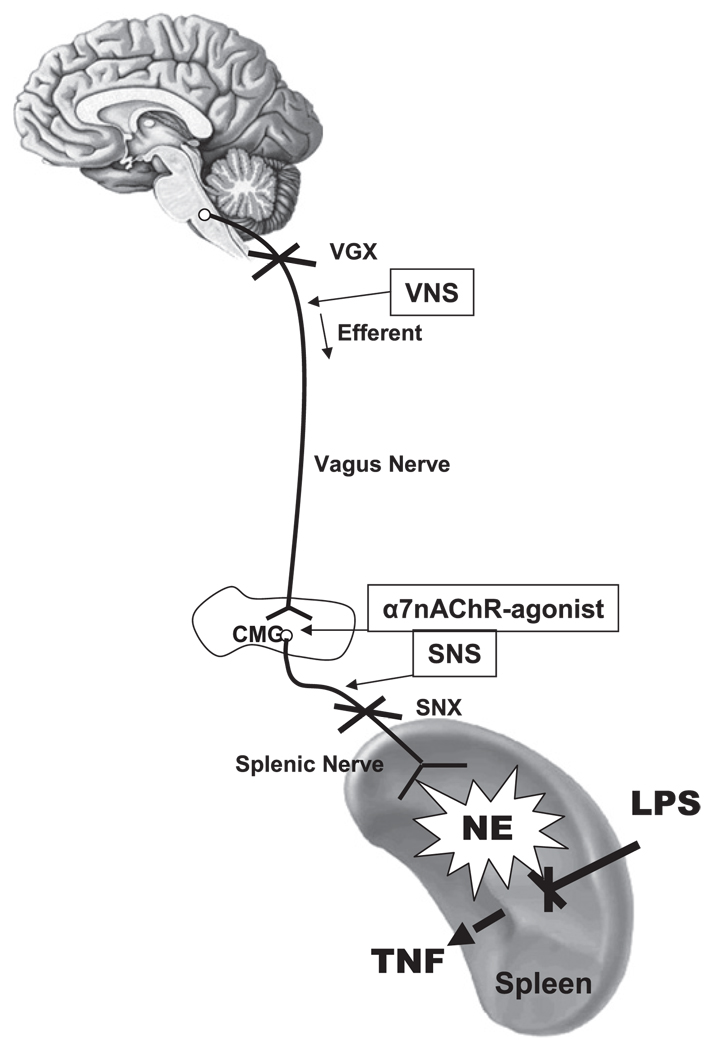
FIGURE 5.
Vagus nerve modulates systemic norepinephrine via the α7nAChR, the splenic nerve, and the spleen. Vagotomy (VGX) prevents afferent vagus nerve toward the CNS. VNS and α7nAChR agonists activate the splenic nerve to produce norepinephrine (NE) via the α7nAChR. Our results suggest that acetylcholine released by the vagus nerve in the celiac mesenteric ganglia (CMG) activates postsynaptic α7nAChR of the splenic nerve, leading to the release of norepinephrine in the spleen. The anti-inflammatory potential of the vagus nerve and α7nAChR agonists is inhibited by splenectomy or splenic neurectomy (SNX). SNS mimics the anti-inflammatory mechanism of the vagus nerve and α7nAChR agonists to induce norepinephrine and prevent LPS-induced serum TNF levels. SNS re-establishes neuromodulation in α7nAChR knockout mice.
Discussion
Our previous studies indicate that the vagus nerve can control systemic inflammation by inhibiting cytokine production in the spleen (18). These results were surprising because the vagus nerve terminates in the celiac-mesenteric ganglia and does not innervate the spleen (28–30). Thus, the anti-inflammatory mechanism of the vagus nerve or cholinergic agonists was unknown. Now, we propose a new physiological mechanism modulating inflammatory responses in life-threatening conditions. Parasympathetic vagus nerve controls systemic inflammation through the sympathetic noradrenergic splenic nerve via the α7nAChR. In vivo, both vagus nerve and cholinergic agonists modulate systemic inflammation by inducing plasma and splenic norepinephrine through the splenic nerve. Acetylcholine released by the vagus nerve in the celiac-mesenteric ganglia activates postsynaptic α7nAChR of the splenic nerve, leading to the release of norepinephrine in the spleen (Fig. 5). Previous studies already indicated that the vagus nerve can connect with the splenic nerve (28, 32). Laser-scanning confocal microscopy identifies vagal efferent terminals in the celiac-mesenteric ganglia (28). These vagal terminals were regarded either as ectopic parasympathetic junctions or as part of a vagal mechanism for gating of sympathetic ganglionic transmission (28). According to our results, the parasympathetic innervation of the mesenteric ganglia mediates the classical vagal influence on gastrointestinal targets, but also a vagal control of the spleen, which has not been traditionally regarded as a vagal target.
Although stimulation of both the vagus nerve and the splenic nerve increases plasma norepinephrine levels in control animals (without endotoxemia), it is unknown whether such a systemic effect may have significant implications in sepsis. Our studies analyze plasma catecholamines in normal animals to improve the experimental conditions and to avoid the interferences induced by the production of catecholamines by other mechanisms in stressful conditions such as sepsis. Indeed, a large number of studies suggest that, unlike the adrenal glands, the sympathetic production of norepinephrine in the spleen is intended to induce a local effect modulating cytokine production in the spleen. Indeed, recent studies indicate that the splenic nerve can inhibit cytokine production in splenic macrophages (32, 35, 36). Noradrenergic nerve fibers distribute with the vascular systems and innervate the periarteriolar lymphatic sheath, the marginal sinus, and the parafollicular zone. At the marginal sinus, tyrosine hydroxylase-positive fibers run adjacent to macrophages (ED3+ cells). This proximity can suggest a direct interaction between norepinephrine release from the nerve terminals and the macrophages closely associated with them, suggesting that this mechanism intends to induce a “local” modulation within the spleen (35). This strategy will allow inhibiting specific splenic responses without affecting other organs. Previous studies in splenic slices and spleens perfused ex vivo already confirmed that the splenic nerve releases norepinephrine in the spleen (36). Splenic norepinephrine can inhibit cytokine production from macrophages via β-adrenergic receptors (36–39). Our current studies in WT and α7nACh knockout mice now suggest that from a molecular and physiological perspective, the vagus nerve controls splenic noradrenergic fibers specifically via the α7nAChR.
Vagal inhibition of postganglionic catecholaminergic fibers has been recently reported. Vagus nerve can inhibit the sympathetic thoracic nerves to release pancreatic norepinephrine (40). Similar to described in respiratory rate, heartbeat, and hormone secretion, these results concur with the classic notion of the parasympathetic system opposing or inhibiting the sympathetic system to maintain the physiological homeostasis. However, our study indicates that vagus nerve activates sympathetic splenic nerves to release norepinephrine. To our knowledge, this is the first report indicating that both systems can team together to restrain systemic inflammation in experimental sepsis. Furthermore, our results indicate that activation of the α7nAChR is necessary and sufficient for the vagal activation of the splenic nerve. Similar to vagotomized or neurectomized animals, α7nAChR knockout mice are also more susceptible to systemic inflammation during sepsis (22) because of the inactivation of the splenic nerve. Previous studies confirmed the presence of the α7nAChR in postsynaptic sympathetic ganglion (41, 42). In addition to the postsynaptic noradrenergic nerves, multiple studies confirmed that α7nAChR is also expressed in macrophages and splenocytes, where it inhibits cytokine production (20, 43). In vitro, cholinergic agonists inhibit the NF-κB pathway and cytokine production via the α7nAChR expressed in macrophages and splenocytes (12, 22, 23). This mechanism is mediated by the inhibition of the JAK2-induced STAT3 tyrosine phosphorylation (25, 26). Although these results could suggest a “direct” interaction between the vagus nerve and immune cells, our results suggest that both the vagus nerve and α7nAChR agonists require the splenic nerve to control systemic inflammation in sepsis. Thus, the physiological implications of the α7nAChR expressed in immune cells remain unknown, but several studies indicate that rat lymphocytes produce and secrete acetylcholine (44). α7nAChR in macrophages could mediate the anti-inflammatory potential of acetylcholine produced in the spleen by lymphocytes. And yet, our results indicate SNS re-establishes neuromodulation and inhibits serum TNF levels in the α7nAChR knockout animals, suggesting that the α7nAChR in the splenocytes is not required for the anti-inflammatory potential of either the vagus or the splenic nerve. Together, our results indicate that acetylcholine released by the vagus nerve in the celiac-mesenteric ganglia activates postsynaptic α7nAChR of the splenic nerve, leading to the release of norepinephrine in the spleen. The α7nAChR represents a unique molecular link to coordinate the parasympathetic and the sympathetic system and control cytokine production in the spleen. From a physiological perspective, both the parasympathetic vagus nerve and the sympathetic splenic nerve can team together and coordinate to control systemic inflammation in life-threatening conditions such as sepsis.
Acknowledgments
This work was supported by the Hungarian Rosztoczy Foundation (to G.V.). Dr. M. Marks is funded by the National Institutes of Health (P30DA015663). L.U. was supported by the faculty program of the Department of Surgery of the New Jersey Medical School and grants from the American Heart Association (AHA06352230N) and the National Institutes of Health (RO1-GM084125).
We thank Dr. Michael Marks and Bolin Cai for their cooperation.
Abbreviations used in this article
- α7nAChR
α7 nicotinic acetylcholine receptor
- SNS
splenic nerve stimulation
- VNS
vagus nerve stimulation
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 2.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol. Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Ulloa L, Brunner M, Ramos L, Deitch EA. Scientific and clinical challenges in sepsis. Curr. Pharm. Des. 2009;15:1918–1935. doi: 10.2174/138161209788453248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu. Rev. Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 5.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman L. Elaborate interactions between the immune and nervous systems. Nat. Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 7.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 9.Tracey KJ. Reflex control of immunity. Nat. Rev. Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracey KJ. Understanding immunity requires more than immunology. Nat. Immunol. 2010;11:561–564. doi: 10.1038/ni0710-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 13.Cai B, Chen F, Ji Y, Kiss L, de Jonge WJ, Conejero-Goldberg C, Szabo C, Deitch EA, Ulloa L. Alpha7 cholinergic-agonist prevents systemic inflammation and improves survival during resuscitation. J. Cell. Mol. Med. 2009;13(9B):3774–3785. doi: 10.1111/j.1582-4934.2008.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J. Vasc. Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 15.Altavilla D, Guarini S, Bitto A, Mioni C, Giuliani D, Bigiani A, Squadrito G, Minutoli L, Venuti FS, Messineo F, et al. Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock. 2006;25:500–506. doi: 10.1097/01.shk.0000209539.91553.82. [DOI] [PubMed] [Google Scholar]
- 16.van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der Poll T. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, Newcombe RG, Russell MA, Feyerabend C, Thomas GA, et al. Transdermal nicotine for active ulcerative colitis. N. Engl. J. Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 18.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, et al. Sple-nectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, Tracey KJ, van der Poll T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J. Infect. Dis. 2005;191:2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 20.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug. Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 21.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 23.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 25.Peña G, Cai B, Deitch EA, Ulloa L. JAK2 inhibition prevents innate immune responses and rescues animals from sepsis. J. Mol. Med. 2010;88:851–859. doi: 10.1007/s00109-010-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peña G, Cai B, Liu J, van der Zanden EP, Deitch EA, de Jonge WJ, Ulloa L. Unphosphorylated STAT3 modulates alpha 7 nicotinic receptor signaling and cytokine production in sepsis. Eur. J. Immunol. 2010;40:2580–2589. doi: 10.1002/eji.201040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X, Patel N, Johnson SM, et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med. 2008;14:567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J. Auton. Nerv. Syst. 1993;42:153–169. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 29.Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav. Immun. 1993;7:191–204. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- 30.Nance DM, Burns J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav. Immun. 1989;3:281–290. doi: 10.1016/0889-1591(89)90028-7. [DOI] [PubMed] [Google Scholar]
- 31.Vizi ES, Orsó E, Osipenko ON, Haskó G, Elenkov IJ. Neu-rochemical, electrophysiological and immunocytochemical evidence for a nor-adrenergic link between the sympathetic nervous system and thymocytes. Neuroscience. 1995;68:1263–1276. doi: 10.1016/0306-4522(95)00215-5. [DOI] [PubMed] [Google Scholar]
- 32.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. USA. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellinger DL, Felten SY, Lorton D, Felten DL. Origin of noradrenergic innervation of the spleen in rats. Brain Behav. Immun. 1989;3:291–311. doi: 10.1016/0889-1591(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 34.Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J. Comp. Neurol. 2001;439:1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- 35.Felten DL, Ackerman KD, Wiegand SJ, Felten SY. Noradrenergic sympathetic innervation of the spleen: I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J. Neurosci. Res. 1987;18:28–36. 118–121. doi: 10.1002/jnr.490180107. [DOI] [PubMed] [Google Scholar]
- 36.Kees MG, Pongratz G, Kees F, Schölmerich J, Straub RH. Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J. Neuro-immunol. 2003;145:77–85. doi: 10.1016/j.jneuroim.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Elenkov IJ, Haskó G, Kovács KJ, Vizi ES. Modulation of lipopolysaccharide-induced tumor necrosis factor-alpha production by selective alpha- and beta-adrenergic drugs in mice. J. Neuroimmunol. 1995;61:123–131. doi: 10.1016/0165-5728(95)00080-l. [DOI] [PubMed] [Google Scholar]
- 38.Chou RC, Stinson MW, Noble BK, Spengler RN. Beta-adrenergic receptor regulation of macrophage-derived tumor necrosis factoralpha production from rats with experimental arthritis. J. Neuroimmunol. 1996;67:7–16. doi: 10.1016/0165-5728(96)00023-9. [DOI] [PubMed] [Google Scholar]
- 39.Hu XX, Goldmuntz EA, Brosnan CF. The effect of norepinephrine on endotoxin-mediated macrophage activation. J. Neuroimmunol. 1991;31:35–42. doi: 10.1016/0165-5728(91)90084-k. [DOI] [PubMed] [Google Scholar]
- 40.Benthem L, Mundinger TO, Taborsky GJ., Jr Parasympathetic inhibition of sympathetic neural activity to the pancreas. Am. J. Physiol. Endocrinol. Metab. 2001;280:E378–E381. doi: 10.1152/ajpendo.2001.280.2.E378. [DOI] [PubMed] [Google Scholar]
- 41.Lips KS, König P, Schaötzle K, Pfeil U, Krasteva G, Spies M, Haberberger RV, Grando SA, Kummer W. Coexpression and spatial association of nicotinic acetylcholine receptor subunits alpha7 and alpha10 in rat sympathetic neurons. J. Mol. Neurosci. 2006;30:15–16. doi: 10.1385/JMN:30:1:15. [DOI] [PubMed] [Google Scholar]
- 42.Del Signore A, Gotti C, Rizzo A, Moretti M, Paggi P. Nicotinic acetylcholine receptor subtypes in the rat sympathetic ganglion: pharmacological characterization, subcellular distribution and effect of pre- and postganglionic nerve crush. J. Neuropathol. Exp. Neurol. 2004;63:138–150. doi: 10.1093/jnen/63.2.138. [DOI] [PubMed] [Google Scholar]
- 43.Galvis G, Lips KS, Kummer W. Expression of nicotinic acetylcholine receptors on murine alveolar macrophages. J. Mol. Neurosci. 2006;30:107–108. doi: 10.1385/JMN:30:1:107. [DOI] [PubMed] [Google Scholar]
- 44.Rinner I, Kawashima K, Schauenstein K. Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation. J. Neuroimmunol. 1998;81:31–37. doi: 10.1016/s0165-5728(97)00155-0. [DOI] [PubMed] [Google Scholar]