Abstract
Background
Previous studies have shown that high alcohol consumption is associated with low withdrawal susceptiblility, while at the same time, other studies have shown that exposure to ethanol vapor increases alcohol drinking in rats and mice. In the present studies, we sought to shed light on this seeming contradiction by using mice selectively bred for High- (HAP) and Low- (LAP) Alcohol Preference, first, assessing these lines for differences in signs of ethanol withdrawal and second, for differences in the efficacy of intermittent alcohol vapor exposure on elevating subsequent ethanol intake.
Methods
Experiment 1 examined whether these lines of mice differed in ethanol withdrawal-induced CNS hyperexcitability and the development of sensitization to this effect following intermittent ethanol vapor exposure. Adult HAP and LAP lines (replicates 1 and 2), and the C3H/HeNcr inbred strain (included as a control genotype for comparison purposes) received intermittent exposure to ethanol vapor and were evaluated for ethanol withdrawal-induced seizures assessed by scoring handling-induced convulsions (HIC). Experiment 2 examined the influence of chronic intermittent ethanol exposure on voluntary ethanol drinking. Adult male and female HAP-2 and LAP-2 mice, along with male C57BL/6J (included as comparative controls) were trained to drink 10% ethanol using a limited access (2 hr/day) 2-bottle choice paradigm. After stable baseline daily intake was established, mice received chronic intermittent ethanol vapor exposure in inhalation chambers. Ethanol intake sessions resumed 72 hr after final ethanol (or air) exposure for 5 consecutive days.
Results
Following chronic ethanol treatment, LAP mice exhibited overall greater withdrawal seizure activity compared to HAP mice. In Experiment 2, chronic ethanol exposure/withdrawal resulted in a significant increase in ethanol intake in male C57BL/6J, and modestly elevated intake in HAP-2 male mice. Ethanol intake for male control mice did not change from baseline levels of intake. In contrast, HAP-2 females and LAP-2 mice of both sexes did not show changes in ethanol intake as a consequence of intermittent ethanol exposure.
Conclusions
Overall, these results indicate that the magnitude of ethanol withdrawal-related seizures is inversely related to inherited ethanol intake preference. Additionally, intermittent ethanol vapor exposure appears more likely to affect high-drinking mice (C57BL/6J and HAP-2) than low drinkers, even though these animals are less affected by ethanol withdrawal.
Keywords: Ethanol Dependence, Withdrawal Sensitization, Ethanol Drinking, Selected Lines
INTRODUCTION
It is well known that genetic factors play an important role in the development of alcoholism (Mayfield et al., 2008), but it is uncertain what behavioral and physiological mechanisms mediate this effect. Sensitivity to ethanol’s intoxicating, rewarding, or aversive effects, differences in metabolism of ethanol, as well as sensitivity to ethanol dependence and withdrawal may all play a role in the heritability of alcoholism (Green and Grahame, 2008; Metten et al., 1998). Genes also modulate the course of development of alcoholism and the effectiveness of different treatments (Mayfield et al., 2008). Several animal models have been developed, especially using rodents, to better understand the role of genetic factors in the development of alcoholism and to identify possible gene candidates that modulate specific ethanol-related effects.
Different phenotypes for ethanol intake have been identified in strains of mice and rats (Belknap et al., 1993; Overstreet et al., 1999; Rhodes et al., 2007; Yoneyama et al., 2008). Also, several lines of rodents have been developed by selective breeding based on differences in sensitivity to certain effects of ethanol administration (e.g., FAST and SLOW mice) or withdrawal (e.g., Withdrawal-Seizure Prone, or WSP and Withdrawal-Seizure Resistant, or WSR mice). These selected lines of rodents have been subsequently evaluated in models of ethanol self-administration in order to evaluate if their sensitivity to certain ethanol effects is related to voluntary ethanol consumption. For example, FAST mice (selectively bred for high locomotor activation after acute ethanol administration) drink significantly more ethanol than SLOW mice that were derived based on depressed locomotor activity in response to ethanol injections (Risinger et al., 1994). This suggests that inherited sensitivity to ethanol’s effects on locomotor activity may modulate ethanol self-administration.
The reverse approach has been used as well to evaluate the relation between voluntary ethanol intake and sensitivity to different effects of ethanol. Lines of rats (e.g., Alcohol Preferring, or P and Alcohol Non-Preferring, or NP) and mice (High-Alcohol Preferring, or HAP and Low Alcohol Preferring, or LAP) have been obtained by selectively breeding rodents based on their level of free-choice ethanol intake or preference. Then, these lines of rodents have been evaluated for their response to experimenter-administered ethanol challenge. For example, both ethanol preferring P rats and HAP mice are less sensitive to ethanol-induced conditioned taste aversion than NP rats (Froehlich et al., 1988) and LAP mice (Chester et al., 2003b), respectively, suggesting a relationship between resistance to ethanol’s aversive effects and elevated ethanol preference.
HAP and LAP lines of mice have been developed by selectively breeding mice based on their voluntary preference for 10 % ethanol measured over a four-week period (Grahame et al., 1999b; Oberlin et al., 2010). Previous studies conducted with these selected mice indicate that similar to P rats, HAP mice appear to consume ethanol in order to obtain its pharmacological effects, rather than just for its taste (Grahame et al., 1999a). Relative to LAP mice, HAP mice also show somewhat attenuated conditioned place preference following high ethanol doses (Grahame et al. 2001), but enhanced ethanol-induced locomotor sensitization (Grahame et al., 2000).
The studies presented here aim to evaluate the relation between sensitivity to ethanol withdrawal and propensity to drink ethanol. Genetic studies have suggested that there is an inverse relationship between sensitivity to ethanol withdrawal symptoms and propensity to self-administer ethanol (Metten et al., 1998; Rhodes et al., 2007). For example, using both inbred strains and selected lines, Metten et al. (1998) consistently demonstrated that a genetic predisposition for high ethanol preference is associated with relatively lower susceptibility to and milder expression of withdrawal-induced seizures, and the converse for animals that exhibit low preference for ethanol. In the study presented here, HAP and LAP mice were evaluated for signs of CNS hyperexcitability (convulsions) associated with withdrawal from chronic ethanol exposure. The first goal of this study was to evaluate whether the HAP and LAP lines of mice show the expected difference in magnitude of ethanol withdrawal-related symptoms (i.e., increased withdrawal response in LAP mice relative to HAP mice). Prior studies conducted in our laboratory indicate that repeated cycles of chronic ethanol vapor exposure followed by periods of withdrawal induce a significant increase in the magnitude of ethanol withdrawal symptoms (Becker and Hale, 1993; Becker and Littleton, 1996). This exacerbation of ethanol withdrawal signs has been well documented in alcoholics that experience repeated detoxification episodes (Ballenger and Post, 1978; Malcolm et al., 2000a; Malcolm et al., 2000b), and has been modeled in mice (Becker et al., 1997; Becker and Hale, 1993). A second goal of this study was to evaluate if these lines of mice exhibit a difference in the development of sensitization of ethanol withdrawal signs following repeated cycles of chronic ethanol exposure and withdrawal, and if so, whether there is any relationship between differences in baseline withdrawal sensitivity and the tendency to develop sensitization to seizures following repeated withdrawal.
Further, while genetic studies have shown that high withdrawal sensitivity is genetically associated with low voluntary alcohol intake, studies have repeatedly shown that individual mice and rats exposed to ethanol vapor show subsequent increases in ethanol consumption and/or self-administration behavior. Using the inbred C57BL/6 mouse strain, we have shown that repeated cycles of chronic intermittent exposure to ethanol vapor via inhalation produces significant escalation of voluntary ethanol intake (Becker and Lopez, 2004; Griffin et al., 2009a; Lopez and Becker, 2005), and this increased ethanol consumption results in significant elevation of blood and brain ethanol concentrations (Becker and Lopez, 2004; Griffin et al., 2009b). Similar results have been obtained by others using mice and rats (Finn et al., 2007; Funk et al., 2007; Gilpin et al., 2008; O'Dell et al., 2004; Roberts et al., 2000; Valdez et al., 2002). Accordingly, a third goal of the present study was to examine the influence of repeated cycles of chronic intermittent ethanol exposure on voluntary ethanol drinking in mice selectively bred for high (HAP) and low (LAP) ethanol preference. By assessing changes in drinking following repeated experience with chronic ethanol exposure and withdrawal in lines that differ both in baseline withdrawal seizure sensitivity and baseline drinking levels, the study was designed to determine whether genetic differences in these behaviors are related to the ability of repeated cycles of chronic intermittent ethanol exposure to produce escalation of voluntary ethanol intake.
MATERIALS AND METHODS
Subjects
Adult male and female mice from high ethanol preferring (HAP) and low ethanol preferring (LAP) lines were obtained from IUPUI and maintained in MUSC’s animal facilities. Replicate 1 HAP and LAP mice for Experiment 1a were from generation 27 of selection, and replicate 2 mice for Experiment 1b were from generation 25. For Experiment 2, replicate 2 HAP and LAP mice were from generation 33 of selection. Mice were bred at IUPUI and shipped to MUSC at 6–7 weeks of age. Adult male C3H/HeNcr mice (C3H) were obtained from Charles River (Kingston, NY) while adult male C57BL/6J mice (C57) were obtained from Jackson Laboratories (Bar Harbor, ME). Mice had ad lib. access to rodent food (Teklad rodent diet, Harland Teklad, Madison, WI) and water throughout all experiments. Body weights typically were recorded weekly, but performed daily during the weeks mice were exposed to ethanol vapor (or air for control mice). Mice were housed in a temperature and humidity-controlled room in an animal facility accredited by AAALAC. Rooms where mice were housed have timers to schedule a daily 12-hr light/dark cycle (lights on at 0200 hr). Mice were individually housed at the beginning of each study in standard mouse cages with corncob bedding. All procedures were approved by the Institutional Animal Care and Use Committee and followed the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996).
Studies Design and Procedures
Experiment 1: Sensitization of Ethanol Withdrawal Seizure Activity in HAP/LAP Mice
Adult male HAP-1, LAP-1 (Experiment 1a), and male and female HAP-2, LAP-2 (Experiment 1b) mice were used, along with C3H mice that served as a positive control condition in each experiment. Mice were separated in two groups (N= 6–8 mice/group/genotype for Experiment 1a and N= and 7–9 mice/group/genotype/sex for Experiment 1b). Mice in the EtOH group experienced multiple ethanol withdrawal episodes. These mice were exposed to 4 cycles of 16 hr exposure to ethanol vapor in inhalation chambers separated by 8 hr periods of withdrawal. Mice in the CTL group served as controls and received similar handling but were not exposed to ethanol vapor in the inhalation chambers. Details about ethanol vapor exposure are described below. Intensity of ethanol withdrawal seizure activity was evaluated by scoring handling-induced convulsions (HIC) as detailed below. HIC activity was assessed hourly during the first 7 hr of withdrawal on cycles 1–4. HIC activity was evaluated by calculating the area under the curve (AUC) for the 7 hours of testing.
Experiment 2: Evaluation of Voluntary Ethanol Intake in Ethanol-Dependent and Control HAP/LAP Mice
Adult male and female mice from the HAP-2 and LAP-2 lines as well as male C57 mice (included as comparative controls) were trained to drink ethanol 10% v/v vs. water for two hours per day. The 10% ethanol concentration was used because the HAP and LAP lines were selectively bred based on their voluntary intake of this ethanol concentration. After four weeks of intake of 10% ethanol (vs. water) and once stable ethanol intake was established in each of the lines (less than 20% variation in daily intake), mice were further separated into EtOH and CTL groups (N= 7–8/group). Similar to Experiment 1, mice in the EtOH group experienced 4 cycles of 16 hr exposure to ethanol vapor in inhalation chambers separated by 8 hr of withdrawal while mice in the CTL group received similar handling but were not exposed to ethanol vapor in the inhalation chambers. During this period of inhalation treatment, access to ethanol for drinking was suspended. Ethanol drinking sessions resumed 72 hours after the last ethanol or air exposure for 5 consecutive days (Test 1). This procedure was repeated for a second cycle, with mice exposed again to chronic intermittent ethanol (or air) exposure followed by 5 daily drinking test sessions (Test 2). Details about limited access ethanol drinking and ethanol (or air) exposure in inhalation chambers are described below.
Chronic Intermittent Ethanol Exposure
Chronic ethanol vapor exposure for the EtOH group or air exposure for the CTL group was delivered in Plexiglas inhalation chambers (60x36x60 cm) modified after that previously described (Becker and Hale, 1993). Ethanol was volatilized by passing air through an air stone submerged in 95% ethanol. The ethanol vapor was mixed with fresh air and delivered to the chambers at a rate of 5 l/min for C57BL/6J, LAP and HAP mice, and 10 l/min for C3H/HeNcr mice. Airflow was adjusted to maintain ethanol concentration in the chambers in the range of 12–20 mg/l air. We have previously demonstrated that these conditions yield stable blood ethanol levels in the range of 175–250 mg/dl during each cycle of intoxication in C3H/HeNcr (Becker and Hale, 1993) and C57BL/6J mice (Lopez and Becker, 2005). Ethanol concentration was monitored daily using procedures described before (Lopez and Becker, 2005). Prior to entry into the ethanol chambers for each 16 hr exposure period (1600 hr), intoxication was initiated by administration of ethanol (1.6 g/kg; 8% w/v) and blood ethanol concentration (BEC) was stabilized by administration of the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg). The two drugs were injected intraperitoneally in a volume of 20 ml/kg body weight. Mice in the CTL group received similar handling in identical chambers with the ethanol vapor omitted. Mice maintained in the control (air) chambers received injections of saline and pyrazole. The housing conditions in the inhalation chambers were identical to that in the colony room.
Assessment of Handling-induced convulsions (HIC) Activity
Withdrawal HIC testing was conducted by a single experimenter that was unaware of the animals’ experimental history. Briefly, each mouse is gently picked up by the tail, held in place, and then rotated along a 360° arc. Convulsive signs are rated depending on the severity of the response as well as the extent of the handling manipulation required to elicit the behavioral response. Severity of HIC responses was assessed using a previously published scoring system and scale (Becker and Hale, 1993; Crabbe and Kosobud, 1990). Briefly, HIC activity was scored on a 0–7 scale: 0= no activity on tail lift, or after gentle 360° spin; 1= no activity on tail lift, but facial grimace after 360° spin; 2= tonic convulsion after 360° spin; 3= tonic/clonic convulsion after 360° spin; 4= tonic convulsion on tail lift; 5= tonic/clonic convulsion on tail lift, onset delayed by 1 to 2 sec; 6= severe tonic/clonic convulsion on tail lift, no delay in onset; 7= severe tonic/clonic convulsion prior to tail lift.
Assessment of Ethanol Intake: Home Cage Limited Access Procedure
At 30 min before the beginning of the dark cycle (1330 hr), water bottles were removed from the home cage and replaced with two 15 ml graduated tubes containing an appropriate ethanol solution and water as alternative. Following a 2 hr access period, the graduated tubes were removed from the home cage and replaced with water bottles. The amount consumed was recorded daily (± 0.1 ml) and body weights were recorded each week. A modified sucrose fading procedure was used to initiate ethanol drinking (Samson, 1986). This procedure is routinely used in our lab to help mice establish baseline intake (Becker and Lopez, 2004, Lopez and Becker, 2005). Mice were initially trained to drink 10% ethanol (v/v) compounded with 5% sucrose (w/v) in water. The sucrose fading procedure employed corresponded to the following schedule: 10% ethanol/5% sucrose for 2 days, 10% ethanol/2% sucrose for 3 days, and finally, 10% ethanol/0% sucrose for the rest of the experiment. The position of the ethanol and water tubes was alternated on a daily basis. Solutions were presented at room temperature and were prepared fresh each day by mixing ethanol and sucrose with deionized water to arrive at appropriate ethanol (v/v) and sucrose (w/v) concentrations. Throughout all of the experiments, mice were neither food nor water deprived.
Assessment of Blood Ethanol Concentration
Blood samples were collected immediately after the second and last (fourth) 16 hr ethanol exposure cycle for EtOH mice for analysis of blood ethanol concentration (BEC) using methods described before (Lopez and Becker, 2005). Briefly, blood samples (40 µl) were obtained from the retro-orbital sinus with heparinized capillary tubes. Blood samples were centrifuged to obtain 5 µl of plasma and ethanol concentration was measured using an Analox Instrument analyzer (Lunenburg, MA).
Data Analysis
Dependent variables for each experiment were analyzed using mixed factorial ANOVA. Whenever a significant main effect or interaction was observed, further comparisons were conducted using the Newman-Keuls post-hoc test. For all analyses, significance levels were set at p< 0.05.
RESULTS
Experiment 1a: Sensitization of Ethanol Withdrawal HIC Activity in HAP-1/LAP-1 and C3H/He Male Mice
BEC values were averaged by strain and analyzed in a one-way ANOVA (HAP-1 vs. LAP-1 vs. C3H). This analysis indicated that there were no significant differences in BEC values between strains [F(2,20)=3.34, p> 0.05]. BEC values were 179.66 ± 19.61, 216.18 ± 16.03, and 162.52 ± 4.05 mg/dl, for HAP-1, LAP-1, and C3H mice respectively (values are mean ± SEM).
HIC scores recorded for HAP-1, LAP-1, and C3H mice as a function of Group (EtOH vs. CTL) and Time (hour) during each of the four withdrawal cycles are shown in Figure 1 Overall, HIC scores were higher in ethanol-exposed mice compared to controls and HIC activity increased over time following removal from the inhalation chambers for EtOH groups. Further analysis of the data was conducted evaluating the area under the curve (AUC) for the first 7 hours of ethanol withdrawal in EtOH and CTL groups of HAP-1, LAP-1, and C3H mice as a function of withdrawal cycle. The mixed factorial ANOVA [Genotype (3)×Group (2)×Cycle (4)] revealed a significant main effect of Genotype, with overall HIC activity significantly lower in HAP-1 mice compared to LAP-1 and C3H mice [F(2,37)= 4.14, p< 0.05]. ANOVA also revealed a significant Genotype×Group×Cycle interaction [F(6,111)= 2.42, p< 0.05]. As illustrated in Figure 2, post hoc analyses indicated that C3H mice showed the expected sensitization profile (significant increase in HIC activity over successive withdrawal cycles). HAP-1 mice showed a similar trend, but this effect was not statistically significant. LAP-1 mice evidenced no change in HIC activity over the repeated withdrawal cycles. Thus, results from this study indicate that only the C3H mice exhibited withdrawal sensitization, with HAP-1 mice evidencing a non-significant trend for such an effect.
Figure 1.
Handling-induced convulsion scores (HIC) recorded hourly for 7 hours during each cycle of intermittent ethanol vapor exposure for EtOH and CTL HAP-1, LAP-1 and C3H male mice. Values are mean ± SEM.
Figure 2.
Area under the curve (AUC) for handling induced convulsions (HIC) scores for each cycle of 7 hours of ethanol withdrawal testing for LAP-1, HAP-1 and C3H male mice. Values are mean ± SEM.
* indicates a significant difference from CTL group within the same strain during that particular cycle
1, 2, 3 and 4 indicate difference from cycle 1, 2, 3 or 4 respectively, in AUC values within the same group for each strain.
# indicates difference between this particular group and strain when compared to C3H mice
Experiment 1b: Sensitization of Ethanol Withdrawal HIC Activity in HAP-2/LAP-2 and C3H/He Male and Female Mice
Blood ethanol concentrations were analyzed for male and female mice in an ANOVA with Sex (2) and Genotype (3) as main factors. This analysis indicated a significant main effect of sex [F(1,41)=22.23, p<0.001] and a significant interaction between sex and genotype [F(2,41)=11.10, p<0.001]. Post-hoc comparisons indicated that females HAP-2 and LAP-2 had significantly lower BEC values than males HAP-2 and LAP-2. BEC values for C3H males and females were similar. Separate follow-up ANOVAs for males and females were done. For males, ANOVA indicated there were no significant differences in BEC [F(2,19)=3.14, p> 0.05]. BEC values were 221.21 ± 23.30, 229.10 ± 17.81, and 174.42 ± 8.93 mg/dl, for HAP-2, LAP-2, and C3H mice, respectively (values are mean ± SEM). For females, ANOVA indicated a significant effect of strain [F(2,22)=11.95, p<0.01]. Post-hoc comparisons indicated that BEC in C3H mice were significantly higher than in HAP-2 and LAP-2 mice. BEC values (mean ± SEM) were, 143.67 ± 12.22, 131.23 ± 8.20, and 192.92 ± 7.63 mg/dl for HAP-2, LAP-2, and C3H mice, respectively.
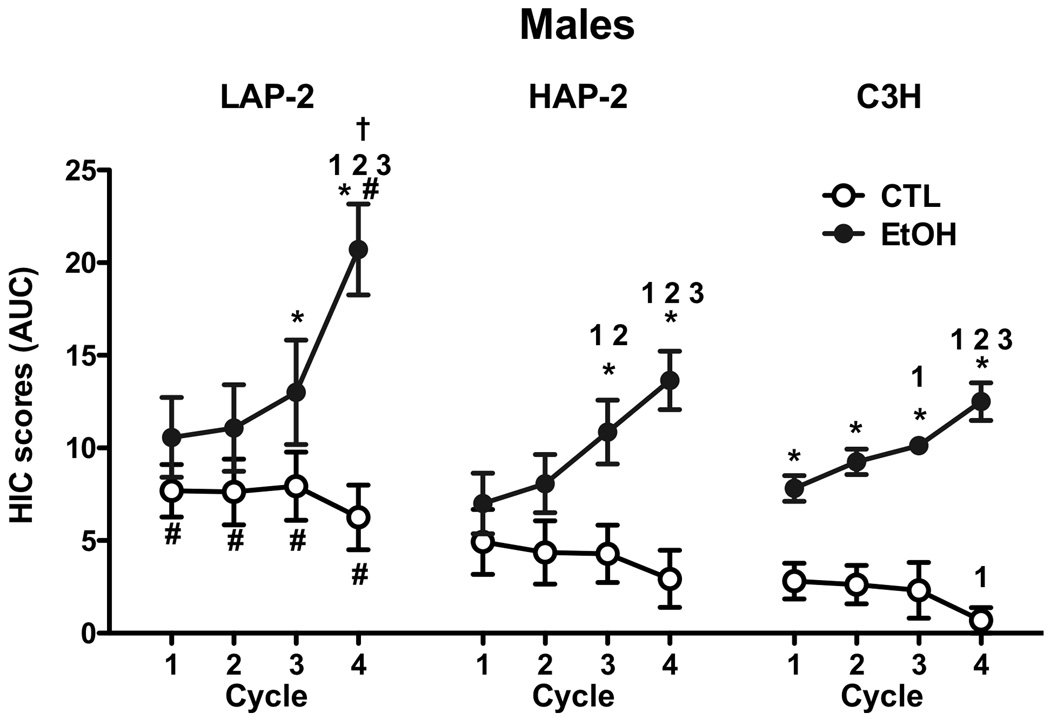
Analysis of HIC activity (7 hr AUC) was first analyzed with an overall ANOVA that included Sex (2), Genotype (3), and Group (2) as between factors and Cycle (4) as repeated measure. This ANOVA indicated a significant Sex×Group×Cycle interaction [F(3,246)=16.53, p<.001), and post-hoc analyses revealed significantly greater HIC activity in males compared to females during Cycle 4. HIC activity was also evaluated for male (Figure 3) and female (Figure 4) mice separately. For males, ANOVA indicated a significant Genotype×Group×Cycle interaction [F(6,117)=2.21, p<0.05]. This interaction allowed for further analyses with 2-way ANOVAs to compare HIC scores for EtOH and CTL groups within each genotype (Group×Cycle) and to compare across the genotypes for EtOH and CTL groups separately (Genotype×Cycle). For LAP-2 males, ANOVA indicated a significant Group×Cycle interaction [F(3,39)= 19.37, p< 0.001], and post-hoc analysis indicated that EtOH mice evidenced higher HIC scores than CTL mice during Cycles 3 and 4 (ps< 0.05). Evidence for sensitization of the HIC response is supported by the fact that HIC activity for the EtOH mice was significantly higher in Cycle 4 than in Cycles 1, 2, and 3 (ps< 0.05). For HAP-2 male mice, ANOVA also indicated a significant Group×Cycle interaction [F(3,36)= 15.56, p< 0.001]. Post-hoc comparisons indicated that HIC scores were higher in the EtOH group compared to CTL on Cycles 3 and 4, and the ethanol-exposed HAP-2 mice displayed significantly higher HIC activity during Cycles 3 and 4 compared to earlier cycles (all ps< 0.05). As expected, C3H mice exhibited progressive intensification of HIC activity over successive withdrawal cycles. ANOVA revealed a significant Group×Cycle interaction [F(3,42)= 12.93, p< 0.001], and post-hoc comparisons showed that HIC scores for the EtOH group were higher than CTL in every cycle (ps< 0.05). Further, HIC scores for the EtOH group were higher in Cycle 3 compared to Cycle 1 and higher in Cycle 4 compared to all previous cycles, while for the CTL group HIC scores were lower in Cycle 4 when compared to the first cycle (ps< 0.05). Analysis of EtOH groups (across all genotypes tested) revealed a significant Genotype×Cycle interaction [F(6,57)= 3.48, p< 0.01]. Post-hoc comparisons indicated that HIC scores for LAP-2 mice during Cycle 4 were higher than for HAP-2 and C3H mice (p< 0.05). Analysis of CTL groups indicated significant main effects of Genotype [F(2,20)= 3.74, p< 0.05] and Cycle [F(3,60)= 6.19, p< 0.01]. Post-hoc comparisons indicated that HIC scores for CTL groups were higher in LAP-2 mice compared to C3H mice, and overall HIC scores were lower during Cycle 4 when compared to all previous cycles (ps< 0.05).
Figure 3.
Area under the curve (AUC) for handling induced convulsions (HIC) scores for each cycle of 7 hours of ethanol withdrawal testing for LAP-2, HAP-2 and C3H male mice. Values are mean ± SEM.
* indicates a significant difference from CTL group within the same strain during that particular cycle
1, 2 and 3 indicate difference from cycle 1, 2 or 3, respectively, in AUC values within the same group for each strain.
# indicates difference between this particular group and strain when compared to C3H mice
† indicates HAP-2 ≠, LAP-2
Figure 4.
Area under the curve (AUC) for handling induced convulsions (HIC) scores for each cycle of 7 hours of ethanol withdrawal testing for LAP-2, HAP-2 and C3H female mice. Values are mean ± SEM.
* indicates a significant difference from CTL group within the same strain during that particular cycle
1 indicate difference from cycle 1 in AUC values within the same group for each strain.
# indicates difference between this particular group and strain when compared to C3H mice
† indicates HAP-2 ≠, LAP-2
A similar strategy was used for analysis of HIC activity in female mice (Figure 4). As in the case for males, ANOVA of female data indicated a significant 3-way interaction between Genotype, Group and Cycle [F(6,129)= 2.78, p< 0.05]. Separate analysis of LAP-2 females revealed significant main effects of Group [F(1,14)= 22.65, p< 0.001] and Cycle [F(3,42)= 10.55, p< 0.001], but no Group×Cycle interaction [F(3,42)= 2.53, p= 0.07]. Post hoc comparisons indicated that EtOH mice displayed higher HIC scores than CTL and that overall HIC activity was lower during Cycle 1 compared to Cycle 4 (ps< 0.05). For female HAP-2 mice, ANOVA indicated a significant Group×Cycle interaction [F(3,42)= 20.96, p< 0.001], and post hoc comparisons indicated that HIC scores were different between CTL and EtOH mice in Cycle 1, and were lower for EtOH mice in Cycle 1 compared to all other cycles (ps< 0.05). Analysis of C3H mice also indicated a significant Group×Cycle [F(3,45)= 4.15, p< 0.001]. Post hoc analyses indicated that ethanol-exposed C3H mice evidenced higher HIC scores than CTL during all test cycles and, in support of a sensitized HIC response, HIC scores for the EtOH mice were also higher in Cycle 4 compared to Cycle 1 (p< 0.05).
Experiment 2: Voluntary Ethanol Intake in Dependent and Control HAP-2/LAP-2 and C57BL/6J Mice
BEC levels during each of the two exposure cycles for all mice tested (HAP-2/LAP-2 male, HAP-2/LAP-2 female, and C57BL/6J male mice) are presented in Table 1. Analysis of these data revealed a significant Genotype/Sex×Cycle interaction [F(4,31)= 9.94, p< 0.05]. Post hoc tests indicated that during the first ethanol exposure cycle, BEC levels were significantly higher in C57 and LAP-2 male mice compared to all other groups (ps<0.05). C57 mice and LAP-2 males had similar BEC during cycle 1. Additionally, BEC values were significantly higher in male compared to female HAP-2 and LAP-2 lines (p< 0.05). During the second exposure cycle, BEC levels were significantly lower for LAP-2 female compared to all other groups (ps< 0.05). Finally, within-group comparisons indicated that BECs were higher during the first compared to the second exposure cycle in C57 male and LAP-2 male groups (ps< 0.05).
Table 1.
Blood ethanol levels (mg/dl) registered during each cycle of chronic intermittent ethanol vapor exposure.
| Group | Cycle 1 | Cycle 2 | N |
|---|---|---|---|
| C57BL/6J male | 283.31 ± 9.50 | 187.51 ± 9.12 | 8 |
| HAP-2 male | 216.03 ± 15.39 | 195.09 ± 12.83 | 7 |
| HAP-2 female | 155.36 ± 8.48 | 168.05 ± 8.52 | 7 |
| LAP-2 male | 269.67 ± 20.67 | 197.38 ± 17.20 | 6 |
| LAP-2 female | 137.88 ± 9.35 | 116.38 ± 7.05 | 8 |
Values are mean ± SEM. See text for details on statistical analyses.
Mean ethanol intake (g/kg) was averaged over the last five days of Baseline, and the five days of each test cycle (Test 1 and Test 2) for male and female HAP-2 and LAP-2 mice as well as male C57 mice (Figure 5). Preliminary analyses of the data that included days as repeated measure indicated that daily ethanol intake did not vary significantly within each phase of the study (Baseline, Test 1 and Test 2). As can be seen, Baseline ethanol intake replicated the profile expected for voluntary ethanol (10%) consumption in HAP-2, LAP-2, and C57 mice (HAP-2>C57>LAP-2). Since mice of different Genotype/Sex group received different levels of ethanol exposure, ethanol intake was evaluated in separate ANOVA for each Genotyope/Sex group. Independent 2-way ANOVA with Group (CTL vs. EtOH) and Cycle (Baseline, Test 1, Test 2) were conducted for each group of mice (male and female HAP-2 and LAP-2 and C57 male mice). In every case ANOVA failed to show a significant interaction between the factors under analysis. However, post-hoc analysis based on the error term of the interaction indicated significant differences between the CTL and EtOH group in Test 2 for the HAP-2 males and Tests 1 and 2 for C57 males. Additionally, one-way ANOVA analyses were conducted to compare CTL and EtOH groups’ level of intake during Tests 1 and 2 versus baseline for each group of mice. In all groups, ethanol intake for CTL mice during the test periods did not differ from baseline levels of intake. Chronic ethanol exposure/withdrawal resulted in a significant increase in ethanol intake in Male HAP-2 [F(2,12) = 4.99, p<0.025] during Test 2 while LAP-2 mice showed an increase in ethanol intake that failed to reach statistical significance [F(2,12) = 2.14, p=.16]. C57 male mice assigned to the EtOH group showed a significant increase in ethanol intake after each intermittent ethanol vapor exposure [F(2,14) = 5.29, p<0.025]. In contrast, HAP-2 and LAP-2 females did not show changes in ethanol intake as a consequence of chronic ethanol exposure.
Figure 5.
Ethanol intake levels (g/kg) during the limited access session of baseline and testing periods (Test 1 and 2). Values are mean ± SEM.
* indicates a significant difference from baseline (p<0.05)
Intermittent ethanol vapor exposure failed to affect water intake or to modify baseline levels of ethanol preference in HAP-2, LAP-2 and C57 mice (data not shown).
DISCUSSION
Results from these experiments are in agreement with previous studies demonstrating an inverse relationship between propensity to voluntarily drink ethanol and sensitivity to ethanol effects (Chester et al., 2003a; Grahame et al., 2001; Grahame et al., 2000). In this particular study, male and female mice selectively bred for high ethanol preference (HAP-1 and HAP-2) exhibited lower susceptibility to withdrawal seizure (HIC) activity when compared to mice selectively bred for low ethanol preference (LAP-1 and LAP-2). In turn, LAP-2 of both sexes evidenced relatively high susceptibility to ethanol withdrawal-induced HIC activity compared to C3H/He mice that were included in the study as a standard inbred strain for comparison. C3H/He mice, which exhibit an intermediate level of ethanol preference (Metten et al., 1998), displayed an intermediate withdrawal seizure (HIC) response. By and large, these findings confirm those of previous studies that demonstrated an inverse genetic correlation between sensitivity to ethanol withdrawal and voluntary ethanol intake (Chester et al., 2003a; Chester et al., 2002; Dess et al., 2005; Metten and Crabbe, 2005; Metten et al., 1998). The present studies expand on the previous work in two important ways: first, we assessed changes in sensitivity to withdrawal-related CNS hyperexcitability following repeated ethanol exposure in mice that differed in baseline ethanol withdrawal sensitivity and baseline ethanol intake, and second, we assessed the effect of repeated ethanol exposure on subsequent ethanol intake in three genotypes, HAP-2, LAP-2, and C57BL/6J mice.
Sensitization of ethanol withdrawal HIC activity was demonstrated in all genotypes tested, as evidenced by HIC activity during the final withdrawal cycle being greater than during the first withdrawal period. This effect was most evident in C3H/He mice as well as LAP-2 and HAP-2 males. A sex-related difference in expression of sensitized HIC activity following repeated withdrawal experience was observed in all genotypes tested. As reported in previous studies, C3H/He females exhibited a more modest sensitization response (Veatch et al., 2007). There were also some differences in HIC activity between groups that were not exposed to ethanol. LAP-2 control males showed a higher level of seizure activity than C3H/He male mice. Taken together, these findings suggest that increases in withdrawal HIC severity as a function of experience with repeated episodes of ethanol exposure and withdrawal are apparently not related systematically to either baseline HIC activity or to baseline ethanol intake. That baseline ethanol intake and baseline ethanol withdrawal susceptibility are inversely genetically correlated would suggest that ethanol withdrawal is protective: that is, it prevents animals from drinking ethanol. Data consistent with this interpretation were obtained by Chester, Blose, and Froehlich (2005). Twice daily intragastric intubation sufficient to cause symptoms of ethanol withdrawal nonetheless reduced subsequent ethanol intake in high-drinking HAD-2 rats, but not low-drinking LAD-2 rats. Contrasting strongly with this datum are findings from Becker and colleagues (Becker and Lopez, 2004; Griffin et al., 2009a; Griffin et al., 2009b; Lopez and Becker, 2005) as well other groups (Dhaher et al., 2008; Finn et al., 2007; O'Dell et al., 2004; Roberts et al., 1996; Roberts et al., 2000; Valdez et al., 2002) showing that ethanol vapor exposure increases subsequent ethanol consumption and ethanol seeking behavior. Results of these studies are consistent with the interpretation that chronic ethanol exposure and withdrawal experience increases subsequent ethanol seeking and consumption, perhaps through negative reinforcement-like mechanisms. On the other hand, it is not clear precisely which effects of ethanol (and/or withdrawal) increase subsequent ethanol consumption, because ethanol has many actions when given repeatedly over long periods of time, such as causing affective changes (Gilpin et al., 2008), ataxia, aphagia, and tolerance to these effects (Griffiths et al., 1973), along with the widely reported withdrawal seizures and hyperexciteability (Becker, 2000).
Seen in this context, the present studies are important because we assessed changes in ethanol intake in mice that differed greatly in baseline intake as well as withdrawal seizure susceptibility. Baseline ethanol intake levels showed the expected genetic differences; i.e., significantly higher consumption in HAP than in LAP mice (Grahame et al., 1999a; Grahame et al., 1999b; Oberlin et al., 2010). Inbred male C57L/6J mice showed intake levels in between that of HAP and LAP lines. Chronic intermittent ethanol exposure produced a significant increase in ethanol (10% v/v) intake in C57BL/6J mice. This result replicates previous findings with this mouse strain using a higher ethanol solution for intake test (15% v/v, Becker and Lopez, 2004; Finn et al., 2007; Griffin et al., 2009a; Griffin et al., 2009b; Lopez and Becker, 2005). HAP-2 male mice also showed an increase in voluntary ethanol intake after chronic intermittent ethanol exposure, while females did not. Induction of ethanol dependence in LAP-2 male mice produced a mild but not significant increase in ethanol intake. These findings are inconsistent with those of Chester et al. (2005) in showing increases, rather than decreases in alcohol consumption following ethanol exposure in the selectively bred High Preferring lines. However, they are consistent with previous findings indicating that the high drinking, but low-withdrawal susceptible C57BL/6J strain (this strain showed the least alcohol withdrawal of 15 strains tested by Metten and Crabbe, 2005) demonstrates increased alcohol consumption following chronic intermittent ethanol vapor exposure. This suggests that mouse genotypes with a relatively high propensity to drink ethanol, but a tempered sensitivity to withdrawal symptoms may be optimal for demonstrating escalation of drinking in this dependence model.
There are at least two plausible interpretations of this pattern. First, animals may need to encounter ethanol’s pharmacological actions when consuming ethanol in order to show dependence/withdrawal-related increases in alcohol intake. That is, a sufficient amount of ethanol needs to be consumed for the negative reinforcing capacity of the drug to be established. According to this view, mice not consuming sufficient quantities of ethanol to obtain its pharmacological effects (such as the LAP-2 mice tested here, who drink ethanol at a rate far below the murine capacity for ethanol metabolism) did not increase their ethanol intake, in spite of relatively severe ethanol withdrawal, because they would not consume a sufficient amount of ethanol to allow for a decrease in ethanol withdrawal resulting from ingested ethanol. HAP-2 and C57BL/6J mice, on the other hand, likely encounter the pharmacological effects of ethanol when consuming it (Grahame et al., 1999a), making it possible for these animals to show dependence/withdrawal-related increases in consumption. It is also possible that the positive reinforcing effects of ethanol need to be firmly established before the negative reinforcing effects of the drug develop with continued ethanol exposure (Meisch, 1983; Meisch, 1984). In this case, the relatively low amount of ethanol consumed along with nominal resultant BEC levels registered in LAP mice may preclude the ability of ethanol to serve as both a positive and negative reinforcer in these mice.
A second plausible interpretation of the current findings is that some effect of ethanol exposure other than withdrawal-related convulsions underlies ethanol dependence/withdrawal- induced increases in ethanol consumption and self-administration. That is, because animals most likely to show increases in drinking were least likely to show withdrawal-related convulsions, these changes themselves are unlikely candidates for mediating increases in drinking seen following repeated cycles of chronic intermittent ethanol exposure. Other withdrawal-related changes, such as alterations in mood and affect (Koob, 2009) or non-withdrawal-related changes such as chronic tolerance (e.g., Kalant, 1993) may be involved. This interpretation would be consistent with the fact that a genetic susceptibility to withdrawal-related convulsions is protective against ethanol consumption (see discussion above).
Tempering these conclusions is the fact that we were unable to completely equate blood ethanol concentrations across genotypes in these studies. The level of chronic ethanol vapor exposure in these studies was set to induce reliable withdrawal sensitization in C3H/He mice (Becker and Hale, 1993; Veatch et al., 2007) and escalation of ethanol intake in C57BL/6J mice (Griffin et al., 2009a; Lopez and Becker, 2005). In the first experiment, blood ethanol levels recorded in all three genotypes were similar for male mice. Blood ethanol levels recorded in HAP-2 and LAP-2 female mice were lower than in C3H/He females. However, there was no difference in blood ethanol levels between HAP-2 and LAP-2 females. Therefore, differences in withdrawal HIC scores between these selected lines cannot be related to differences in intensity of ethanol intoxication. For the second experiment, there were some significant differences in blood ethanol levels between groups defined by strain and sex during the two cycles of chronic intermittent ethanol vapor exposure. However, these differences are not likely related to the profile of results observed for voluntary ethanol intake in ethanol-dependent mice. During the first cycle of exposure, the level of intoxication in C57BL/6J mice was higher than in male and female HAP-2 and female LAP-2. This may explain why C57BL/6J male mice showed an increase in ethanol intake during Test 1 that was not observed in these other groups exposed to ethanol vapor. However, blood ethanol levels were not significantly different between C57BL/6J and LAP-2 males but only LAP-2 males did not show elevated ethanol intake. Importantly, in Test 2, blood alcohol levels were similar in all sexes and genotypes, except LAP-2 females. Thus, the important outcomes observed in Test 2 – increases in ethanol consumption following ethanol vapor in HAP-2 males, and C57BL/6J males, but not in the other genotype/sex combinations, are unlikely to be due to differences in ethanol exposure levels among these genotypes. It has been suggested that there is a threshold level of intoxication during ethanol vapor exposure required to induce increased voluntary ethanol intake in C57BL/6J mice (Griffin et al., 2009a). Future studies can evaluate whether the intensity of ethanol-withdrawal induced seizures and elevated ethanol intake in HAP-2 and LAP-2 to would vary as a function of ethanol intoxication during ethanol vapor exposure cycles.
Overall, these results indicate a genetic association between high sensitivity to ethanol withdrawal seizures and low ethanol preference. In addition, increases in voluntary ethanol intake following chronic intermittent ethanol vapor exposure are more likely to be observed in genotypes with lowest baseline levels of ethanol withdrawal.
Acknowledgements
Supported by National Institute on Alcohol Abuse and Alcoholism grants AA014095 and AA10761 to HCB, AA13483 to NJG, and AA015512 to Lawrence Lumeng.
The authors wish to thank Laura A. Ralston for excellent technical support.
REFERENCES
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Hale RL. Exacerbation of ethanol withdrawal seizures in mice with a history of multiple withdrawal experience. Pharmacol Biochem Behav. 1997;57:179–183. doi: 10.1016/s0091-3057(96)00303-6. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal "kindling". Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Littleton JM. The alcohol withdrawal "kindling" phenomenon: clinical and experimental findings. Alcohol Clin Exp Res. 1996;20:121A–124A. doi: 10.1111/j.1530-0277.1996.tb01760.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Further evidence of an inverse genetic relationship between innate differences in alcohol preference and alcohol withdrawal magnitude in multiple selectively bred rat lines. Alcohol Clin Exp Res. 2003a;27:377–387. doi: 10.1097/01.ALC.0000056619.98553.50. [DOI] [PubMed] [Google Scholar]
- Chester JA, Lumeng L, Li TK, Grahame NJ. High- and low-alcohol-preferring mice show differences in conditioned taste aversion to alcohol. Alcohol Clin Exp Res. 2003b;27:12–18. doi: 10.1097/01.ALC.0000046340.06154.9F. [DOI] [PubMed] [Google Scholar]
- Chester JA, Price CS, Froehlich JC. Inverse genetic association between alcohol preference and severity of alcohol withdrawal in two sets of rat lines selected for the same phenotype. Alcohol Clin Exp Res. 2002;26:19–27. [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Effects of chronic alcohol treatment on acoustic startle reactivity during withdrawal and subsequent alcohol intake in high and low alcohol drinking rats. Alcohol Alcohol. 2005;40(5):379–387. doi: 10.1093/alcalc/agh172. [DOI] [PubMed] [Google Scholar]
- Crabbe J, Kosobud A. Alcohol withdrawal seizures: Genetic animal models. In: Porter RJ, Mattson RH, Cramer JA, Diamond I, editors. Alcohol and Seizures: Basic Mechanisms and Clinical Concepts. Philadelphia: F.A. Davis Company; 1990. pp. 126–139. [Google Scholar]
- Dess NK, O'Neill P, Chapman CD. Ethanol withdrawal and proclivity are inversely related in rats selectively bred for differential saccharin intake. Alcohol. 2005;37:9–22. doi: 10.1016/j.alcohol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcohol Clin Exp Res. 2008;32:197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK. Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol Biochem Behav. 1988;31:215–222. doi: 10.1016/0091-3057(88)90336-x. [DOI] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Chapter 9:Unit 9. Curr Protoc Neurosci. 2008:29. doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Chester JA, Rodd-Henricks K, Li TK, Lumeng L. Alcohol place preference conditioning in high- and low-alcohol preferring selected lines of mice. Pharmacol Biochem Behav. 2001;68:805–814. doi: 10.1016/s0091-3057(01)00476-2. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L. Limited access alcohol drinking in high- and low-alcohol preferring selected lines of mice. Alcohol Clin Exp Res. 1999a;23:1015–1022. [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L. Selective breeding for high and low alcohol preference in mice. Behav Genet. 1999b;29:47–57. doi: 10.1023/a:1021489922751. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Rodd-Henricks K, Li TK, Lumeng L. Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high- and low-alcohol preferring mice. Psychopharmacology (Berl) 2000;151:252–260. doi: 10.1007/s002130000388. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009a;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology. 2009b;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths PJ, Littleton JM, Ortiz A. A method for the induction of dependence to ethanol in mice. British Journal of Pharmacology. 1973;47:669P–670P. [PMC free article] [PubMed] [Google Scholar]
- Kalant H. Problems in the search for mechanisms of tolerance. Alcohol Alcohol. 1993 Suppl 2:1–8. [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56 Suppl 1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Herron JE, Anton RF, Roberts J, Moore J. Recurrent detoxification may elevate alcohol craving as measured by the Obsessive Compulsive Drinking scale. Alcohol. 2000a;20:181–185. doi: 10.1016/s0741-8329(99)00073-7. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Roberts JS, Wang W, Myrick H, Anton RF. Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol. 2000b;22:159–164. doi: 10.1016/s0741-8329(00)00114-2. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Harris RA, Schuckit MA. Genetic factors influencing alcohol dependence. Br J Pharmacol. 2008;154:275–287. doi: 10.1038/bjp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisch R. Relationship between physical dependence on ethanol and reinforcing properties of ethanol in animals. NIAAA Research Monographs. 1983;13:27–32. [Google Scholar]
- Meisch RA. Alcohol self-administration by experimental animals. In: Smart RG, Cappell HD, Glaser FB, Israel Y, Kalant H, Popham RE, Schmidt W, Sellers EM, editors. Research Advances in Alcohol and Drug Problems. vol 8. New York: Plenum Press; 1984. pp. 23–45. [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behavioral Neuroscience. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Matson LM, Henderson AN, Grahame NJ. Derivation and characterization of replicate High- and Low- Alcohol Preferring lines of mice and a high-drinking Crossed HAP line. Behav Genet. 2010 doi: 10.1007/s10519-010-9394-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Rezvani AH, Parsian A. Behavioural features of alcohol-preferring rats: focus on inbred strains. Alcohol Alcohol. 1999;34:378–385. doi: 10.1093/alcalc/34.3.378. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Malott DH, Prather LK, Niehus DR, Cunningham CL. Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology (Berl) 1994;116:207–216. doi: 10.1007/BF02245064. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased Ethanol Self-Administration and Anxiety-Like Behavior During Acute Ethanol Withdrawal and Protracted Abstinence: Regulation by Corticotropin-Releasing Factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcohol Clin Exp Res. 2007;31:477–485. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]