Abstract
Inhibiting single cytokines produced modest effects in clinical trials, in part because the cytokines werenot specific for sepsis, and sepsis may require cellular strategies. Previous studies reported that mast cells (MCs) fight infections in early sepsis. In this study, we report that MC stabilizers restrain serum TNF levels and improve survival in wild-type but not in MC-deficient mice. Yet, MC depletion in knockout mice attenuates serum TNF but does not improve survival in sepsis. Serum HMGB1 was the only factor correlating with survival. MC stabilizers inhibit systemic HMGB1 levels and rescue mice from established peritonitis. MC stabilizers fail to inhibit HMGB1 secretion from macrophages, but they prevent apoptosis and caspase-3 activation in sepsis. These results suggest that MC stabilization provides therapeutic benefits in sepsis by inhibiting extracellular release of HMGB1 from apoptotic cells. Our study provides the first evidence that MCs have major immunological implications regulating cell death in sepsis and represent a pharmacological target for infectious disorders in a clinically realistic time frame.
Severe sepsis is a major clinical challenge in modern medicine, representing the third cause of death in developed countries (1, 2). There are very few treatment options, and the mortality rate in sepsis remains extremely high, from 30–70% depending on the underlying cause and the organs affected (3). Severe sepsis remains a scientific challenge with >30 unsuccessful clinical trials (3). Recombinant human activated protein C is the only treatment approved by the U.S. Food and Drug Administration. Yet, this treatment is approved only for a small subset of patients with severe sepsis due to the risk of hemorrhage. One of the principal challenges of sepsis is the two factors of the pathology: the infection and the inflammatory responses of the host. Sepsis is commonly originated by an infection, and the development of new antibiotics can control and prevent infections (2). However, despite the advances in antibiotics and intensive care, sepsis remains the most common cause of death in hospitalized patients, killing over 250,000 patients and accounting for 9.3% of the overall deaths in the United States annually (1, 2). In addition to the infection, sepsis is characterized by detrimental inflammatory responses produced by the host. Immune cytokines trigger defensive inflammatory responses to fight the infection. But, overzealous cytokine production can be worse than the original infection, causing deleterious inflammatory responses leading to multiple organ failure (4–7). Many studies have shown the contribution of particular cytokines to the pathogenesis of sepsis. Among others, inhibition of TNF, ILs, migration inhibitory factor, or HMGB1 provided promising results in experimental sepsis. Yet, none of these cytokines appears to be specific for sepsis, and, so far, the inhibition of single cytokines has produced modest effects in clinical trials for sepsis (8). A potential explanation is that sepsis is not produced by a single cytokine, and hence, successful treatments may require inhibiting several cytokines rather than a single cytokine. Thus, most recent strategies have focused on analyzing the cellular sources of those detrimental inflammatory responses as an alternative strategy for treating sepsis.
The inflammatory responses in sepsis are complex, and their cellular sources remain hitherto controversial. Most of the inflammatory cytokines in sepsis were first described in macrophages, and thus most of the cellular studies focused on those cells. Recent studies revealed the unique potential of mast cells (MCs) to produce prompt inflammatory responses and to fight the infection in the early stages of sepsis. In agreement with this mechanism, MC-deficient mice have increased susceptibility and mortality in polymicrobial peritonitis (9). As the number of peritoneal macrophages does not differ between MC-deficient mice and normal littermates (9, 10), macrophages are an unlikely candidate to explain these differences. Adoptive transfer of MCs from wild-type mice re-establishes natural protection against infection in reconstituted knockout mice (9). MC-induced protection appears to be mediated by TNF because anti-TNF neutralizing Abs prevent this effect (9). Unlike resting macrophages (11), T cells (12) or B cells (13), MCs store notable quantities of TNF available for immediate release (14). MCs are the only resident cell type capable of storing TNF in cytoplasmic granules that is rapidly released immediately postinfection (14, 15). MC-produced TNF modulates neutrophil influx and bacterial clearance at sites of infection (16). Most of the studies emphasized the role of the MC in the early stages of sepsis using experimental models of moderate sepsis, which show high survival even in the absence of antibiotics. Although these results provided valuable information about the physiological implications of MCs in early stages of the infection, little is known about the pharmacological implications of MCs in the clinical settings of sepsis. In this study, we analyze the potential contribution of MCs to experimental models of sepsis resembling clinical settings, characterized by the advanced stage of the infection and a higher mortality despite the use of antibiotics. Our results are the first evidence that MCs contribute to the pathogenesis of sepsis, and MC stabilization improves survival by preventing apoptosis in sepsis.
Materials and Methods
Animal experiments
Adult male Sprague-Dawley rats (Taconic Farms, Germantown, NY), wild-type C57BL/6J (Taconic Farms), or MC-deficient WBB6F1-KitW/KitW-v and counterpart WBB6F1 Kit-+/+ mice (The Jackson Laboratory, Bar Harbor, ME) were randomly grouped, and investigators were blinded to the experimental treatment. All animal experiments were performed in accordance with the National Institutes of Health Guidelines under protocols approved by the Institutional Animal Care and Use Committee of the University of Medicine & Dentistry of New Jersey-New Jersey Medical School. Experimental models were performed as we previously reported in Wang et al. (17). Endotoxemia: endotoxin (Escherichia coli LPS 0111:B4; Sigma-Aldrich, St. Louis, MO) was dissolved in sterile, pyrogen-free PBS and sonicated for 30 min immediately before use. Cecal ligation and puncture (CLP): animals were anesthetized with ketamine (75 mg/kg i.m., Fort Dodge, Fort Dodge, Iowa) and xylazine (20 mg/kg i.m.; Boehringer Ingelheim, St. Joseph, MO) and subjected to abdominal incision and ligation of the cecum at 7.0 mm from the cecal tip away from the ileocecal valve. The ligated cecal stump was punctured once with a 22-gauge needle, and stool was extruded (~1 mm) to ascertain patency of puncture. The abdominal wound was closed in two layers, peritoneum and fascia separately to prevent leakage of fluid. All animals received antibiotic (7 mg/kg enrofloxacine i.p.) immediately postsurgery and provided every 12 h during 3 d. Unless otherwise indicated, cromoglycate (100 mg/kg i.p) and doxantrazole (10 mg/kg i.p) were administered twice at 16 h and 40 mins before the septic challenge. In the delayed treatments, the administration of cromoglycate was started at 24 h after the septic challenge and given every 12 h for 3 d.
Ex vivo organ and cell cultures
Briefly, spleen and lung were collected and washed with cold 1× PBS and cut into 3-mm3 fragments. Two explants (total weight ~2 mg) were incubated per well (2.0 cm2) of a 24-well plate with RPMI 1640 medium containing 1% penicillin, 1% streptomycin, and 1% gentamycin at 37°C in 5% CO2/air. Whole blood was collected in syringes with 0.02 ml of heparin and incubated in 96-well plates. Tissue and whole blood were stimulated with vehicle or LPS and treated with a concentration range of cromoglycate. TPH1 cells (American Type Culture Collection, Manassas, VA) were cultured in RMPI 1640 medium with L-glutamine (Life Technologies, Rockville, MD) supplemented with 10% heat-inactivated FBS (Hyclone Laboratories, Logan, UT) at 37°C in a humidified incubator with 5% CO2. For experiments, cells were transferred to 24-well polystyrene culture plates at 3 × 105 cells/well in 1 ml medium/well. After overnight incubation, the medium was removed and replaced with serum-free RMPI 1640 medium. Cells were pretreated with cromoglycate for 40 min prior to LPS challenge (100 ng/ml).
Cytokine measurements
TNF was analyzed as previously described in Huston et al. (18). TNF in serum and organs was analyzed at 90 min after the septic challenge. Organs were washed with cold 1× PBS then homogenized (Homogenizer, Omni International, Kennesaw, GA) in lyses buffer (50 mM Tris-HCl, 150 mM NaCl, 0.5% NaPO4, 50 mM NaF, 0.2 mM NaPO4, 25 ug/ml aprotonin, 25 ug/ml pepstatin A, and 1 mM PMSF) and incubated for 10 min on ice. The resulting suspension was centrifuged (10,000 × g for 25min at 4°C), and the supernatant was collected. TNF concentration in organs was normalized according to total protein concentration (nanograms per gram tissue). HMGB1 was analyzed as previously described in Huston et al. (17). Mouse monoclonal anti-HMGB1Ab (1µg/ml) (Ab12029, Abcam, Cambridge, MA) was used for coating the wells in ELISA plates. Captured HMGB1 protein was analyzed using polyclonal anti-HMGB1 Ab (Ab 18256, Abcam) at a final concentration of 1000 ng/ml and the reaction then visualized by adding anti-rabbit IgG-HRP Ab (1:2000) (Cell Signaling Technology, Beverly, MA). Multicytokine analyses for IL-1A, IL-1B, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12A, IL-13, IL-17A, G-CSF, and GM-CSF) were measured using a Multi-Analyte Profiler ELISArray Kit (SABiosciences, Frederick, MD).
Cell death
Spleens were mechanically disrupted with a 100-um pore cell strainer (BD Falcon, BD Biosciences, San Jose, CA), erythrocytes lysed in solution (Gentra Systems, Big Lake, MN) for 10 min, and intact cells washed twice in PBS. Splenocytes (1×105 cells) were suspended in 200µl lysis buffer supplied by the manufacturer and incubated for 30 min at room temperature. After pelleting nuclei (200 × g for 10 min), cytoplasmatic mononucleosome- and oligonucleosome-associated histone DNA complexes were analyzed using the Cell Death Detection ELISA plus kit (Roche Molecular Biochemicals, Mannheim, Germany). In situ cell death was analyzed with TUNEL. Spleens were fixed in 10% (v/v) buffered formalin (pH 7.2) and permeabilized following the manufacturer’s instructions. After washing, the 3′-OH ends of DNA fragments were stained as described by the manufacturer (In Situ Apoptosis Detection kit POD, Roche Diagnostic Systems, Somerville, NJ). Sections were counterstained with 7-aminoactinomycin D to define splenic structures. Stained sections were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and examined at ×20 on a laser-scanning confocal microscope (Radiance 2100, Bio-Rad, Hercules, CA). Caspase-3 was analyzed from splenic homogenates and normalized by protein concentration. Protein samples were separated by 4–12% gradient SDS-PAGE gels, transferred, probed with purified anti–caspase-3 and anti-cleaved caspase-3 Ab (Cell Signaling Technology), and processed with the Immune-Lite Chemiluminescent assay kit (Bio-Rad).Films were scanned with a silver image scanner (Silverscaner II, Lacie Limited, Beaverton, OR), and the relative intensity was quantified by using National Institutes of Health ImageJ 1.59 software (Bethesda, MD).
Statistical analyses
All data were expressed as mean ± SD. Statistical analyses were performed using ANOVA with Bonferroni’s correction. Analyses of normality and homogeneity of variance were performed to verify the assumptions of ANOVA. ANOVA was used to compare all treatments and specific pairwise comparisons as stated in the experiments. The Student t test was used to compare mean values between the two experimental groups. Statistical analyses of survival were determined using the log-rank test. Kaplan-Meier product-limit estimates of the survival functions were plotted using Graph-Pad (version 5, GraphPad, San Diego, CA). Tests resulting in p values of <0.05 were considered statistically significant.
Results
MC stabilizers prevented systemic inflammation and improved survival in sepsis
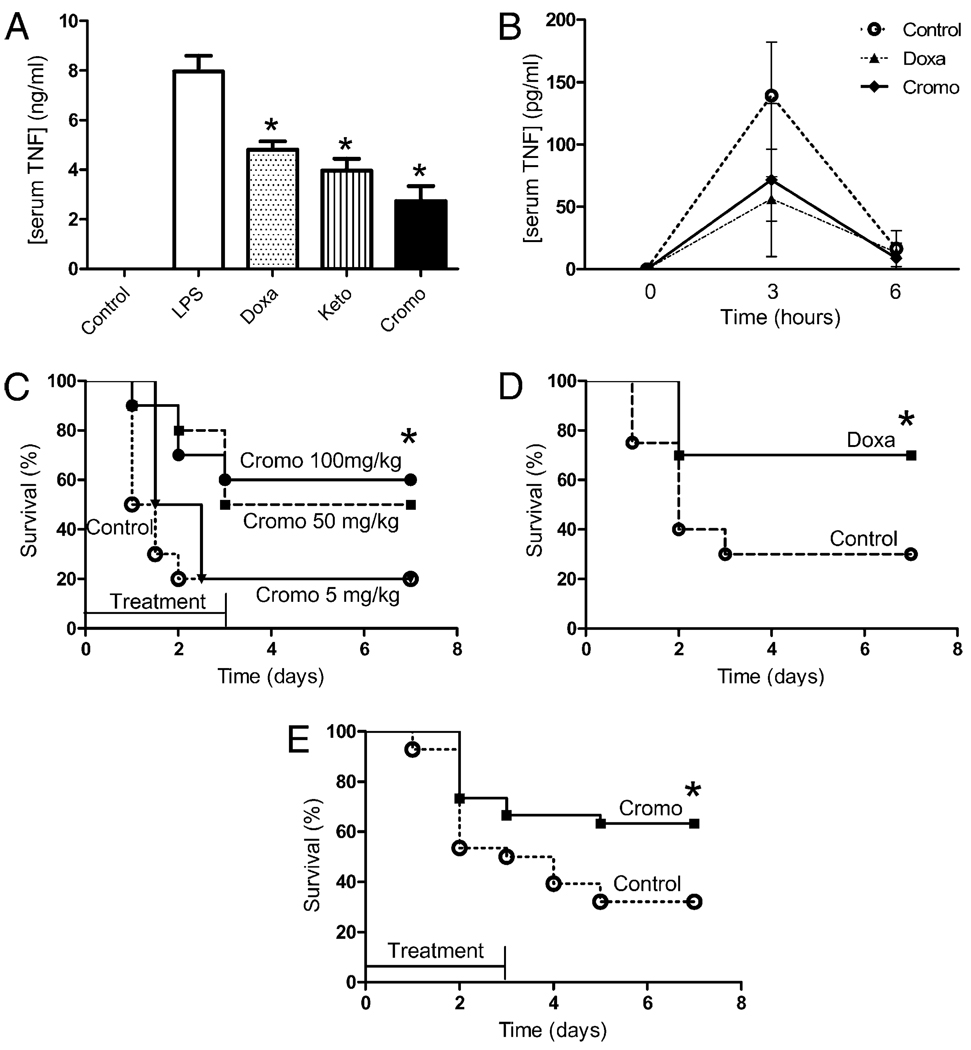
The implications of the MC in sepsis were first analyzed by using the most characteristic MC stabilizers including doxantrazole, ketotiphen, or cromoglycate. All three MC stabilizers significantly blunted serum TNF levels by >50% in endotoxemic mice (Fig. 1A). Yet, cromoglycate was the most efficient by inhibiting serum TNF response by over 60%. The anti-inflammatory potential of these stabilizers was also confirmed in Sprague-Dawley rats and analyzed at different time points, indicating that cromoglycate and doxantrazole induce a lasting inhibition and did not merely delay the TNF responses in vivo (Fig. 1B). This anti-inflammatory effect provides therapeutic benefits, and cromoglycate improved survival by 40% (Fig. 1C). Similar results indicate that cromoglycate inhibits serum levels of MC-derived tryptase (Supplemental Fig. 1). These two effects were dose-dependent, and the lowest concentration of cromoglycate failed to induce survival benefits. Likewise, MC stabilization with doxantrazole also improved survival by 40% in endotoxemia (Fig. 1D). The therapeutic potential of these MC stabilizers was also analyzed in polymicrobial sepsis induced by CLP, a more clinically relevant model with polymicrobial peritonitis induced by the cecal puncture and the necrotic tissue induced by the cecal ligation. MC stabilization with cromoglycate attenuated the clinical manifestations of endotoxemia (including lethargy, diarrhea, piloerection, and huddling) and improved survival by 40% in polymicrobial peritonitis (Fig. 1E). Animals were observed for 2 wk, and no late deaths were found, suggesting that MC stabilizers conferred a lasting protection and did not merely delay the pathology.
FIGURE 1.
MC stabilization prevented systemic inflammation and improved survival in sepsis. A, C57BL/6J mice were treated with endotoxin (6 mg/kg i.p.) and vehicle: doxantrazole (Doxa; 10 mg/kg i.p.), ketotiphen (Keto; 25 mg/kg i.p.), or cromoglycate (Cromo; 50 mg/kg i.p.). Serum TNF levels were analyzed 90 min later by ELISA (n = 4/group). *p < 0.05 versus LPS. B, Adult male Sprague-Dawley rats were treated with endotoxin and vehicle: doxantrazole (Doxa) orcromoglycate (Cromo;50mg/kg i.p.). Serum TNF levels were analyzed at different time points (n = 4/group). *p < 0.05 versus LPS. C, Animals were treated with endotoxin and vehicle (control)or a concentration range of cromoglycate (n = 10/group). *p < 0.05, survival log-rank test versus control. D, Animals were treated with endotoxin and vehicle (control) or doxantrazol (10 mg/kg) (n = 10/group). *p < 0.05, survival log-rank test versus control. E, Animals were subjected to polymicrobial sepsis induced by CLP and treated with vehicle (control) or cromoglycate (100 mg/kg) started before the septic challenge and given every 12 h for 3 d (n = 30/group). *p < 0.05, survival log-rank test versus control. These results are representative of three different experiments.
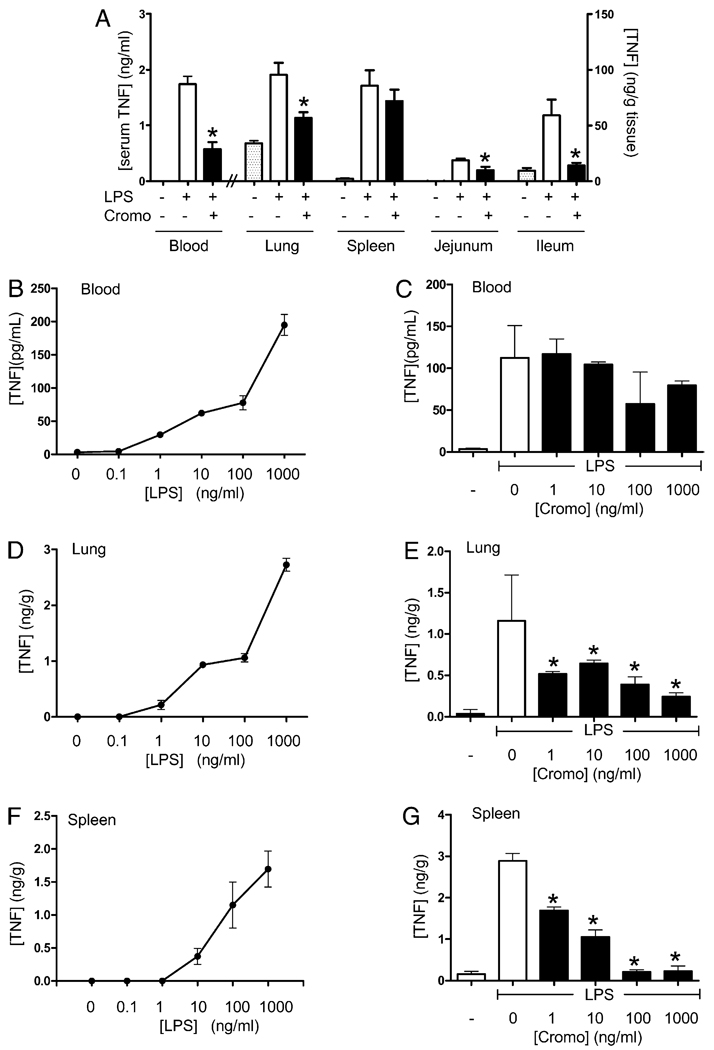
The anti-inflammatory mechanism of the MC stabilizers in sepsis was analyzedinthe major organs (Fig. 2A). The highest TNF responses and concentrations were found in the spleen, lung, and through the gastrointestinal tract including the jejunum, ileum, and colon. In vivo, the most significant effects of MC stabilizers were found in the lung and through the gastrointestinal tract (jejunum, ileum, and colon), where cromoglycate consistently inhibited TNF levels by >40%. Yet, cromoglycate failed to modulate TNF levels in the spleen of endotoxemic mice. Because MCs display functional heterogeneity based on their location (19), we analyzed whether splenic MCs may have particular features preventing the effect of cromoglycate. MC stabilizers were analyzed in ex vivo culture of blood, lung, and spleen. Ex vivo organ cultures were treated with a concentration range of LPS and cromoglycate, and TNF was analyzed at 3 h posttreatment. All organ cultures had a typical dose response to endotoxin, yet lung and spleen showed higher sensitivity than blood (Fig. 2B, 2D, 2F). This pattern of responses is similar to that reported in vivo (18). Cromoglycate failed to inhibit TNF production in the blood, even at high, non-physiological concentrations of 1 mg/ml (Fig. 2C). Cromoglycate inhibited TNF production in the lung by ~60%, a proportion similar to that described in vivo (Fig. 2E). Unlike in vivo, cromoglycate inhibited TNF production in the spleen by >90% with an estimated EC50 of 4 ± 0.5 ng/ml (Fig. 2G). These results suggest that MC stabilizers can improve survival in experimental sepsis by inhibiting TNF responses from resident macrophages in the critical organs of sepsis and particularly in the lung.
FIGURE 2.
MC stabilization attenuated inflammatory responses. A, C57BL/ 6J mice were treated with endotoxin and vehicle (control) or MC stabilizer cromoglycate (Cromo; 100 mg/kg i.p.). TNF concentration in major organs including lung, spleen, jejunum, and colon was determined by ELISA and normalized to organ protein (n = 6/group). The anti-inflammatory potential of cromoglycate was further analyzed in ex vivo organ cultures. Blood (B), lung (D), and spleen (F) were cultured ex vivo and treated with a concentration range of LPS to establish a dose response ex vivo. C, E, and G, The cultures were treated with LPS (100 ng/ml) and the indicated concentration of cromoglycate. TNF was measured in the supernatant at 3 h post-treatment (n = 9/group). These results are representative of three different experiments. *p < 0.05 versus LPS.
MC-deficient mice had lower TNF responses but similar mortality and HMGB1 responses in sepsis
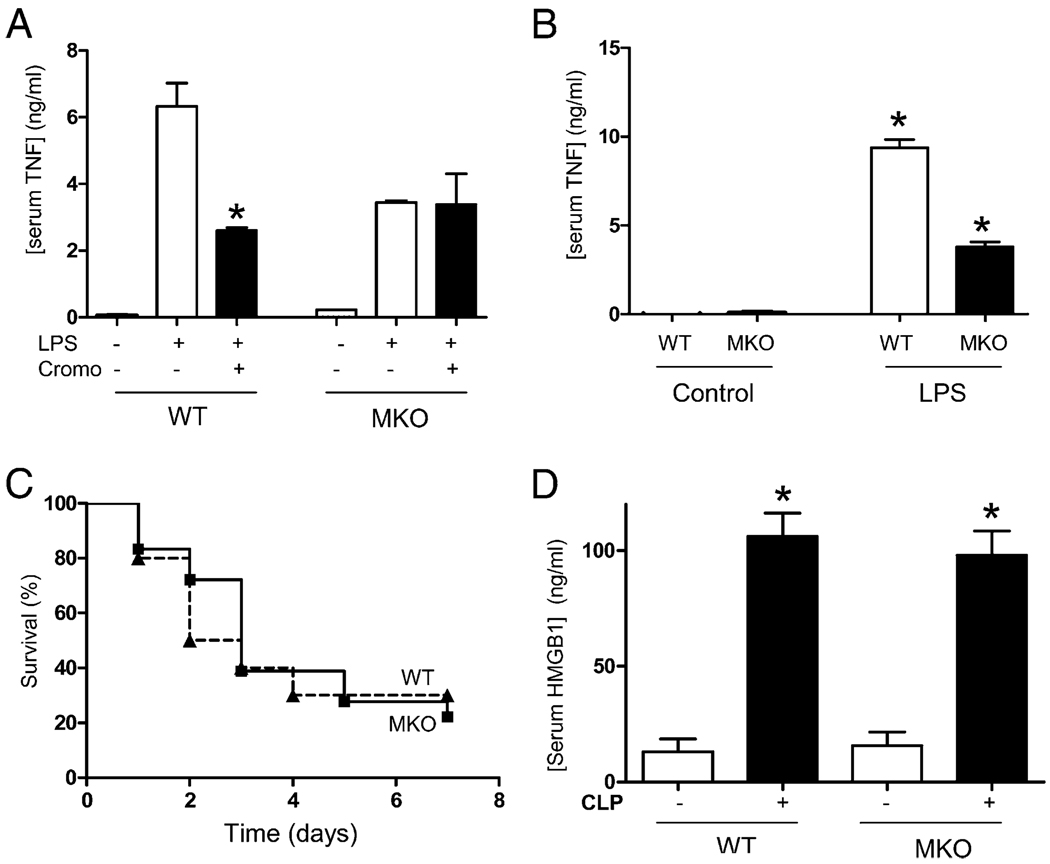
The specificity of MC stabilizers was analyzed in MC-deficient WBB6F1-KitW/KitW-v mice and the wild-type littermate WBB6F1+/+ mice. Cromoglycate attenuated serum TNF levels in wild-type but not in MC-deficient mice (Fig. 3A). These results also indicate that MC-deficient mice had significantly lower TNF responses to endotoxin than the wild-type littermate. To confirm this hypothesis, animals were treated with different concentrations of endotoxin and serum TNF responses analyzed in vivo. MC-deficient mice had statistically significant lower serum TNF responses to endotoxemia than the wild-type mice (Fig. 3B). However, these diminished TNF responses in the MC-deficient mice did not correlate with survival benefits. Indeed, MC-deficient and wild-type littermate mice had a similar mortality kinetic in polymicrobial peritonitis treated with antibiotics (Fig. 3C). Animals were observed for 2 wk, and no late deaths were found, indicating that MC depletion in knockout mice did not prevent or delay mortality in polymicrobial sepsis with antibiotics. To further determine the role of the MCs in late stages of sepsis, we analyzed the cytokine profile of the serum of septic animals at 24 h after the onset of sepsis. Around 10% of the animals start to die at ~24 h, and thus, this is the latest time point possible to analyze all the animals while they remain alive. Cytokines were analyzed with the multianalyte profiler to determine TNF, HMGB1, IL-1A, IL-1B, IL-2, IL-4, IL-5, IL-6,IL-10, IL-12A, IL-13, IL-17, G-CSF, and GM-CSF. HMGB1 was the most abundant cytokine with ~105 ± 8 ng/ml serum. Furthermore, serum HMGB1 levels in wild-type mice were statistically similar to those found in MC-deficient mice (Fig. 3D). Although MC-deficient mice had lower serum TNF responses than WT mice, they both had similar mortality and a similar increase in serum HMGB1 responses during sepsis.
FIGURE 3.
MC-deficient mice had lower TNF responses but similar HMGB1 responses and mortality in sepsis. A, Wild-type WBB6F1+/+ (WT) or MC-deficient WBB6F1-KitW/KitW-v (MKO) mice were treated with endotoxin (6 mg/kg i.p.) and cromoglycate (Cromo). n = 5/group. *p < 0.05 versus LPS. B, WT or MKO mice were treated with endotoxin (15 mg/kg i.p.). Serum TNF levels were analyzed at 90 min by ELISA (n = 5/group). *p < 0.05 versus LPS. C, WT or MKO mice were subjected to CLP and Kaplan-Meier survival analysis (n = 25/group). p = NS, survival log-rank test. D, WT or MKO mice underwent CLP, and HMGB1 serum levels were analyzed at 24 h (n = 5/group). *p < 0.05 versus control. These results are representative of three different experiments.
MC stabilizers inhibited serum HMGB1 and rescued animals from sepsis
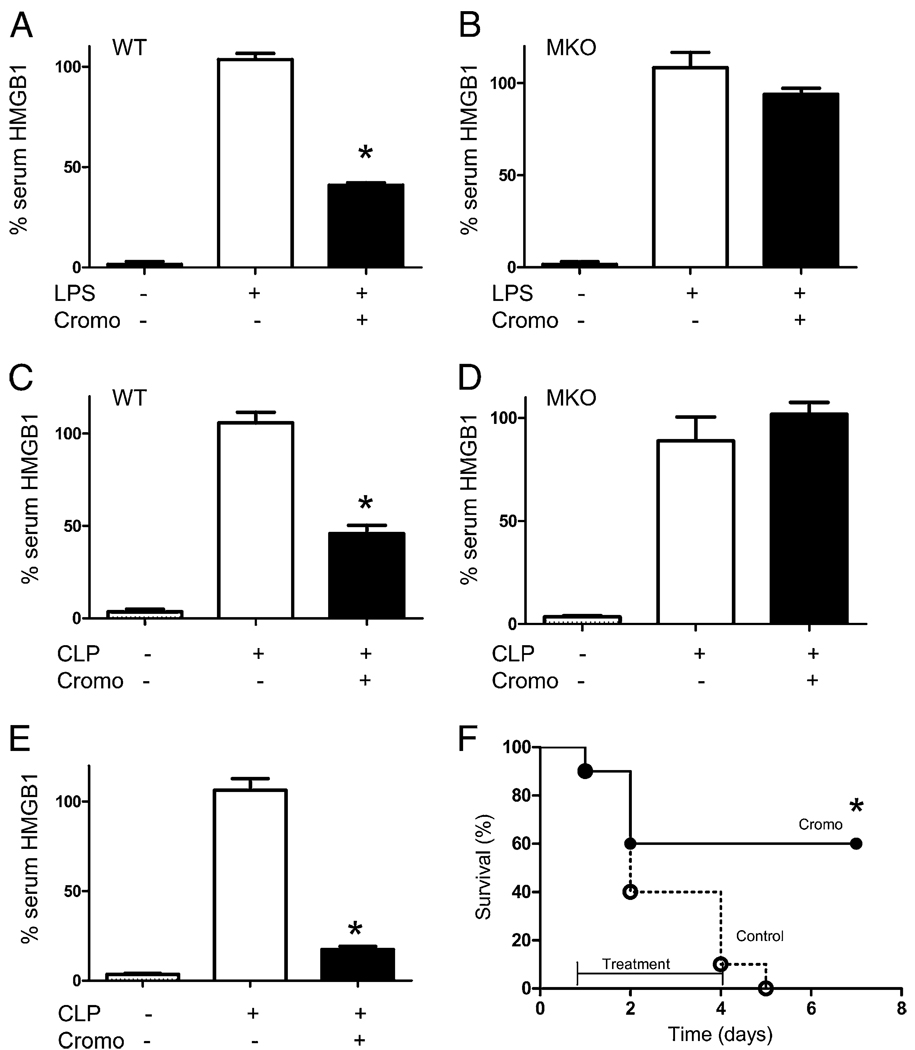
We analyzed whether treatment with MC stabilizers modulates serumHMGB1 levels, a characteristic late mediator of sepsis. HMGB1 regulation by MC stabilization was analyzed in both endotoxemia and CLP. Both endotoxemia and polymicrobial peritonitis induced lethal serum HMGB1 responses. MC stabilization with cromoglycate starting before LPS blunted serum HMGB1 levels by ~60% in wild-type but not in the MC-deficient mice (Fig. 4A, 4B). Similar results were found in polymicrobial sepsis, in which cromoglycate blunted serum HMGB1 levels by ~60% in wild-type but not in MC-deficient mice (Fig. 4C, 4D). Because HMGB1 is a late cytokine produced by macrophages at ~20 h after endotoxin stimulation, we analyzed whether cromoglycate can inhibit serum HMGB1 levels when the treatment is delayed 1 d after septic challenge. Treatment with cromoglycate was started at 24 h after CLP, and serum HMGB1 levels were analyzed at 44 h by ELISA. Delayed treatment with cromoglycate dramatically attenuated serum HMGB1 levels ~80% (Fig. 4E). Because this treatment was significantly efficient, we analyzed whether delayed MC stabilization could rescue mice from polymicrobial sepsis. Animals underwent CLP, and the treatment with cromoglycate was started 24 h later. This is a time point at which mice show clear signs of sepsis, including fever, lethargy, diarrhea, and huddling, and ~10% of animals will die in the next few hours. Delayed administration of cromoglycate attenuated the clinical manifestations of sepsis and rescued 60% of the animals (Fig. 4F). Animals were monitored for 2 wk, and no late deaths were observed, indicating that MC stabilization conferred a lasting protection. These results suggest that MCs contribute to systemic inflammation during sepsis, and their stabilization can rescue animals from established sepsis in a clinically realistic time frame.
FIGURE 4.
MC stabilization inhibited systemic HMGB1 response to sepsis and rescued animals from polymicrobial sepsis. WT (A, C) or MKO (B, D) mice were treated with cromoglycate (Cromo; 100 mg/kg i.p.) 16 h and 40 min before endotoxin (6 mg/kg i.p.). Animals were subjected to endotoxemia (A, B) or CLP (C, D), and serum HMGB1 levels were analyzed by Western blot at 24 h after the procedure (n = 9/group). *p < 0.05 versus LPS. E and F, WT mice were subjected to CLP. Treatment with vehicle (control) or cromoglycate was started at 24 h after the CLP procedure. E, Serum HMGB1 levels were analyzed at 44 h after the induction of sepsis. n = 5/group. *p < 0.05 versus control. F, Survival was analyzed and represented in a Kaplan-Meier analysis (n = 10/group). *p < 0.01, survival log-rank test versus control. These results are representative of three different experiments.
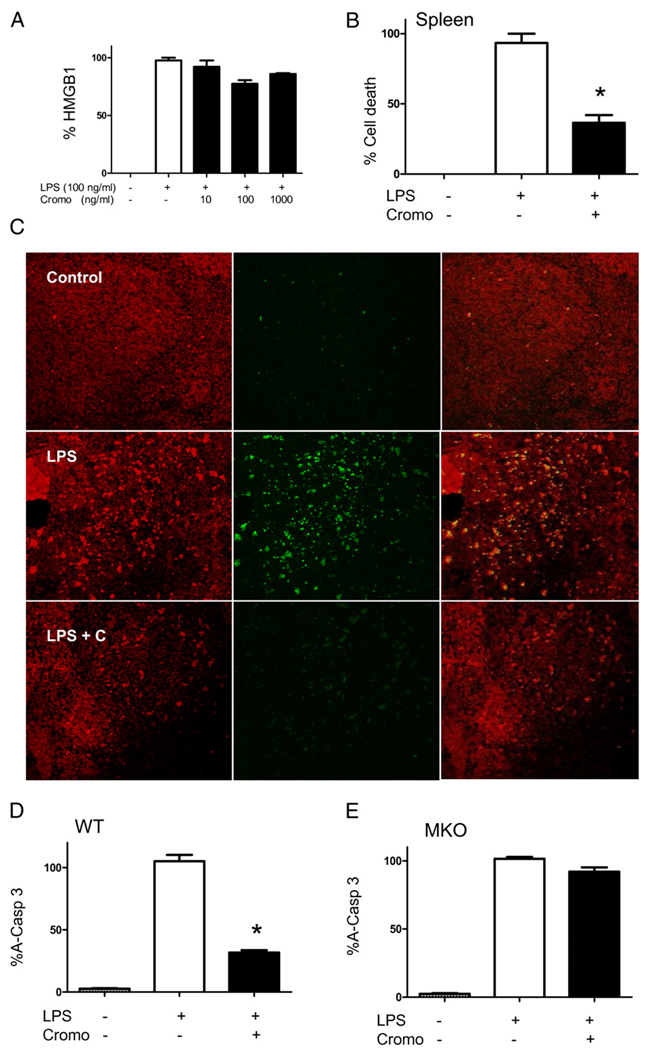
MC stabilization with cromoglycate prevented apoptosis in sepsis
To study the mechanism of HMGB1 regulation, we first analyzed whether cromoglycate inhibits LPS-induced HMGB1 secretion from macrophages. Cromoglycate failed to inhibit extracellular HMGB1 secretion from peritoneal macrophages (Fig. 5A). Because serum HMGB1 can be released from apoptotic lymphocytes and mediates the apoptosis-induced sepsis lethality (20), we analyzed whether cromoglycate prevents cell death in sepsis. Cromoglycate dramatically prevented cell death in the spleen of septic mice as determined by cytoplasmic histone-associated DNA fragments (Fig. 5B). We further confirmed these results by TUNEL staining of splenic sections from septic mice (Fig. 5C). In agreement with previous studies, bacterial endotoxin induced apoptosis predominately in the germinal center of the spleen. Cromoglycate dramatically diminished the apoptotic staining in the white pulp of the germinal center. Because apoptosis is mediated by death receptor and mitochondrial-mediated apoptosis converge in the activation of caspase-3 (21), we analyzed whether MC stabilization with cromoglycate modulates caspase-3 during sepsis. Bacterial endotoxin induces significant activation of caspase-3, and MC stabilization with cromoglycate inhibited caspase-3 activation in the spleen of septic mice by >60% (Fig. 5D). This effect was specific for MCs because cromoglycate failed to prevent apoptosis and caspase-3 activation in MC-deficient mice (Fig. 5E). These results strongly suggest that MC stabilization with cromoglycate can prevent apoptosis in sepsis by inhibiting caspase-3 activation.
FIGURE 5.
MC stabilization modulates apoptosis (cell death) in sepsis. A, Macrophages were pretreated with a concentration range of cromoglycate and activated with LPS (100 ng/ ml). Extracellular HMGB1 was analyzed in the conditioned media at 24 h poststimulation (n = 4/group). p < 0.05 versus LPS. B, WT mice were treated with vehicle or cromoglycate (+) 16 h and 40 min before LPS (6 mg/kg i.p.). Spleens were collected at 24 h, and DNA fragmentation was analyzed by the cytoplasmic mononucleosome- and oligonucleosome-associated histone assay. n = 4/group. *p < 0.05 versus LPS. C, WT mice were pretreated with vehicle or cromoglycate (+ C) before endotoxin (6 mg/kg i.p.). Splenic sections were stained for 7-aminoactinomycin D (red) or in situ cell death (TUNEL) (green) (original magnification ×40). Pictures are representative of four different animals. WT (D) or MKO (E) mice were treated with cromoglycate 16 h and 40 min before endotoxin (6 mg/kg i.p.). A-Casp 3 was analyzed at 24 h posttreatment. n = 4/ group. *p < 0.05 versus LPS. These results are representative of three different experiments. A-Casp 3, activated caspase-3.
Discussion
Because previous studies on MCs emphasized their unique potential to induce prompt inflammatory responses against infection, the stabilization of MCs is expected to increase the risk of infection and to be detrimental in sepsis. However, our results indicate that treatment with MC stabilizers rescues animals from established sepsis. Although the molecular details of MC stabilization were not fully determined, MC stabilizers block calcium channels essential for MC degranulation (22, 23). Without intracellular calcium, the secretory vesicles cannot fuse to the cell membrane, and MCs cannot degranulate their inflammatory factors. The specificity of our treatments was confirmed using MC-knockout animals as follows: 1)MCstabilization with cromoglycate attenuated serum TNF levels in wild-type but not in MC-knockout mice; 2) cromoglycate started before LPS blunted serum HMGB1 levels by ~60% in wild-type but not in MC-deficient mice; 3) similar results were found in polymicrobial sepsis, and cromoglycate blunted serum HMGB1 levels by ~60% in wild-type but not in MC-knockout mice; and 4) sepsis causes apoptosis in the spleen of both wild-type and MC-knockout mice. However, cromoglycate attenuated apoptosis in the wild-type but not in MC-knockout mice. Because our current study focuses on the role of the MC in the late stages of sepsis, we analyzed the cytokine profile at 24 h after the onset of sepsis. Around 10% of the animals start to die at this time, and thus, this is the latest time point possible to analyze serum cytokines while all the animals remain alive. Although the array at a single time point may not fully reveal the effects of other early cytokines with different times of production, HMGB1 was the most abundant cytokine at the late stages of sepsis. Unlike other cytokines, serum HMGB1 levels were decreased by MC stabilizers but not in MC-knockout animals, similar to those results described for survival and apoptosis. These results provide novel conceptual perspectives for the MCs as modulators of apoptosis and the damage-associated molecular pattern in infectious diseases. To our knowledge, this is the first study supporting MCs as the first known physiological modulator of apoptosis in sepsis, improving survival in sepsis by preventing apoptosis. Previous studies confirmed that apoptosis plays a critical role in sepsis, and pharmacological inhibition of apoptosis can improve survival (24). Our results show that MC genetic depletion in knockout mice does not prevent apoptosis in sepsis, indicating that apoptosis is not dependent on MCs. Unlike genetic deletion, MC stabilization prevents apoptosis and improves survival in sepsis. These results suggest that MCs can induce therapeutic benefits in sepsis through a mechanism independent of degranulation. Future studies will be required to analyze the potential release of antiapoptotic factors from MCs via a degranulation-independent mechanism. Similar to apoptosis, MC stabilization, but not MC-knockout animals, prevents systemic HMGB1 responses in sepsis. HMGB1 was originally identified as a nuclear DNA-binding protein that functions as a structural cofactor. However, HMGB1 can be released into the extracellular milieu, where it functions as an inflammatory factor (7, 25–27) contributing to abrupt cardiac standstill (28, 29), intestinal derangement (30), and acute lung injury (31) in sepsis. HMGB1 is presented in the extracellular mileu through two mechanisms: active secretion from macrophages (17, 25, 32) or passive release from apoptotic or necrotic cells (20, 33, 34). Since MC stabilizers did not target macrophages and the number of peritoneal macrophages is similar in both MC-deficient and normal littermates mice (9, 10), these results suggest that MC stabilizers attenuate serum HMGB1 by preventing its extracellular release from apoptotic cells. In this sense, HMGB1 represents a novel family of inflammatory cytokines composed of intracellular proteins that, when present in the extracellular milieu, are recognized by the innate immune system as necrotic markers (26, 35) or damage-associated molecular patterns (36, 37). This emerging family of specific intracellular proteins represents optimal chemotactic markers selected by the innate immune system to recognize tissue damage and initiate reparative responses (32, 33, 36). In agreement with these results, recent studies indicate that apoptotic cells released HMGB1 to the extracellular mileu, and HMGB1 contributes to apoptosis-mediated lethality in sepsis (20, 38–40). Together, our results strongly support a new immunological role of MCs in modulating cell death during infectious diseases and preventing apoptosis-induced inflammation and lethality in sepsis.
Little is known about the pharmacological implications of MC in the clinical settings of sepsis, which are normally associated with the advanced stages of the pathology and high mortality despite the use of antibiotics. Previous studies avoided the use of antibiotics to emphasize the unique potential of MC to fight bacterial infection in the early stages of sepsis. In these conditions, MC-deficient mice have higher mortality in moderate sepsis (9) because of their limited bacterial clearance (16). However, our studies indicate that MC-deficient mice have similar mortality to wild-type littermate animals when treated with antibiotics. These results suggest that the prompt inflammatory responses of the MC can prevent infections in the early stages of sepsis, but this benefit is overridden in the advanced stages and the use of antibiotics. These two considerations are typical in clinical settings of sepsis, and they are characteristic factors limiting the clinical translation of experimental strategies. A classical example is that anti-TNF therapies can prevent sepsis in the early stages when the treatment is started before the challenge (41), but these approaches failed in clinical trials, because they are ineffective when administered after the septic challenge (4, 42, 43). In contrast, HMGB1 appears as a late mediator of sepsis (25), and anti-HMGB1 therapies are more effective when delayed after the septic challenge (44, 45). A remarkable implication of our study is that MC stabilizers improve survival even when the treatment is started 1 d postinduction of peritonitis. By comparison with other targets, administration of anti-TNF Abs increased mortality when administered after cecal perforation (42). Antimacrophage migration inhibitory factor Abs are ineffective if administered >8 h postinduction of peritonitis (46). The therapeutic window for MC stabilization is also significantly wider than for lysophosphatidylcholine, which is only effective when treatment occurs within 10 h after cecal puncture (47). These comparisons are particularly remarkable because the implications of MCs in the late stages of sepsis have been traditionally underestimated because of their early responses to endotoxin. These results open the potential of harnessing MCs for the treatment of sepsis in a clinically realistic time frame.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Spaniard Ministry of Sanidad y Consumo (CM05/00055, to L.R.). L.U. is funded by the faculty program of the Department of Surgery of the University of Medicine and Dentistry of New Jersey-New Jersey Medical School, grants from the U.S. Army Medical Research Command (USAMRMC#05308004), the American Heart Association (AHA06352230N), and the National Institutes of Health (RO1-GM084125).
Abbreviations used in this paper
- A-Casp 3
activated caspase-3
- CLP
cecal ligation and puncture
- MC
mast cell
- MKO
mast cell-deficient
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR, et al. Academic Medical Center Consortium Sepsis Project Working Group. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–240. [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC. Caring for the critically ill patient: challenges and opportunities. JAMA. 2007;298:456–458. doi: 10.1001/jama.298.4.456. [DOI] [PubMed] [Google Scholar]
- 4.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr. Opin. Pharmacol. 2004;4:378–385. doi: 10.1016/j.coph.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat. Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 7.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol. Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Abraham E, Laterre PF, Garbino J, Pingleton S, Butler T, Dugernier T, Margolis B, Kudsk K, Zimmerli W, Anderson P, et al. Lenercept Study Group. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit. Care Med. 2001;29:503–510. doi: 10.1097/00003246-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Echtenacher B, Männel DN, Hültner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Ramos BF, Jakschik BA. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992;258:1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- 11.Old LJ. Tumor necrosis factor (TNF) Science. 1985;230:630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 12.Cuturi MC, Murphy M, Costa-Giomi MP, Weinmann R, Perussia B, Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J. Exp. Med. 1987;165:1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung SS, Jung LK, Walters JA, Chen W, Wang CY, Fu SM. Production of tumor necrosis factor/cachectin by human B cell lines and tonsillar B cells. J. Exp. Med. 1988;168:1539–1551. doi: 10.1084/jem.168.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 15.Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J. Exp. Med. 1991;174:103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 18.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galli SJ. New concepts about the mast cell. N. Engl. J. Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 20.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 22.Spataro AC, Bosmann HB. Mechanism of action of disodium cromoglycate—mast cell calcium ion influx after a histamine-releasing stimulus. Biochem. Pharmacol. 1976;25:505–510. doi: 10.1016/0006-2952(76)90378-6. [DOI] [PubMed] [Google Scholar]
- 23.White JR, Ishizaka T, Ishizaka K, Sha’afi R. Direct demonstration of increased intracellular concentration of free calcium as measured by quin-2 in stimulated rat peritoneal mast cell. Proc. Natl. Acad. Sci. USA. 1984;81:3978–3982. doi: 10.1073/pnas.81.13.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat. Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 26.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 27.Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17:189–201. doi: 10.1016/j.cytogfr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Mantell LL, Parrish WR, Ulloa L. HMGB1 as a therapeutic target for infectious and inflammatory disorders. Shock. 2006;25:4–11. doi: 10.1097/01.shk.0000188710.04777.9e. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Sama AE, Wang H. Role of HMGB1 in cardiovascular diseases. Curr. Opin. Pharmacol. 2006;6:130–135. doi: 10.1016/j.coph.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic mono-layers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 31.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J. Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 32.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 34.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Mu¨ller S, Iannacone M, Traversari C, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulloa L, Batliwalla FM, Andersson U, Gregersen PK, Tracey KJ. High mobility group box chromosomal protein 1 as a nuclear protein, cytokine, and potential therapeutic target in arthritis. Arthritis Rheum. 2003;48:876–881. doi: 10.1002/art.10854. [DOI] [PubMed] [Google Scholar]
- 36.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 38.Huston JM, Wang H, Ochani M, Ochani K, Rosas-Ballina M, Gallowitsch-Puerta M, Ashok M, Yang L, Tracey KJ, Yang H. Splenectomy protects against sepsis lethality and reduces serum HMGB1 levels. J. Immunol. 2008;181:3535–3539. doi: 10.4049/jimmunol.181.5.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang W, Bell CW, Pisetsky DS. The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J. Immunol. 2007;178:6495–6503. doi: 10.4049/jimmunol.178.10.6495. [DOI] [PubMed] [Google Scholar]
- 40.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 41.Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu. Rev. Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 42.Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J. Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 43.Remick D, Manohar P, Bolgos G, Rodriguez J, Moldawer L, Wollenberg G. Blockade of tumor necrosis factor reduces lipopolysaccha-ride lethality, but not the lethality of cecal ligation and puncture. Shock. 1995;4:89–95. doi: 10.1097/00024382-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc. Natl. Acad. Sci. USA. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hültner L, Heumann D, Ma¨nnel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 47.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, et al. Therapeutic effects of lysophos-phatidylcholine in experimental sepsis. Nat. Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.