Abstract
Background
Moderate prenatal alcohol exposure can contribute to neurodevelopmental impairments and disrupt several neurotransmitter systems. We examined the timing of moderate level alcohol exposure, serotonin transporter gene polymorphic region variation (rh5-HTTLPR), and levels of primary serotonin and dopamine metabolites in cerebrospinal fluid (CSF) in rhesus monkeys.
Methods
Thirty-two 30-month old rhesus monkeys (Macaca mulatta) from four groups of females were assessed: (1) early alcohol-exposed group (n = 9), in which mothers voluntarily consumed 0.6 g/kg/day alcohol solution on gestational days 0 – 50; (2) middle-to-late gestation alcohol-exposed group (n = 6), mothers consumed 0.6 g/kg/day alcohol solution on gestational days 50 – 135; (3) a continuous-exposure group (n = 8), mothers consumed 0.6 g/kg/day alcohol solution on gestational days 0 – 135; and (4) controls (n = 9), mothers consumed an isocaloric control solution on gestational days 0 – 50, 50 – 135, or 0 – 135. Serotonin transporter promoter region allelic variants (homozygous s/s or heterozygous s/l versus homozygous l/l) were determined. We examined CSF concentrations of the 5-HT and DA metabolites, 5-hydroxyindoleacetic acid (5-HIAA) and homovanillic acid (HVA), respectively, at baseline and 50 hours after separation from cage-mates, when the monkeys were 30 months old.
Results
Early- and middle-to-late gestation-alcohol exposed monkeys carrying the short allele had lower concentrations of 5-HIAA in CSF relative to other groups. Concentrations of 5-HIAA in CSF were lower for s allele carriers and increased from baseline relative to pre-separation values, while 5-HIAA levels in l/l allele carriers were not affected by separation. Monkeys carrying the short allele had lower basal concentrations of HVA in CSF compared to monkeys homozygous for the long allele.
Conclusion
Carrying the s allele of the 5-HT transporter increased the probability of reduced 5-HIAA in early- and middle-to-late gestation alcohol-exposed monkeys and reduced HVA at baseline. These findings that prenatal alcohol exposure altered central 5-HT activity in genetically sensitive monkeys raise questions about whether abnormal serotonin biological pathways could underlie some of the psychiatric disorders reported in fetal alcohol spectrum disorder (FASD).
Keywords: serotonin, rhesus monkey, prenatal alcohol exposure, serotonin transporter gene, 5-hydroxyindoleacetic acid
INTRODUCTION
Fetal alcohol spectrum disorder (FASD) is associated with a range of adverse effects that can be observed in children prenatally exposed to alcohol (Riley and McGee, 2005). Fetal alcohol syndrome (FAS), which includes growth retardation, craniofacial anomalies, CNS dysfunction, and cognitive and behavioral impairments, is the most serious of the FASD outcomes of prenatal alcohol exposure (Autti-Rämö, 2002; Mattson and Riley, 1998; Jones and Smith, 1973; Streissguth et al., 2004). Alcohol-related neuro-developmental disorder (ARND) is the term used to describe prenatally alcohol-exposed children with problems that are primarily neurobehavioral, including cognitive effects, hyperactivity, impulsivity, reduced attention span, and lack of inhibition (Streissguth et al., 1986; Mattson et al., 2001).
Individuals with FASD have been shown to be at high risk for secondary disabilities, including mental health problems, such as depression and anxiety (O’Connor et al., 2000; Streissguth et al., 2004). Indeed, such mental health problems were considered to be among the most severe manifestations of FAS in adulthood (Lemoine et al., 2003). The underlying mechanisms of depression and anxiety in individuals with FASD are not fully understood. Because animal studies have linked prenatal alcohol exposure to deficiencies in the serotonergic (5-HT) neurotransmitter system and because deficits in the 5-HT system are thought to underlie depression and anxiety, it is possible that 5-HT system deficits and subsequently altered behavior and brain function might contribute to the high prevalence of mental health problems noted in individuals with FASD (Holsboer et al., 1995; Nemeroff et al., 2002; Nestler et al., 2002; Streissguth et al., 1996).
Development of the monoamine neurotransmitter serotonin (5-HT) system plays a major role in the plasticity of the brain and is linked to a wide range of behaviors, physiological mechanisms, and disease processes (Azmitia, 2001). 5-HT neurons project diffusely to a wide range of brain regions, including the cortex, amygdala, and hippocampus Hensler et al., 1994) and play a role in a wide range of functions, including food intake, sleep, pain, sensory function, sexual activity, and endocrine function, as well as the integration of emotional, cognitive, and motor function (Lesch 1997). The 5-HT system is involved in the regulation of the HPA axis (Chaouloff, 1993; Lanfumey et al., 2000) and the expression of depression and anxiety.
Prenatal alcohol exposure has been found to alter the development of the 5-HT system. In rodents, moderate level prenatal alcohol exposure decreased 5-HT neurons, density of 5-HT reuptake sites and induced an abnormal density of 5-HT1A receptors in the dorsal and median raphe nuclei, where 5-HT neurons reside (Druse et al., 1991; Druse and Paul, 1989; Kim et al., 1997; Tajuddin and Druse, 2001). Moderate fetal alcohol exposure (20–25% ethanol-derived calories, BAC’s from 44 to 142 mg/dl) at mid-gestation (from embryologic day 7 or 8 until sacrifice) compromised midline neural tube development (Zhou et al., 2001). Because the midline of the neural tube mediates neural differentiation, abnormal midline neural tube development can alter the development of midline neurons, including the raphe, from which 5-HT-rich neurons are derived, as well as septal nuclei and appropriate crossing of commissural fibers (Sari et al., 2001; Zhou et al., 2002). Moreover, while the neural tube midline defects were no longer apparent near or after birth, the long-term consequences were fewer 5-HT neurons in the raphe (Zhou et al., 2003).
The timing of prenatal alcohol exposure has been found to be critical, given evidence that prenatal alcohol exposure effects are related to the region of the brain or cell type undergoing rapid development at that time (Goodlett and Johnson, 1999). Moreover, it is possible that genetic factors might increase or decrease an organism’s sensitivity to fetal alcohol effects during a sensitive period, i.e., an epoch of development during which the effects of experience can alter neuronal development and connectivity (Bear, 1995).
During early pregnancy, prenatal alcohol exposure results in craniofacial anomalies typical of FAS, and also causes midline brain abnormalities, and reduced cerebellar Purkinje cell number in humans, sheep, and rodents (Graham et al., 1988; Ramadoss et al., 2007; Sulik et al., 1981). Exposure on gestational day 8 in mice significantly reduced the volume in the right olfactory bulb, right hippocampus, and cerebellum, while the septal region, pituitary, and ventricles were larger than those of the control group. These findings differ from results found at gestation days 7 and 9 (of 19–21 day gestation period), and suggest that a unique pattern of alcohol-induced deficits is associated with a specific time period of alcohol exposure (Parnell et al., 2009). In pigtailed macaques, once-weekly alcohol dosing during early gestation (weeks 0–6) (at a dose comparable to six drinks in humans) was sufficient to cause deficits that were as severe as those detected from drinking throughout pregnancy (weeks 0 – 24) (Clarren et al., 1992). Along similar lines, we reported that early gestation alcohol exposure in rhesus macaques was as deleterious to neonatal neurobehavior as was continuous exposure throughout pregnancy (Schneider et al., 2005). Moreover, moderate dose alcohol exposure occurring during the early gestation period, a time period that is comparable to the first trimester in humans, reduced striatal dopamine (DA) D2R binding while middle-to-late gestation exposure induced effects in the opposite direction, up-regulation of striatal DA D2R binding, relative to the early and continuous-exposed monkeys (Schneider et al., 2005). These findings suggest that the prenatal exposure also affects the DA system. The major metabolite of DA in monkeys is homovanillic acid (HVA). The effects of differences in the timing of alcohol exposure on HVA were determined in addition to measures of 5-HT system activity.
While numerous studies have investigated the role of timing of prenatal alcohol exposure on offspring outcome, few studies have examined how the timing of exposure interacts with genetic influences. Controlled studies of prenatal alcohol exposure relative to genotype can only be done in nonhuman primates. One genotype that has recently received attention is the serotonin transporter gene promoter (5-HTTLPR), which has a length polymorphism. Carrying the short (s/s or s/l) combination of alleles compared to the long (l/l) polymorphism has been shown to result in reduced transcription of the 5-HTT gene, which may translate into altered uptake of synaptic 5-HT (Lesch et al. 1996). In rhesus macaques (Macaca mulatta), a 21 base pair (bp) insertion/depletion polymorphism (rh5-HTTLPR) that is present in the orthologous region and which is functionally similar to the human 5-HTTLPR variant has been shown to influence transcriptional efficiency (Bennett et al., 2002).
Our previous work showed that prenatal alcohol-exposed monkeys carrying the serotonin transporter gene polymorphic region (rh5-HTTLPR) short (s) allele exhibited increased neonatal irritability and higher levels of ACTH during weaning at 6 months of age compared with prenatal alcohol exposed monkeys homozygous for the long allele and monkeys not prenatal alcohol exposed, regardless of genotype (Kraemer et al., 2008). Moreover, we found that early gestation alcohol exposure induced behavioral under-responsivity or blunting of responses to non-noxious tactile stimuli in monkeys carrying the short rh5-HTTLPR allele compared to early alcohol-exposed monkeys homozygous for the long allele and monkeys from middle-to-late alcohol-exposed pregnancies and controls, regardless of genotype (Schneider et al., 2009). Others have reported that monkeys carrying a copy of the rh5-HTTLPR short allele differed in concentrations of 5-HT metabolites in cerebral spinal fluid (Bennett et al., 2002), neonatal visual orienting to stimuli (Champoux et al., 2002) and ACTH levels during social separation when compared to monkeys with the l/l genotype (Barr et al., 2004). These effects occur in relation to a gene-by-environment interaction dependent on early social rearing conditions, that is, being reared by mothers versus in peer group housing.
In the present study, we tested the hypothesis that moderate prenatal alcohol exposure during a particular gestation period would interact with rh5-HTTLPR genotype (l/l vs. l/s and s/s) to alter CNS 5-HT and DA functioning, as measured by cerebrospinal fluid concentrations (CSF) of the 5-HT metabolite, 5-hydroxyindoleacetic acid (5-HIAA) and the DA metabolite, homovanillic acid (HVA) in young adult rhesus monkeys. We measured 5-HIAA and HVA at baseline and 50 hours after separation from cage-mates when the monkeys were 30 months of age. The response to separation provides an indicator of response to stressors in relation to prenatal treatment and genetic factors.
MATERIALS AND METHODS
Maternal Alcohol Treatments
Healthy adult female rhesus monkeys within the breeding colony that voluntarily and reliably consumed 0.6 g/kg/day of a 6% volume/volume (v/v) alcohol solution sweetened with NutraSweet (300 mg/100 ml) (Equal Sweetener, Merisant US, Inc., Chicago, IL) were used in this study. Prior to breeding, blood samples were obtained 60 minutes after consumption of 0.6g/kg/day alcohol, which produced average blood alcohol concentrations of 20 – 50 mg/dL. This dosage is comparable to an average-size woman consuming two drinks daily. Females that consumed alcohol prior to breeding were randomly assigned to the control group or one of three experimental groups (see below) with timing of prenatal alcohol exposure as the independent variable. The monkeys assigned to the alcohol-consuming groups voluntarily consumed the alcohol solution daily at 1600 hours. Water was available ad libitum, including during the period when the alcohol solution was available. The animals had no chow left by the time of day that the alcohol was introduced. The control mothers consumed a sucrose solution that was designed to be approximately equivolemic and equicaloric (8g/100 ml water) to the alcohol solution. All females were housed under identical conditions, undisturbed except for necessary routine animal husbandry. These studies were conducted in accordance with the Institutional Animal Care and Use Committee.
Subjects
The offspring subjects in this study were 32 male and female rhesus monkeys (Macaca mulatta), members of an ongoing longitudinal study investigating the effects of moderate level prenatal alcohol exposure, during early or middle-to-late gestation, on brain and neurobehavioral function. The alcohol exposure periods were selected to approximate the embryological and fetal periods in human development, respectively. The embryological periods are similar in rhesus macaques and humans, with major organogenesis essentially complete by approximately day 45 in the rhesus macaque and day 56 in the human (Newell-Morris and Fahrenbruch, 1985). The species differ with regard to the fetal period in that the duration in humans is almost twice that of the macaque. By day 135, the macaque has reached a percentage of brain growth similar to that of a human newborn (Newell-Morris and Fahrenbruch, 1985).
Controls consisted of 9 monkeys in which mothers voluntarily consumed an isocaloric control solution on gestational days 0 through 50, 50 through 135, or 0 through 135. Nine monkeys were born to female rhesus monkeys that consumed 0.6g/kg/day alcohol solution on gestational days 0 through 50. Six monkeys were from female rhesus monkeys that consumed 0.6 g/kg/day alcohol solution on gestational days 50 through 135, approximating the second and third trimesters. Eight monkeys were from mothers that consumed 0.6g/kg/day alcohol solution on days 0 through 135, a combination of all three trimesters.
The rearing conditions and previous testing of these subjects were described in detail elsewhere (Schneider et al., 2001). Briefly, all infant monkeys were housed with their mothers in individual cages during the first 6 months of life. They were separated briefly from their mothers weekly and tested for neonatal neurobehavioral function during the first month of life. At 6 months of age, they were separated permanently from their mothers and reared in mixed-sex peer groups consisting of 5–6 monkeys from similar prenatal conditions. When the monkeys were 30 months old, they were removed from their social groups and individually housed for 3 days for assessments. They were subsequently pair-housed with same-sex peers from similar treatment groups. They were maintained on a diet of Purina Monkey Chow supplemented 3 times weekly with fresh fruit. All housing conditions were light (8 dark and 16 light) and temperature (21 ± 0.5 degree C) controlled. When the monkeys were approximately 6–9 years old blood samples were collected for genotyping.
Offspring testing procedure
At 30 months of age, all subjects were separated from their peer cage-mates and housed individually in wire mesh cages for 3 days. All subjects were separated from their peers at 0900 hr on Separation Day 1.
CSF samples
All CSF was collected at 1300 hr 1 week prior to separation (basal) and at 1300 hr on Day 3 of peer separation. Approximately 1.0 ml (0.8–1.2 ml) was withdrawn from the cisterna magna using a 25-gauge 0.625-in hypodermic needle and syringe 5–7 min after ketamine anesthesia (10 mg/kg) and within 10 min of initial disturbance. Ketamine anesthesia does not affect measures of biogenic amine or metabolite within this time period (Bacopoulos etal., 1979; Chernow et al., 1982). Samples were flash frozen in dry ice and stored at −70° C and later assayed using high performance liquid chromatography multi-channel quantitative assays for amines and metabolites in duplicate (Schmidt et al., 1990).
Assay Methods
The CSF samples were stored at −70 degrees C. Subsequently they were filtered through 10,000-MW cutoff molecular filters (Amicon Corp.), diluted with mobile phase, and analyzed directly by high-performance liquid chromatography (HPLC). The HPLC system consists of a Waters 510 HPLC pump, a WISP model 712 refrigerated auto-injector system, a Waters NOVA-Pak 4-μm, 1 × 15-cm C-18 reverse phase Rad Pak radial compression column installed in a Waters RCM-100 compression module (protected by a 1-cm C-18 guard column insert), an ESA 3-electrode detector system, and a Spectra/Physics model 4270 integrator. The mobile phase used for determining the neurotransmitter metabolites consisted of a 84:16 mixture of 60 mmol/L citrate buffer (pH 4.65): methanol, which also contained sodium heptane sulphonate (0.40 mmol/L) and sodium EDTA (0.27 mmol/L). The solutions were filtered (0.22 μm nylon filter) and degassed prior to use. The flow rate was 21.7 μl/sec and the elution times in minutes of the compound of interest at ambient temperature was 11.8. The three-electrode ESA detector system was operated in a reductive screen mode. The conditioning electrode was set at 0.60 V in order to oxidize the compound of interest. The first electrode in the analytical cell was set at 0.10 V, and the second electrode, whose output was recorded, was set at −.30 V in order to reduce the compound of interest. The assay was standardized using an external standard mixture of the authentic compound, which was used to recalibrate the automatic integration and quantitation of the compound after every four samples. The minimum sensitivity was approximately 100 fmol for HVA and for 5-HIAA. The coefficient of variation for repeated between-day analyses (N = 10) of a pooled test sample of primate CSF was 2.3% to 4.5%.
DNA extraction and Genotyping
Blood samples for genotyping were collected when the animals were approximately 6–9 years old. DNA was isolated from whole blood using standard extraction methods. Using a protocol modified from that of (Lesch et al 1997), rh-5HTTLPR was amplified from 25ng of genomic DNA with oligonucleotide primers (stpr5, 5′-GGCGTTGCCGCTCTGAATGC; intl, 5′-CAGGGGAGATCCTGGGAGGG) in 15 μl reactions using Platinum Taq and the PCRX Enhancer System kit, according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Amplifications were performed on a PerkinElmer thermocycler (9700) with one cycle at 96°C/5 min followed by 30 cycles of 94°C/15 sec, 60°C/15 sec, 72°C/30 sec, and a final 3-minute extension at 72°C. Amplicons were separated by electrophoresis on 10% polyacrylamide gels, and the short (s, 398bp) and long (l, 419bp) alleles of the rh5-HTTLPR were identified by visualization following ethidium bromide staining.
Data analysis
Data for 5-HIAA and HVA were analyzed using a mixed analysis of variance (ANOVA) for early alcohol exposure (early, not early) x mid-late alcohol exposure (mid-late, not mid-late) x genotype ((l/l, l/s and s/s) with separation (baseline, 50 hour after separation) treated as a repeated measure. The factorial manipulation of early and mid-late exposure yielded 4 treatment groups: controls, early-exposed, mid-late exposed, and both early and mid-late exposed or continuously-exposed. There was a significant main effect of time (baseline versus 50 hour post-separation), therefore, data were also analyzed for baseline and post-separation separately using early alcohol exposure (early, not early) x mid-late alcohol exposure (mid-late, not mid-late) x genotype ((l/l, l/s and s/s) ANOVAs. Since there were four pairs of half-siblings in the sample, data were re-analyzed after removing one of each pair, reducing the sample size by four. We carried out analyses on all 16 possible sets omitting four half-siblings. The mean effect sizes were comparable across analyses to the results reported here leading to the conclusion that our findings were not due to half-siblings in our sample. The Huyhn-Feldt adjustment of p-levels was used to adjust for possible violations of the sphericity assumption for effects involving repeated measures. Post-hoc tests were conducted using the Tukey-Kramer method (Keppel and Wickens, 2004). Consistency of individual differences in CSF levels of 5-HIAA and HVA from baseline to 50 hour post-separation was evaluated using Pearson-product correlations.
RESULTS
Cerebrospinal fluid (CSF) concentrations of the 5-HT metabolite, 5-hydroxyindoleacetic acid (5-HIAA) and the dopamine (DA) metabolite, homovanillic acid (HVA)
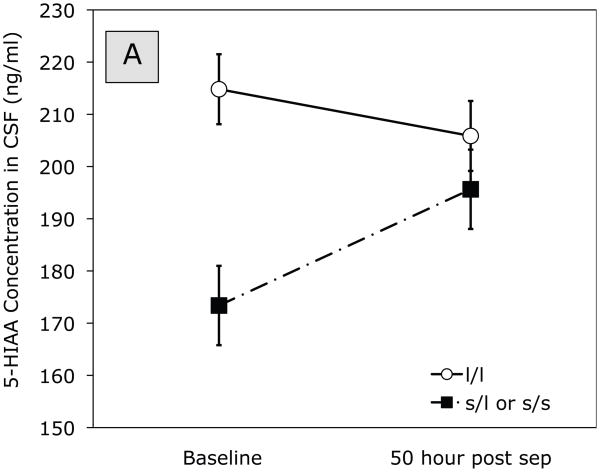
ANOVA of 5-HIAA at baseline and 50 hr post separation indicated a significant Separation (baseline, 50 hr post-separation) x Gene effect, F (1,24) = 4.38, p = 0.047. The means in Figure 1A show that, at baseline, monkeys carrying the short allele showed lower concentrations of 5-HIAA in CSF than those homozygous for the long allele. Moreover, there was little change from baseline to 50 hr post-separation for monkeys homozygous for the long allele. Monkeys carrying the short allele, on the other hand, showed a marked increase of 5-HIAA in CSF from baseline pre-separation concentrations to the 50-hour post-separation time point. Thus, 5-HIAA concentrations in CSF differed at baseline, but not at 50 hours post-separation, depending on whether the monkey carried one or more copies of the short allele compared to carrying two copies of the long rh5-HTTLPR allele.
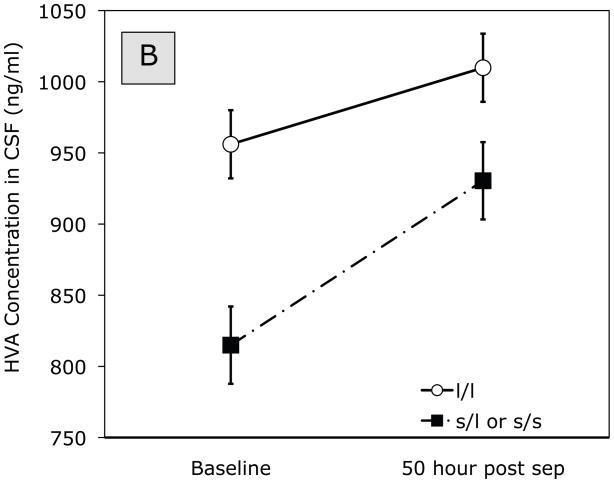
Figure 1.
Effect of timing (baseline, 50 hour post separation) and genotype on (A) 5-HIAA and (B) HVA concentration in CSF. Solid line represents monkeys homozygous for the long rh5-HTTLPR allele. Dashed line represents monkeys carrying the short (l/s or s/s) allele.
ANOVA of HVA concentrations in CSF at baseline and 50 hr post separation indicated a significant main effect of Separation (baseline (pre-separation), 50 hr post-separation), F (1,24) = 10.08, p = 0.004. HVA concentrations increased from baseline to 50 hrs post separation (see Figure 1B). At baseline, there was a significant effect for genotype, F (1,24) = 5.09, p = 0.03, in that monkeys carrying the short allele had lower pre-separation HVA concentrations compared to those homozygous for the long allele. A marginal Early x Late x Genotype effect, F(1, 24) = 3.33, p = 0.08, showed that Early-exposed and Mid-late alcohol-only monkeys carrying the short variant tended to show lower HVA concentrations compared to the other groups.
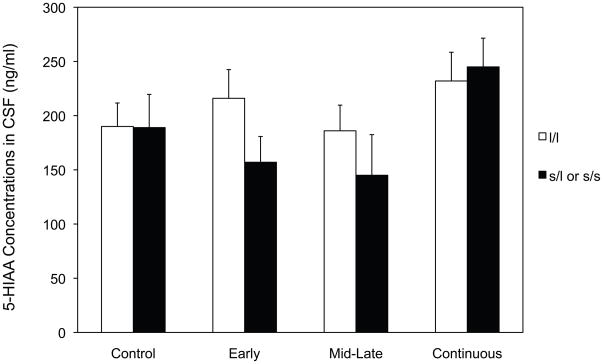
ANOVA of 5-HIAA concentrations also showed a significant Early x Mid-late x Gene interaction, F (1, 24) = 5.89, p = 0.023 (Figure 2). Averaged across both pre- and post-separation, monkeys carrying the short (l/s or s/s) allele in the Early-only and Mid-late-only alcohol-exposed groups showed the lowest concentrations of 5-HIAA in CSF.
Figure 2.
Effect of prenatal alcohol exposure and genotype on CSF concentrations of 5-HIAA averaged across time (baseline and post-separation). White bars represent monkeys homozygous for the long rh5-HTTLPR allele. Black bars represent monkeys carrying the short (l/l or s/s) allele.
In follow-up analyses of 5-HIAA concentrations in CSF at baseline (pre-separation), there were significant effects for Early alcohol exposure, F (1,24) = 5.97, p = 0.021 (188.65 vs 212.84, for Early vs Not Early, respectively), and for Gene, F (1, 24) = 7.16, p = 0.013 (195.64 vs 205.86 for short and long allele, respectively). There was also a significant Early x Mid-late x Gene interaction, F(1,24) = 5.43, p = 0.029. As in Figure 2, Early-only and Mid-late-only alcohol-exposed monkeys carrying the short variant showed the lowest baseline concentrations of 5-HIAA relative to other groups.
Follow-up analyses at 50 hours post-separation showed a significant Early x Mid-late Alcohol interaction, F (1, 24) = 5.23, p= 0.03, with Early-only and Mid-late-only alcohol-exposed monkeys again showing lower post separation concentrations of 5-HIAA relative to Continuous-exposed and Controls.
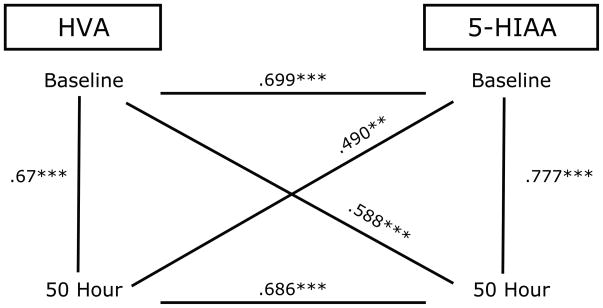
Correlations for 5-HIAA and HVA
We examined relationships between 5-HIAA and HVA concentrations in CSF within and across sampling times (pre- and post-separation) using Pearson r correlation. Figure 3 shows high consistency between HVA and 5-HIAA levels across and within time points. HVA and 5-HIAA were positively related to each other at all time points.
Figure 3.
Correlation between 5-HIAA and HVA concentration in CSF across and within sampling time points (baseline, 50 hr post separation) **p<0.01, ***p<0.001
DISCUSSION
This study provides evidence for a gene-environment interaction between timing of exposure to prenatal alcohol and rh5-HTTLPR genotype on indices of central serotonergic (5-HT) and dopaminergic (DA) function. There were three principal findings in the present study.
First, CSF 5-HIAA levels were lower at baseline (pre-separation) for s allele carriers compared to l/l allele carriers. Separation resulted in increased 5-HIAA in the s allele carriers relative to their pre-separation values. CSF 5-HIAA levels in l/l allele carriers were not affected by separation. Overall, s allele carriers had reduced concentrations of the DA metabolite HVA in CSF. Separation resulted in increased HVA concentrations regardless of genotype.
Second, a significant Gene x Early x Mid-late gestation alcohol exposure interaction for CSF concentrations of the 5-HT metabolite, 5-HIAA, was found. Prenatal alcohol exposure during early or middle-to-late gestation reduced 5-HIAA levels in monkeys carrying the short variant of the rh5-HTTLPR compared to control and continuous-exposed monkeys, regardless of genotype, and to monkeys homozygous for the long allele, regardless of alcohol exposure.
Third, there were significant and substantial correlations between 5-HIAA and HVA concentrations across and within time points. This important for two reasons. One is that the high correlation of metabolite levels, in either 5-HIAA or HVA across time periods suggests that inter-individual comparisons and intra-individual values are stable and “trait-like”. This stability is evident and measureable within the first 2–3 months of postnatal development (Kraemer et al., 1989; Clarke et al., 1995), and the present study confirms that individual differences persist through 30 months of age in rhesus monkeys. The second is that 5-HT and DA metabolite concentrations are highly correlated in every study where both are reported (see below). The meaning of this is uncertain, but it may reflect some intrinsic relationship between DA and 5-HT system activity that is reflected at the whole brain level and measurable in CSF. This finding will be discussed further in the context of its relevance to the first two findings.
Considering the first finding, previous studies have shown that in rhesus monkeys reared without mothers, a model of early adversity which produces long-lasting effects on brain structure and behavioral and endocrine stress reactivity, maternal deprivation interacted with the s allele to predict CSF concentrations of 5-HIAA, neonatal behavior, and stress responsivity (Bennett et al., 2002; Barr et al., 2004; Champoux et al., 2002). It is likely that HTTLPR genotype interacts with other types of perturbations that impact brain development. Individual differences in CSF 5-HIAA concentrations have been observed early in life in rhesus monkeys and remain remarkably stable throughout life (Clarke et al., 1995; Kraemer et al., 1989; Higley et al., 1996; Shannon et al., 2005).
Moreover, the levels of 5-HIAA concentration in CSF have been shown to reflect underlying risks for certain types of behavioral abnormalities in monkeys, including impaired impulse control, aggression, dysregulated circadian activity patterns, and excessive alcohol consumption (Fairbanks et al., 2001; Higley et al., 1996; Higley and Bennett, 1999). To our knowledge, central serotonergic function and HTTLPR genotype have not yet been evaluated in individuals with FASD. However, many of the mental health problems in such individuals, including depression and anxiety, and substance abuse as well, could be linked to prenatal alcohol-induced alterations of 5-HT function in genetically vulnerable individuals. Thus this study raises the question of whether that abnormal 5-HT biological pathways underlie some of the psychiatric disorders noted in FASD.
The s allele serotonin transporter gene promoter polymorphism is associated with reduced transcription of the gene that regulates uptake of synaptic serotonin (Lesch et al. 1996). Higher uptake of synaptic serotonin has been linked to anxiety and mood disorder. For this reason, the selective serotonin reuptake inhibitors (SSRIs) are treatments used for anxiety and mood disorders (Berton and Nestler 2006). In fact, most drugs used to treat a wide range of psychiatric disorders act, at least in part, through serotonergic mechanisms. It could be hypothesized that both prenatal alcohol exposure and carrying the short rh 5-HTTLPR allele have effects on the development of 5-HT pathways, and when exposure to alcohol during gestation as well as genetic endowment which compromises the 5-HT system are present, maladaptation in 5-HT function and related brain function could result, with behavioral consequences.
Given that 5-HT must be taken into the synapse to be metabolized into 5-HIAA, lower pre-separation 5-HIAA levels in monkeys carrying the s allele are consistent with the idea that 5-HT reuptake and perhaps release may typically be lower as well. The increase in 5-HIAA following separation in carriers of the s allele is remarkable for two reasons. First, previous developmental studies indicate that carriers of the s allele who are exposed to early adversity (peer versus maternal rearing) exhibit reduced CSF 5-HIAA by comparison to carriers of the s allele that have not been challenged early on (Bennett et al., 2002). These results are similar to the findings of the present study in that prenatal alcohol exposure seems to have effects on CSF measures in carriers of the s allele compared with carriers of the l/l alleles. These findings are consistent with the idea that carrying the s allele and having low CSF 5-HIAA might be stable and “trait-like” markers of risk for increased sensitivity to negative effects of adverse experiences later in life. Second, the effects of separation stress or early social isolation on CSF 5-HIAA has been measured in several previous studies, and no effects were observed (Higley et al., 1991; Kraemer et al., 1989; Kraemer et al., 1991). However, these studies did not consider variation in genotype as a variable. The results of the present study indicate that there may actually be a differential response of the 5-HT system to stressors in adulthood depending on genotype. The results presented in Figure 1a suggest that separation stress may produce greater demands for increased 5-HT output in carriers of the s allele by comparison to carriers of the l/l allele; or, that tonic activity of the 5-HT system in carriers l/l allele is sufficient to meet demands, even of separation, and no increase is observed when the individual is stressed. As far as the DA system is concerned, HVA concentrations increased from baseline to 50 hours post-separation in both l/l and s carriers indicating that separation is a stressor for both groups and produces increased activation of the DA system. This increase in DA activation is most likely associated with increased motor activity as a result of social separation and is consistent with prior work showing that chronic stress increases synaptic dopamine concentrations (Papp et al., 1993).
The rh5-HTTLPR genotype was also associated with altered HVA concentrations in CSF at baseline. Monkeys carrying the short allele had lower basal concentrations of HVA than carriers of the l/l alleles. The finding of lower DA activity, as indexed by CSF HVA concentrations, in short allele carriers, raises an interesting question: Why would a genotype that is associated with reduced transcription of the gene that regulates uptake of synaptic 5-HT (Lesch et al. 1996) have an effect on HVA concentrations in CSF? Interestingly, the 5HT and DA transporters are closely related evolutionarily, sharing close protein sequences and functional sensitivity (Zhou et al., 2002). DA/5-HT interactions have been found in numerous studies and serotonin is considered to play a major role in reward processing, along with dopamine (Kranz et al., 2010). Serontonergic neurons project to neurons in the mesolimbic dopamine system (Parent, et al., 1981) and are involved in the regulation of dopamine transmission through several serotonergic receptor subtypes (Kapur and Remington 1996, Alex and Pehek, 2007). In addition, DA neurons in the ventral tegmental area and substantia nigra express a range of 5-HT receptor types that can be activated by 5-HT input from the dorsal raphe nucleus (Herve et al., 1987). In mice, cross-neuronal type uptake exists between the 5-HT and DA transporters, such that if one transporter is dysfunctional or inadequate, the other can compensate or serve as a back-up mechanism (Zhou et al., 2002). Our third finding of consistently high correlations between 5-HIAA and HVA levels across and within time points provides further evidence that DA and 5-HT are closely related.
With regard to our second major finding, the sensitivity to timing of prenatal alcohol exposure, the literature indicates that the development of the serotonin system is sensitive to numerous environmental and genetic influences (Higley et al., 1993; Rogers 2004). Serotonin plays a critical role in plasticity of the brain. Serotonin is implicated in numerous behaviors, physiological mechanisms, and disease processes (Azmitia, 2001). Serotonin neurons are one of the first types of brainstem neurons to emerge during early development, with a wide range of projections to numerous brain regions, playing an important role in neurogenesis (Kligman and Marshak, 1985).
Exposure to alcohol during early gestation or mid-to-late gestation affected serotonin function as indexed by lower CSF 5-HIAA concentrations in rhesus monkeys carrying the short rh5-HTTLPR variant. This finding is consistent with reports of altered serotonergic function in rodents exposed to alcohol (Druse et al., 1991; Tajuddin and Druse, 1999; 2001; Zhou et al., 2001). This is, however, the first study to expand these findings to include prenatal alcohol-exposed monkeys carrying the short serotonin transporter gene promoter polymorphism, compared to prenatally alcohol-exposed monkeys with the l/l allele and controls regardless of genotype.
It was interesting to find that s allele carriers from the early and middle-to-late exposure groups showed reduced 5-HIAA concentrations, whereas monkeys from the continuous alcohol-exposure group, exposed for a longer period of time, did not. The mechanisms underlying the gene-environment interaction are not known. Prenatal alcohol exposure operates on the same neurotransmitters that are influenced by certain genes. For example, there is a large rodent literature linking prenatal alcohol exposure to low serotonin function as indexed by a decrease in serotonin (5-HT) neurons, reduced density of serotonin reuptake sites and abnormal density of 5-HT1A receptors (Druse et al., 1991; Tajuddin and Druse, 1999; 2001; Zhou et al., 2001). Moreover, the 5-HT1A receptor, altered by prenatal alcohol exposure develops early in the brainstem and later in regions maturing later during gestation, such as the cerebellum and visual cortex (Bar-Peled et al., 1991). The 5-HT1A receptor in the adult brain regulates adult neurogenesis and maintains the adult state of neurons (Azmitia, 2001).
Findings in the literature suggest that alcohol effects vary during gestation but they may not be easily interpreted, or predicted, in relation to timing and duration of exposure. For example, in rats, alcohol exposure during E14 and E15 lowered the number of cells in the ventral lateral nucleus of the thalamus, whereas alcohol exposure E11 – E20 did not, suggesting that the timing of both the onset and the offset of exposure is critical (Livy et al., 2001). The mechanisms underlying this phenomenon have not been determined. It is possible that continuous exposure afforded the opportunity for neuroadaptation of the serotonin system in the continuous exposed group, whereas shorter periods of exposure did not. Also, it could be that substantial developmental effects are actually caused by withdrawal effects after alcohol has been present for a significant time period. Withdrawal from alcohol in the early exposure group in itself could present a challenge in the early exposed group. Perhaps the effects in the shorter exposure groups result from a combination of onset/offset challenges and lack of neuroadaptation, compared to the results for the group exposed throughout pregnancy. More studies are clearly needed to understand this phenomenon further.
Clinical studies are clearly needed to follow up these results and explore the role of allelic variation of 5-HTTLPR on DA- and 5-HT-dependent behaviors in children with FASD as well as long-term mental health outcomes. Moreover, recent studies in humans and rhesus monkeys suggest that individuals carrying the short 5-HTT allele might be more sensitive to both negative and positive rearing environments (Belsky and Pluess, 2009; Boyce and Ellis, 2005: Suomi, 2006). Examining mental health outcomes in children with FASD as a function of prenatal alcohol exposure, 5-HTTLPR variant, and early rearing environment could be an important avenue for future studies. Better knowledge of how allelic variation in certain genes interacts with prenatal alcohol exposure may lead to developing better intervention strategies for children with FASD and ultimately to improved prevention of adverse mental health outcomes in this population. Finally, studies examining the relationship between variation in the rh5-HTTLPR allele in prenatal alcohol-exposed monkeys and several other molecular indices of DA and 5-HT function, (DA D1 receptor, DA transporter and 5-HT1A receptor availability using PET) are currently underway in our laboratory and could lead to improved knowledge of the neurobiological substrates of prenatal alcohol exposure, leading to improved intervention strategies.
It is interesting that there were few main effects of genetic variant on CSF 5-HIAA concentrations. This finding is consistent with the notion that susceptibility genes have very minor effects by themselves, but rather, when interacting with other gene variants and environmental factors, produce disorders (Kendler, 2005). The idea is that susceptibility genes represent particular allelic variations of common genes or normal allelic variation. Thus, the genes may affect physiological pathways that could render psychological disorders more or less inevitable, but the genes do not cause the disorder directly (Rutter et al., 2006). Rather they may contribute to genetically influenced sensitivities to specific environments.
A limitation of this study is that the candidate gene was limited to one functional 5-HTT marker (vs. haplotype). Candidate loci that affect functioning of other neurotransmitter system are undoubtedly important and relevant to understanding prenatal alcohol effects. Another limitation is that while this study has a relatively large sample size for a primate study, the sample size is smaller than that used with human studies. However, the experimental control the primate model affords cannot be achieved in human studies, making primate studies invaluable for addressing gene x environment influences. Finally, while experiments with nonhuman primates permit control of the dose and timing of prenatal alcohol exposure and inclusion of candidate genes similar to humans, caution is needed in generalizing from monkeys to humans. Thus, it is critical to replicate these findings in human studies. If fetal alcohol-exposed children with vulnerable genotypes can be identified early in life, targeted and effective intervention studies are possible early in life at the peak of brain plasticity. These studies ultimately have the potential to significantly reduce the pain and suffering associated with the devastating effects of fetal alcohol spectrum disorder.
Table 1.
Genotype distribution as a function of prenatal treatment conditions:
| Genotype | control | mid-late | Early | Continuous |
|---|---|---|---|---|
| l/l | 6 | 4 | 4 | 4 |
| l/s or s/s | 3 | 2 | 5 | 4 |
Acknowledgments
This study was supported by AA10079 and AA12277 from the National Institute of Alcoholism and Alcohol Abuse to M.L. Schneider.
References
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autti-Rämö I. Foetal alcohol syndrome-a multifaceted condition. Dev Med Child Neurol. 2002;44:141– 144. doi: 10.1017/s0012162201001839. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Modern views on an ancient chemical: Serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Bacopoulos NG, Redmond DE, Roth RH. Serotonin and dopamine metobolites in brain regions and cerebrospinal fluid of a primate species: effects of ketamine and fluphenazine. J Neurochem. 1979;32:1215–1218. doi: 10.1111/j.1471-4159.1979.tb11048.x. [DOI] [PubMed] [Google Scholar]
- Bar-Peled O, Gross-Isseroff R, Ben-Hur H, Hoskins I, Groner Y, Biegon A. Fetal human brain exhibits a prenatal peak in the density of serotonin 5-HT1A receptors. Neurosci Lett. 1991;127:173–176. doi: 10.1016/0304-3940(91)90787-t. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55:733–8. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bear MF. Critical periods in visual system development. In: Conn PM, editor. Neuroscience in Medicine. Vol. 33. Lippincott; Philadelphia: 1995. pp. 465–483. [Google Scholar]
- Belsky J, Pluess M. The nature (and nurture?) of plasticity in early human development. Perspect Psychol Sci. 2009;4:345–351. doi: 10.1111/j.1745-6924.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–22. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-development theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Brain Res Rev. 1993;18:1–32. doi: 10.1016/0165-0173(93)90005-k. [DOI] [PubMed] [Google Scholar]
- Chernow B, Lake CR, Cruess D, Coyle J, Hughes P, Balestrieri F, Casey L, Rainey TG, Fletcher JR. Plasma, urine, and CSF catecholamine concentrations during and after ketamine anesthesia. Crit Care Med. 1982;10:600–603. doi: 10.1097/00003246-198209000-00009. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Astley SJ, Gunderson VM, Spellman D. Cognitive and behavioral deficits in nonhuman primates associated with very early embryonic binge exposures to ethanol. Pediatrics. 1992;121:789–796. doi: 10.1016/s0022-3476(05)81917-1. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Kammerer CM, George KP, Kupfer DJ, McKinney WT, Spence MA, Kraemer GW. Evidence for heritability of biogenic amine levels in the cerebrospinal fluid of rhesus monkeys. Biological Psychiatry. 1995;38:572–577. doi: 10.1016/0006-3223(95)00042-4. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Kuo AP, Tajuddin N. Effects of in utero ethanol exposure on the developing serotonergic system. Alcohol Clin Exp Res. 1991;15:678–684. doi: 10.1111/j.1530-0277.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Paul LH. Effects of in utero ethanol exposure on serotonin uptake in cortical regions. Alcohol. 1989;5:455–459. doi: 10.1016/0741-8329(88)90082-1. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT. Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neruopsychopharmacology. 2001;24:370–378. doi: 10.1016/S0893-133X(00)00211-6. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Temporal windows of vulnerability within the third trimester equivalent: why “knowing when” matters. In: Hannigan JH, Goodlett CR, Spear LP, Spear NE, editors. Alcohol and Alcoholism: Effects on Brain and Development. Earlbaum Associates; Mahwah: 1999. pp. 59–91. [Google Scholar]
- Graham JM, Jr, Hanson JW, Darby BL, Barr HM, Streissguth AP. Independent dysmorphology evaluations at birth and 4 years of age for children exposed to varying amounts of alcohol in utero. Pediatrics. 1988;81:772–8. [PubMed] [Google Scholar]
- Hensler JG, Ferry RC, Labow DM, Kovachich GB, Frazer A. Quantitative autoradiography of the serotonin transporter to assess the distribution of serotonergic projections from the dorsal raphe nucleus. Synapse. 1994;17:1–15. doi: 10.1002/syn.890170102. [DOI] [PubMed] [Google Scholar]
- Herve D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435:71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- Higley JD, Bennett AJ. Central nervous system serotonin and personality as variables contributing to excessive alcohol consumption in non-human primates. Alcohol Alcohol. 1999;34:402–418. doi: 10.1093/alcalc/34.3.402. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res. 1996;20:629–642. doi: 10.1111/j.1530-0277.1996.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Higley JD, Thompson WW, Champoux M, Goldman D, Hasert MF, Kraemer GW, Scanlan JM, Suomi SJ, Linnoila M. Paternal and maternal genetic and environmental contributions to cerebrospinal fluid monoamine metabolites in rhesus monkeys (Macaca mulatta) Arch Gen Psychiatry. 1993;50:615–23. doi: 10.1001/archpsyc.1993.01820200025003. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology. 1991;103(4):551–556. doi: 10.1007/BF02244258. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Lauer CJ, Schreiber W, Krieg JC. Altered hypothalmic-pituitary-adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–347. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999– 1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis L, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis: A researcher’s handbook. 4. Pearson Prentice Hall; Upper Saddle River: 2004. [Google Scholar]
- Kim JA, Gillespie RA, Druse MJ. Effects of maternal ethanol consumption and buspirone treatment on 5-HT1A and 5-HT2A receptors in offspring. Alcohol Clin Exp Res. 1997;21:1169–1178. [PubMed] [Google Scholar]
- Kligman D, Marshak DR. Purification and characterization of a neurite extension factor from bovine brain. Proc Natl Acad Sci. 1985;82:7136–9. doi: 10.1073/pnas.82.20.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer GW, Ebert MH, Schmidt DE, McKinney WT. A longitudinal study of the effect of different social rearing conditions on cerebrospinal fluid norepinephrine and biogenic amine metabolites in rhesus monkeys. Neuropsychopharmacology. 1989;2:175–89. doi: 10.1016/0893-133x(89)90021-3. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, Ebert MH, Schmidt DE, McKinney WT. Strangers in a strange land: A psychobiological study of infant monkeys before and after separation from real or inaninmate mothers. Child Development. 1991;62:548–566. [PubMed] [Google Scholar]
- Kraemer GW, Moore CF, Newman TK, Barr CS, Schneider ML. Moderate level fetal alcohol exposure and serotonin transporter gene promoter polymorphism affect neonatal temperament and limbic-hypothalamic-pituitary-adrenal axis regulation in monkeys. Biol Psychiatry. 2008;63:317– 324. doi: 10.1016/j.biopsych.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, La Cour CM, Froger N, Hamon M. 5-HT-HPA interactions in two models of transgenic mice relevant to major depression. Neurochem Res. 2000;25:1199–1206. doi: 10.1023/a:1007683810230. [DOI] [PubMed] [Google Scholar]
- Lemoine P, Harousseau H, Borteyru JP, Menuet JC. Children of alcoholic parents--observed anomalies: discussion of 127 cases. Ther Drug Monit. 2003;25:132–6. doi: 10.1097/00007691-200304000-00002. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm. 1997;104:1259–66. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: Effects of binge- like alcohol exposure on the ventro-lateral nucleus of the thalamus. Alcohol Clin Exp Res. 2001;25:774–780. [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis L, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. Comorbidity of mood and anxiety disorders: the rule, not the exception? Am J Psychiatry. 2002;159:3–4. doi: 10.1176/appi.ajp.159.1.3. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Newell-Morris L, Fahrenbruch CE. Practical and evolutionary considerations for use of the nonhuman primate model in prenatal research. In: Watts ES, editor. Nonhuman primate models for human growth and development. Liss; New York: 1985. pp. 9–40. [Google Scholar]
- O’Connor MJ, Kasari C. Prenatal alcohol exposure and depressive features in children. Alcohol Clin Exp Res. 2000;24:1084 – 1092. [PubMed] [Google Scholar]
- Papp M, Muscat R, Willner P. Subsensitivity to rewarding and locomotor stimulant effects of a dopamine agonist following chronic mild stress. Psychopharmacology. 1993;110:152–158. doi: 10.1007/BF02246965. [DOI] [PubMed] [Google Scholar]
- Parent A, Descarries L, Beaudet A. Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1981;6:115–1138. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Parnell SE, O’Leary-Moor SK, Godi EA, Dehar DB, Johnso BW, Johnso GA, Styne MA, Suli KK. Magnetic Resonance Microscopy Defines Ethanol-Induced Brain Abnormalities in Prenatal Mice: Effects of Acute Insult on Gestational Day. Alcohol Clin Exp Res. 2009;33:1001–1011. doi: 10.1111/j.1530-0277.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Chen WJ, West JR, Cudd TA. Temporal vulnerability of fetal cerebellar Purkinje cells to chronic binge alcohol exposure: ovine model. Alcohol Clin Exp Res. 2007;31:1738–45. doi: 10.1111/j.1530-0277.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- Rathbun W, Druse MJ. Dopamine, serotonin, and acid metabolites in brain regions from the developing offspring of the ethanol-treated rats. J Neurochem. 1985;44:57–62. doi: 10.1111/j.1471-4159.1985.tb07112.x. [DOI] [PubMed] [Google Scholar]
- Rawat AK. Development of histaminergic pathways in brain as influenced by maternal alcoholism. Res Commun Chem Pathol Pharmacol. 1980;27:91–103. [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Day NL, Taylor PM. The effect of prenatal alcohol, marijuana, and tobacco exposure on neonatal behavior. Infant Behav Dev. 1989;12:199–209. [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rogers DT, Barron S, Littleton JM. Neonatal ethanol exposure produces a hyperalgesia that extends into adolescence, and is associated with increased analgesic and rewarding properties of nicotine in rats. Psychopharmacology (Berl) 2004;171:204–211. doi: 10.1007/s00213-003-1574-z. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47:226–61. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Sari Y, Powrozek T, Zhou FC. Alcohol deters the outgrowth of serotonergic neurons at midgestation. J Biomed Sci. 2001;8:119–125. doi: 10.1007/BF02255980. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Roznoski M, Ebert M. Qualitative and quantitative high performance liquid chromatographic analysis of monoamine neurotransmitters and metabolites in cerebrospinal fluid and brain tissue using reductive electrochemical detection. Biomed Chromatogr. 1990;4:215–220. doi: 10.1002/bmc.1130040509. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Barnhart TE, Larson JA, DeJesus OT, Mukherjee J, Nickles RJ, Converse AK, Roberts AD, Kraemer GW. Moderate-level prenatal alcohol exposure alters striatal dopamine system function in rhesus monkeys. Alcohol Clin Exp Res. 2005;29:1685–97. doi: 10.1097/01.alc.0000179409.80370.25. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Becker EF. Timing of moderate alcohol exposure during pregnancy and neonatal outcome in rhesus monkeys (Macaca mulatta) Alcohol Clin Exp Res. 2001;25:1238–45. [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Larson JA, Barr CS, Dejesus OT, Roberts AD. Timing of moderate level prenatal alcohol exposure influences gene expression of sensory processing behavior in rhesus monkeys. Front Integr Neurosci. 2009;3:1–9. doi: 10.3389/neuro.07.030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, Higley JD. Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. Am J Psychiatry. 2005;162:1658–1664. doi: 10.1176/appi.ajp.162.9.1658. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL. Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE) Centers for Disease Control and Prevention (CDC), University of Washington, Fetal Alcohol and Drug Unit; 1996. [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, Parrish-Johnson JC, Kirchner GL, Martin DC. Attention, distraction and reaction time at age 7 years and prenatal alcohol exposure. Neurobehav Toxicol Teratol. 1986;8:717–25. [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental & Behavioral Pediatrics. 2004;25:228–38. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: Embryogenesis in a mouse model. Science. 1981;214:936–938. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Risk, resilience, and gene X environment interactions in rhesus monkeys. Ann N Y Acad Sci. 2006;1994:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Tajuddin N, Druse MJ. In utero ethanol exposure decreased the density of serotonin neurons: Maternal ipsapirone treatment exerted a protective effect. Brain Res Dev Brain Res. 1999;117:91–97. doi: 10.1016/s0165-3806(99)00102-9. [DOI] [PubMed] [Google Scholar]
- Tajuddin NF, Druse MJ. A persistent deficit of serotonin neurons in the offspring of the ethanol- fed dams: Protective effects of maternal ipsapirone treatment. Dev Brain Res. 2001;129:181–188. doi: 10.1016/s0165-3806(01)00199-7. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Li TK, Goodlett CR, Azimitia EC. Deviations in brain early serotonergic development as a result of fetal alcohol exposure. Neurotox Res. 2002;4:337–342. doi: 10.1080/10298420290030532. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek T, Goodlett CR, Li TK. Moderate alcohol exposure compromises neural tube midline development in prenatal brain. Dev Brain Res. 2003;144:43–55. doi: 10.1016/s0165-3806(03)00158-5. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK, Goodlett CR, Li T. Prenatal alcohol exposure retards the migration and development of serotonin neurons in fetal C57BL mice. Brain Res Dev Brain Res. 2001;126:147–55. doi: 10.1016/s0165-3806(00)00144-9. [DOI] [PubMed] [Google Scholar]