Abstract
The association between pre-transplant serum albumin concentration and post-transplant outcomes in kidney transplant recipients is unclear. We hypothesized that in transplant-waitlisted hemodialysis patients, lower serum albumin concentrations are associated with worse post-transplant outcomes.
Linking the 5-year patient data of a large dialysis organization (DaVita) to the Scientific Registry of Transplant Recipients, we identified 8961 hemodialysis patients who underwent first kidney transplantation. Mortality or graft failure and delayed graft function (DGF) risks were estimated by Cox regression (hazard ratio [HR]) and logistic regression (Odds ratio [OR]), respectively.
Patients were 48±13 years old and included 37% women and 27% diabetics. The higher pre-transplant serum albumin was associated with lower mortality, graft failure and DGF risk even after multivariate adjustment for case-mix, malnutrition-inflammation complex and transplant related variable. Every 0.2 g/dL higher pre-transplant serum albumin concentration was associated with 13% lower all-cause mortality (HR=0.87 [95% confidence interval: 0.82-0.93]), 17% lower cardiovascular mortality (HR=0.83[0.74-0.93]), 7% lower combined risk of death or graft failure (HR=0.93[0.89-0.97]), and 4% lower DGF risk (OR=0.96[0.93-0.99]).
Hence, lower pre-transplant serum albumin level is associated with worse post-transplant outcomes. Clinical trials to examine interventions to improve nutritional status in transplant-wait-listed hemodialysis patients and their impacts on post-transplant outcomes are indicated.
Keywords: Hypoalbuminemia, kidney transplantation, malnutrition-inflammation complex, mortality, cardiovascular death, graft failure, delayed graft function (DGF)
Introduction
In patients with advanced chronic kidney disease (CKD) who require maintenance dialysis treatment, kidney transplantation is the treatment of choice and usually associated with better outcomes than dialysis therapy. (1-4) However, unfavorable post-transplant outcomes such as cardiovascular events, mortality or graft failure requiring dialysis treatment are still not uncommon.(5-7) Many previous studies examined predictors of mortality or graft failure in kidney transplant recipients, but only few analyzed the association between pre-transplant parameters during dialysis therapy era and post-transplant outcomes.(8-11) Another unfavorable post-transplant event is delayed graft function (DGF) in the immediate post-transplantation period, defined as the need for at least one session of dialysis treatment in the first week after receiving a kidney transplant.(12) The occurrence of DGF may significantly complicate the immediate and long-term post-transplant management by increasing morbidity and mortality,(13, 14) prolonging patient hospitalization (15) and inflating health care costs.(15-17)
In maintenance dialysis patients a low serum albumin level is a marker of more severe co-morbidity, worse general health status and malnutrition-inflammation complex, also known as protein-energy wasting (PEW).(18) Hypoalbuminemia is a strong predictor of both cardiovascular (19) and all-cause mortality(20) in the entire range of CKD including non-dialysis dependent CKD patients and those undergoing dialysis treatment.(21) However, the association between pre-transplant hypoalbuminemia during dialysis therapy of post-transplant outcomes is no clear. It is possible that during the dialysis period conditions related to PEW cause irreversible damage in kidney-transplant waitlisted patients contributing to increased risk of adverse outcomes after transplantation. To our knowledge no study has thoroughly examined the association between pre-transplant serum albumin level and short-term outcome such as DGF and long-term outcomes such as mortality, and graft failure after kidney transplantation. We hypothesized that lower pre-transplant serum albumin during the weeks immediately prior to kidney transplantation is associated with worse post-transplant patient and graft survival and DGF in a large prospective cohort of incident kidney transplant recipients across the United States.
Materials and Methods
Patients
We linked data of all kidney transplant recipients listed in the Scientific Registry of Transplant Recipients (SRTR) up to June 2007 to a list of individuals with CKD who underwent maintenance hemodialysis treatment from July 2001 to June 2006 in one of the outpatient dialysis facilities of a US-based large dialysis organization (DaVita Inc, prior to its acquisition of former Gambro dialysis facilities) using patients' social security numbers. The study was approved by the Institutional Review Committees of both Los Angeles Biomedical Research Institute at Harbor-UCLA and DaVita Clinical Research.
Clinical and Demographic Measures
The creation of the national DaVita hemodialysis patient cohort has been described previously.(22-26) Demographic data and details of medical history were collected, when information on age, gender, race, type of insurance, marital status, presence of diabetes, height, post-hemodialysis dry weight (to calculate averaged body mass index [BMI]) and dialysis vintage. Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the day of kidney transplantation.
Laboratory Measures
Blood samples were drawn using uniform techniques in all of the DaVita dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida, typically within 24 hours. All laboratory values were measured by automated and standardized methods in the DaVita Laboratory. Most laboratory values were measured monthly, including serum urea, creatinine, albumin, calcium, phosphorus, bicarbonate, and total iron binding capacity (TIBC). Serum ferritin and intact PTH were measured at least quarterly. Hemoglobin was measured at least monthly in essentially all patients and weekly to bi-weekly in most patients. Most blood samples were collected pre-dialysis with the exception of the post-dialysis serum urea nitrogen to calculate urea kinetics. Kt/V (single pool) was calculated using urea kinetic modeling equations as described elsewhere.(24) The last 3-month-averaged pre-transplant serum albumin was used in our analyses. All measured serum levels were averaged into one single value. We divided pre-transplant serum albumin into almost equal quintiles (< 3.77, 3.77-<3.97, 3.97-<4.13, 4.13-<4.3 and ≥4.3 g/dL).
Statistical Methods
Data were summarized using proportions, means (±standard deviation [SD]) or medians (interquartile range [IQR]) as appropriate. For all-cause and cardiovascular mortality and graft failure, defined as re-initiation of dialysis treatment or re-transplantation, time to event was used in all survival analyses. For the DGF, defined as the need for any dialysis therapy in the first week after transplantation,(12) time to event was not accounted for. Survival analyses to calculate hazard ratios (HR) and 95% confidence interval (95%CI) of death or graft failure employed Cox proportional hazards regression. In the mortality analyses the patients were followed until event (death) or censoring (graft failure or end of follow-up period) whichever happened first. In the graft failure analyses the patients were followed until event (graft failure) or censoring (death or end of follow-up period) whichever happened first. In the combined outcome analyses patients were followed until event (death or graft failure) or censoring (end of follow-up period) whichever happened first. We also tested the linearity all of survival models using fractional polynomials and restricted cubic splines. Logistic regression models were employed to estimate the odds ratio (OR) and 95%CI of post-transplant DGF. We also examined p-values for trends across quintiles of serum albumin.
For each regression analysis, four level of multivariate adjustment were examined: (I) A minimally adjusted (referred to as “unadjusted”) model that included serum albumin as the predictor and entry calendar quarter (q1 through q20) as the covariate; (II) Case-mix adjusted models that included the above plus age, gender, race-ethnicity (African Americans and other self-categorized Blacks, Non-Hispanic Whites, Asians, Hispanics and others), diabetes mellitus, dialysis vintage (<6 mo, 6 mo to 2 yrs, 2-<5 yrs and ≥5 yrs), primary insurance (Medicare, Medicaid, private and others), marital status (married, single, divorced, widowed and other or unknown), standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a dialysis catheter, and residual renal function during the entry quarter; (III) The malnutrition-inflammation-complex syndrome (MICS) adjusted models which included all of the covariates plus 10 surrogates of nutritional status and inflammation measured during the last calendar quarter before transplantation including body mass index (BMI) and 8 laboratory variables, i.e. nPCR as an indicator of daily protein intake, also known as the normalized protein nitrogen appearance (nPNA)(27),) and serum or blood concentrations of TIBC, ferritin, phosphorus, calcium, bicarbonate, peripheral white blood cell count (WBC), lymphocyte percentage, and hemoglobin; and (IV) Case-mix, MICS and transplant data adjusted models included all of the above plus 6 transplant-related variables: (1) donor type (deceased or living), (2) donor age, (3) panel reactive antibody (PRA) titer (last value prior to transplant), (4) number of HLA mismatches, (5) cold ischemia time and (6) extended donor criteria (EDC) using standard definition (donor history of hypertension and/or serum creatinine of donor > 1.5 mg/dL and/or cause of death in donor is cerebrovascular event). All analyses were carried out with SAS version 9.1, SAS Institute Inc., Cary, North Carolina and STATA version 11.1 (STATA Corporation, College Station, TX).
Results
The original 5-year (7/2001-6/2006) national database of all DaVita patients included 164,789 adult subjects. This database was linked via social security numbers to the national SRTR registry that included all transplant waitlisted people and kidney transplant recipients up to 06/2007. Out of 65,386 DaVita patients who were identified in the SRTR database, 17,629 had undergone one or more kidney transplantation during their life time, but only 14,508 dialysis patients had undergone their first kidney transplantation. After excluding those without electronically recorded serum albumin levels in the last quarter prior to transplantation (n=3128), peritoneal dialysis patients (n=2016) subjects who lacked data from the baseline quarter (n=330) and those with outlier values for age (n=73), there were 8,961 hemodialysis patients who underwent their first kidney transplantation during the observation period and who were followed until death, graft failure, loss of follow up, or survival until June 30th 2007. There were 719 deaths (8.0%) and 785 graft failures (8.8%) irrespective of subsequent deaths. The median follow-up time was 804 days (interquartile range was: 385-1329 days). The basic characteristics of waitlisted, but non-transplanted, patients have been described elsewhere.(28) Table 1 shows the clinical, demographic and laboratory data of the 8,961 transplanted hemodialysis patients across 5 quintiles of pre-transplant serum albumin levels. Hypoalbumiemic patients were older, included more women and diabetics, and had incrementally higher crude mortality, graft failure and DGF rates.
Table 1.
Quintiles of serum albumin in 8,961 hemodialysis patients who underwent renal transplantation between 7/2001 and 6/2006, including selected clinical and laboratory values in each group. (Mean±SD of serum albumin in the entire cohort was 4.02±0.37 g/dL)
| Albumin range (g/dl) [mean±SD] |
Total population | < 3.77 [3.49±0.32] |
3.77--<3.97 [3.88±0.06] |
3.97--<4.13 [4.05±0.04] |
4.13--<4.30 [4.23±0.05] |
>=4.30 [4.50±0.15] |
p-for trend |
|---|---|---|---|---|---|---|---|
| N [%] | 8961 [100] | 1834 [20] | 1768 [20] | 1789 [20] | 1892 [21] | 1678 [19] | |
| Age (years) | 48±13 | 51±13 | 50±13 | 49±13 | 47±13 | 43±13 | <0.001 |
| Deaths (n) [Crude Death Rate %] | 719 [8] | 234 [13] | 165 [9] | 137 [8] | 117 [6] | 66 [4] | <0.001 |
| Graft failure (n) [Crude Death Rate %] | 785 [9] | 205 [11] | 158 [9] | 139 [8] | 158 [8] | 125 [7] | <0.001 |
| DGF (n) [Crude DFG %] | 1951 [22] | 462 [25] | 376 [21] | 394 [22] | 404 [21] | 315 [19] | <0.001 |
| Gender (% women) | 37 | 47 | 45 | 38 | 33 | 24 | <0.001 |
| Race (% African-American) | 27 | 27 | 29 | 26 | 27 | 27 | 0.613 |
| Diabetes mellitus (%) | 27 | 36 | 31 | 28 | 23 | 16 | <0.001 |
| BMI (kg/m2) | 26.6±5.7 | 27.0±6.3 | 27.1±5.7 | 26.8±5.4 | 26.5±6.0 | 25.6±4.9 | <0.001 |
| Kt/V (dialysis dose) | 1.61±0.35 | 1.59±0.36 | 1.63±0.32 | 1.62±0.36 | 1.61±0.34 | 1.61±0.36 | 0.543 |
| Dialysis Vintage (%): | |||||||
| 0-6 months | 11 | 19 | 10 | 9 | 9 | 11 | <0.001 |
| 6-24 months | 27 | 27 | 26 | 27 | 27 | 28 | 0.296 |
| 2-5 years | 37 | 32 | 39 | 38 | 39 | 37 | 0.005 |
| >5 years | 25 | 21 | 25 | 26 | 25 | 25 | 0.097 |
| nPCR (g/kg/day), (mean ± SD) | 1.05±0.25 | 0.98±0.27 | 1.05±0.25 | 1.07±0.25 | 1.08±0.24 | 1.08±0.25 | <0.001 |
| KRU (ml/min) (mean ± SD) | 0.42±1.36 | 0.40±1.38 | 0.34±1.17 | 0.38±1.27 | 0.47±1.48 | 0.50±1.45 | <0.001 |
| Dialysis Catheter (%) | 17 | 24 | 16 | 15 | 15 | 14 | <0.001 |
| Serum creatinine (mg/dL) | 10.6±3.2 | 9.2±2.9 | 10.4±3.0 | 10.7±3.1 | 11.1±3.1 | 11.7±3.2 | <0.001 |
| Blood hemoglobin (g/dL) | 12.3±1.2 | 11.8±1.4 | 12.2±1.2 | 12.3±1.1 | 12.4±1.1 | 12.5±1.1 | <0.001 |
| WBC (×103/l) | 6.8±2.0 | 7.2±2.4 | 7.0±2.0 | 6.8±1.9 | 6.6±1.9 | 6.5±1.9 | <0.001 |
| Number of HLA mismatch | 3.5±1.8 | 3.5±1.9 | 3.6±1.8 | 3.6±1.8 | 3.6±1.8 | 3.5±1.9 | 0.355 |
| PRA (%) | 10.1±24.0 | 11±25 | 11±25 | 10±24 | 10±24 | 9±22 | 0.002 |
| Donor age (years) | 39±15 | 40±15 | 39±15 | 39±15 | 39±15 | 37±15 | <0.001 |
| Donor Type (% Living) | 32 | 35 | 31 | 30 | 30 | 34 | 0.174 |
| EDC kidney (%) | 19 | 21 | 19 | 19 | 19 | 14 | <0.001 |
| Cold Ischemia time (hours) | 14.4±10.6 | 14.1±11.2 | 14.6±10.6 | 14.9±10.4 | 14.3±10.3 | 13.9±10.7 | 0.454 |
Footnote:
Values in parentheses represent the proportion of the HD patients in each albumin category. Values in brackets indicate the crude death rate or crude graft failure rate in the indicated group during the 6 years of observation.
PRA: panel reactive antibody (last value prior to transplant). WBC: White Blood Cell count. BMI: Body Mass Index. EDC: Extended Donor Criteria KRU: residual renal function. nPCR: normalized protein catabolic rate
Table 2 shows the calculated HR of all-cause and cardiovascular death and/or graft failure for every 0.2 g/dL higher pre-transplant serum albumin level. Higher serum albumin was associated with 13% lower all-cause mortality over the 6-year observation period after adjusting for several relevant clinical and transplant-related variables. Similar associations were found for cardiovascular death. Every 0.2 g/dL higher pre-transplant serum albumin level was also associated with 4% higher graft failure risk and 7% lower combined risk of death or graft failure after full multivariable adjustment. To further examine the linearity of the association of pre-transplant serum albumin with post-transplant outcomes, we calculated HR across five quintiles of serum albumin using the lowest quintile (albumin <3.77 g/dL) as the reference as shown in Table 3. Similar associations were observed including 53% lower all-cause death risk, 74% lower cardiovascular death risk, and 32% lower combined graft failure and all-cause death risk in the highest vs. lowest serum albumin quintiles (Table 3). Figure 1 shows the cubic spline models for the association of the entire range of pre-transplant serum albumin with post-transplant outcomes consistent with the findings in Tables 2 and 3.
Table 2.
Hazard ratio (95% confidence intervals) of post-transplant death (all-cause or cardiovascular) or graft failure for each 0.2 g/dL higher pre-transplant serum albumin values using Cox regression analyses in 8961 hemodialysis patients who underwent renal transplantation and observed for up to 6 years (7/2001-6/2007)
| minimally adjusted | + case-mix adjusted* | + MICS adjusted** | + transplant data adjusted *** | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Graft failure censored all-cause death | 0.85 (0.82-0.87) | <0.001 | 0.86 (0.83-0.90) | <0.001 | 0.87 (0.83-0.91) | <0.001 | 0.87 (0.82-0.93) | <0.001 |
| Graft failure censored cardiovascular death | 0.81 (0.77-0.86) | <0.001 | 0.82 (0.76-0.88) | <0.001 | 0.83 (0.76-0.91) | <0.001 | 0.83 (0.74-0.93) | 0.001 |
| All-cause death censored graft failure | 0.92 (0.89-0.96) | <0.001 | 0.89 (0.86-0.93) | <0.001 | 0.91 (0.87-0.95) | <0.001 | 0.96 (0.90-1.02) | 0.150 |
| Combined all-cause death or graft failure | 0.90 (0.87-0.92) | <0.001 | 0.89 (0.86-0.92) | <0.001 | 0.91 (0.88-0.94) | <0.001 | 0.93 (0.89-0.97) | 0.002 |
Footnote:
Case-mix adjusted models adjusted for: age, gender, race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a dialysis catheter, and residual renal function during the entry quarter;
MICS adjusted models which included all of the covariates plus body mass index (BMI), the normalized protein nitrogen appearance (nPNA) and serum or blood concentrations of TIBC, ferritin, phosphorus, calcium, bicarbonate, peripheral white blood cell count (WBC), lymphocyte percentage, and hemoglobin;
Case-mix, MICS and transplant data adjusted models included all of the above plus donor type, donor age, panel reactive antibody (PRA) titer (last value prior to transplant), number of HLA mismatches, cold ischemia time and extended donor criteria (EDC)
Table 3.
Hazard ratio (95% confidence intervals) of post-transplant death and/or graft failure across the pre-transplant serum albumin categories using Cox regression analyses in 8961 hemodialysis patients who underwent renal transplantation and who were observed over a 6-year observation period (7/2001-6/2007): (A) Post-transplant graft failure censored all-cause death. (B) Post-transplant graft failure censored cardiovascular death. (C) All-cause death censored graft failure; and (D) Combined all mortality or graft failure
| Pre-Tx albumin | Minimally adjusted | Case-mix adjusted* | Case-mix & MICS adjusted** | Case-mix & MICS & Transplant data adjusted *** | ||||
|---|---|---|---|---|---|---|---|---|
| (g/dL) | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| (A) post-transplant graft failure censored all-cause death | ||||||||
| <3.77 (ref.) | 1.0 | n/a | 1.0 | n/a | 1.0 | n/a | 1.0 | n/a |
| 3.77- <3.97 | 0.68 (0.56-0.83) | <0.001 | 0.67 (0.55-0.83) | <0.001 | 0.73 (0.59-0.91) | 0.005 | 0.79 (0.60-1.04) | 0.094 |
| 3.97-<4.13 | 0.55 (0.44-0.68) | <0.001 | 0.55 (0.44-0.69) | <0.001 | 0.59 (0.46-0.75) | <0.001 | 0.65 (0.48-0.88) | 0.005 |
| 4.13- <4.30 | 0.46 (0.37-0.57) | <0.001 | 0.54 (0.43-0.68) | <0.001 | 0.57 (0.44-0.77) | <0.001 | 0.66 (0.48-0.91) | 0.011 |
| >=4.3 | 0.30 (0.23-0.40) | <0.001 | 0.44 (0.33-0.58) | <0.001 | 0.45 (0.33-0.62) | <0.001 | 0.47 (0.30-0.67) | <0.001 |
| (C) post-transplant graft failure censored cardiovascular death | ||||||||
| <3.77 (ref.) | 1.0 | n/a | 1.0 | n/a | 1.0 | n/a | 1.0 | n/a |
| 3.77- <3.97 | 0.59 (0.40-0.86) | 0.006 | 0.59 (0.40-0.87) | 0.007 | 0.64 (0.43-0.97) | 0.035 | 0.73 (0.44-1.21) | 0.218 |
| 3.97-<4.13 | 0.45 (0.30-0.68) | <0.001 | 0.44 (0.29-0.68) | <0.001 | 0.50 (0.32-0.79) | 0.003 | 0.58 (0.33-1.01) | 0.054 |
| 4.13- 4.3 | 0.25 (0.15-0.41) | <0.001 | 0.28 (0.17-0.47) | <0.001 | 0.34 (0.19-0.58) | <0.001 | 0.38 (0.19-0.74) | 0.005 |
| >=4.3 | 0.23 (0.13-0.39) | <0.001 | 0.30 (0.14-0.57) | <0.001 | 0.28 (0.14-0.55) | <0.001 | 0.26 (0.11-0.63) | 0.002 |
| (B) all-cause death censored graft failure | ||||||||
| <3.77 (ref.) | 1.0 | n/a | 1.0 | n/a | 1.0 | n/a | 1.0 | n/a |
| 3.77- <3.97 | 0.75 (0.61-0.92) | 0.007 | 0.67 (0.54-0.82) | <0.001 | 0.74 (0.59-0.92) | 0.007 | 0.83 (0.62-1.12) | 0.220 |
| 3.97-<4.13 | 0.64 (0.51-0.79) | <0.001 | 0.56 (0.45-0.70) | <0.001 | 0.64 (0.50-0.81) | <0.001 | 0.77 (0.57-1.05) | 0.102 |
| 4.13- <4.30 | 0.71 (0.58-0.88) | 0.001 | 0.62 (0.50-0.77) | <0.001 | 0.70 (0.55-0.88) | 0.003 | 0.86 (0.63-1.18) | 0.355 |
| >=4.3 | 0.66 (0.52-0.82) | <0.001 | 0.56 (0.44-0.71) | <0.001 | 0.63 (0.48-0.82) | 0.001 | 0.83 (0.59-1.17) | 0.287 |
| (D) combined all-cause mortality or graft failure | ||||||||
| <3.77 (ref.) | 1.0 | n/a | 1.0 | n/a | 1.0 | n/a | 1.0 | n/a |
| 3.77- <3.97 | 0.76 (0.65-0.88) | <0.001 | 0.71 (0.60-0.83) | <0.001 | 0.77 (0.65-0.91) | 0.002 | 0.83 (0.67-1.03) | 0.088 |
| 3.97-<4.13 | 0.62 (0.53-0.73) | <0.001 | 0.58 (0.49-0.69) | <0.001 | 0.64 (0.53-0.77) | <0.001 | 0.71 (0.57-0.90) | 0.004 |
| 4.13- 4.3 | 0.61 (0.52-0.71) | <0.001 | 0.60 (0.51-0.72) | <0.001 | 0.66 (0.55-0.79) | <0.001 | 0.79 (0.63-1.00) | 0.053 |
| >=4.3 | 0.51 (0.43-0.61) | <0.001 | 0.54 (0.45-0.66) | <0.001 | 0.59 (0.47-0.72) | <0.001 | 0.68 (0.52-0.89) | 0.005 |
Footnote:
Case-mix adjusted models adjusted for: age, gender, race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a dialysis catheter, and residual renal function during the entry quarter;
MICS adjusted models which included all of the covariates plus body mass index (BMI), the normalized protein nitrogen appearance (nPNA) and serum or blood concentrations of TIBC, ferritin, phosphorus, calcium, bicarbonate, peripheral white blood cell count (WBC), lymphocyte percentage, and hemoglobin;
Case-mix, MICS and transplant data adjusted models included all of the above plus donor type, donor age, panel reactive antibody (PRA) titer (last value prior to transplant), number of HLA mismatches, cold ischemia time and extended donor criteria (EDC)
Figure 1.

Hazard ratio (95% confidence intervals) of post-transplant outcomes across the entire range of the pre-transplant serum albumin level using Cox regression analyses in 8961 long-term hemodialysis transplant patients who underwent renal transplantation and who were observed over a 6-year observation period (7/2001-6/2007). Panel A: Post-transplant graft failure censored all-cause death. Panel B: Post-transplant graft failure censored cardiovascular death. Panel C: Death censored graft failure. Panel D: Combined all-cause mortality or graft failure
Figure 2 shows fully adjusted hazard ratios of all-cause mortality associated with pre-transplant serum albumin levels in selected patient subgroups for each 0.2 g/dL higher pre-transplant serum albumin concentration. The death hazard ratios were below unity in almost all examined subgroups, indicating a lower risk of poor outcomes with higher serum albumin levels. Additional analyses of statistical interaction among selected subgroups were performed, which did not result in statistically significant interaction indicating no clinically meaningful effect modification by the examined characteristics.
Figure 2.
Hazard ratio (95% confidence intervals) of post-transplant graft censored all-cause death for each 0.2 g/dL higher pre-transplant albumin level in selected subgroups of hemodialysis patients who underwent renal transplantation using multivariate Cox regression analyses.
Footnote: “P-value for interaction” indicates whether the association of serum albumin with mortality is significantly different between the subgroups of each demographic (age, gender, race and diabetes), hemoglobin or BMI category.
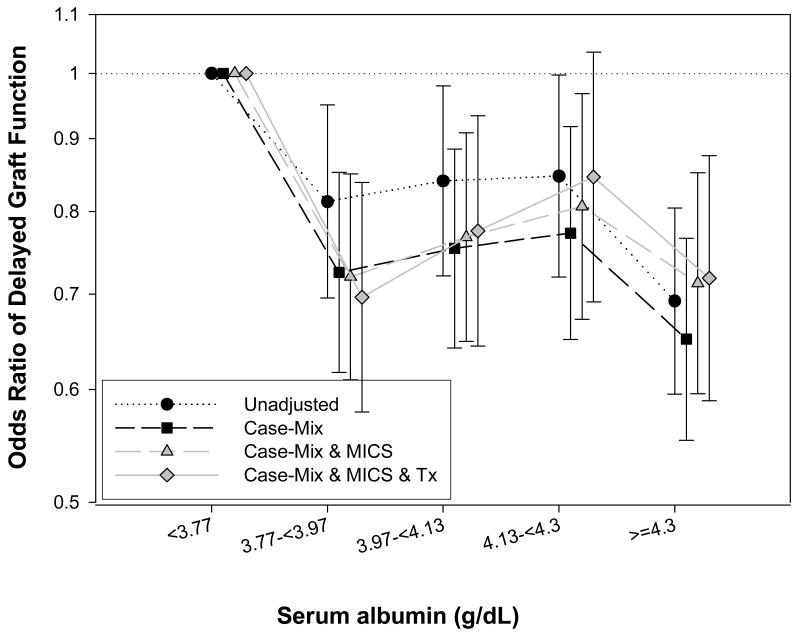
To examine the association of pre-transplant serum albumin with post-transplant DGF, multivariate logistic regression analyses were performed using same covariates as in Cox model. We found that each 0.2 g/dL higher serum albumin was associated with OR (95% CI) of DGF of 0.95 (0.93-0.98), 0.93 (0.91-0.96), 0.95 (0.92-0.98) and 0.96 (0.93-0.99), respectively (p<0.05) in unadjusted, case-mix, MICS and fully adjusted models. As shown in Figure 3 we also examined the albumin-DGF association across 5 quintiles of pre-transplant albumin groups using <3.77 g/dL as the reference group. Patient with albumin >= 4.3 g/dL, 4.13-<4.3 g/dL, 3.97-<4.13 g/dL and 3.77-<3.97 g/dL had 28% (OR: 0.72 [0.59-0.88]), 15% (OR: 0.85 [0.69-1.03]) 24% (OR: 0.76 [0.64-0.93]) and 30% (OR: 0.70 [0.58-0.84]) lower risk of DGF, respectively (p-for trend <0.001 for unadjusted and 0.03 for fully adjusted logistic regression model). Test of interaction did not detect meaningful interactions, except for diabetes where a significant interaction (p=0.047) was noted. Figure 4 shows the association between pre-transplant albumin and post-transplant DGF across same patient subgroups as in Figure 2, indicating similar associations, although 95% CI included unity in some subgroups.
Figure 3.
Odds ratios (and 95% confidence intervals) of post-transplant delayed graft function (DGF) across 5 increments of pre-transplant serum albumin using multivariate logistic regression analyses (Reference: albumin <3.77 g/dL) (p-for trend <0.001 for unadjusted and 0.03 for fully adjusted logistic regression model).
Figure 4.
Odds ratios (and 95% confidence intervals) of post-transplant delayed graft function (DGF) for each 0.2 g/dL higher pre-transplant albumin level in selected subgroups of hemodialysis patients who underwent renal transplantation using multivariate logistic regression analyses.
Footnote: “P-value for interaction” indicates whether the association of serum albumin with DGF is significantly different between the subgroups of each demographic (age, gender, race and diabetes), hemoglobin or BMI category.
Discussion
In 8,961 kidney transplant recipients with comprehensive pre-transplant data during hemodialysis treatment who were followed for up to 6 years post-transplantation, lower pre-transplant serum albumin was associated with higher DGF risk and increased all-cause and cardiovascular mortality and graft loss. The association between pre-transplant low serum albumin and higher mortality was strictly linear and incremental. Assuming that a lower pre-transplant serum albumin level in hemodialysis patients is a surrogate marker of PEW or worse sickness, our findings may have major clinical and public health implications in providing care to transplant waitlisted hemodialysis patients. If our findings are verified in additional studies, assessment of nutritional status and comorbid conditions in waitlisted dialysis patients may become an important task in care to transplant waitlisted patients.
Several potential mechanisms can explain the association between pre-transplant serum albumin and post-transplant outcomes. In prevalent renal transplant recipients serum albumin is a strong predictor of mortality;(29) and lower pre-transplant serum albumin is associated with higher likelihood of post-transplant hypoalbuminemia and PEW.(29, 30) Another potential mechanism may be through cytomegalovirus (CMV) infection, which increases the risk for mortality and graft failure in kidney transplant recipients;(31) low serum albumin level is associated with CMV infection in liver transplant recipients.(32) Another possible explanation is worse co-morbid status, since low serum albumin level is a marker of acute illnesses or chronic wasting diseases, and higher co-morbidity is associated with worse survival in kidney transplanted patients.(33) Malnutrition and severe co-morbidity carried over from the dialysis period may lead to impaired immune function and inadequate host resistance, resulting in increased susceptibility to infections and poor wound healing.(34) Impaired host resistance, aggravated by immune suppressive agents, may predispose to inflammatory diseases with endothelial cell damage and endothelial dysfunction, predisposing to atherosclerotic processes.(35) Endothelial dysfunction is associated with inflammation in kidney transplant recipients(36) and is associated with increased mortality risk.(37)
We found higher risk of post-transplant DGF in hypoalbuminemic hemodialysis patients. DGF is a common post-transplant complication and attributed to ischemia-reperfusion and immunological injury of the graft.(38) The prevalence of DGF varies from 4% to 10% in living donor (38) and 5% to 50% in deceased donor kidney transplants.(39-43) The well known deleterious effects of DGF in the post-transplant period are multiple including complications of immediate post-transplant patient care in the hospital. There may be even long-term impact of DGF. Most,(44, 45) but not all,(46, 47) studies report an association between DGF and reduced long-term graft survival. DGF is also associated with an increased risk of death with graft function in living donor kidney recipients.(48) Whereas DGF is a predictor of poorer short- and long term graft survival, less is known about the risk factors which per se predict the development of DGF. Some of the well-known post-transplant complications such as calcineurin inhibitor toxicity, vascular- or urological complication, rejection and volume depletion are characteristically present in patients with DGF;(12) however, very little is known about the association of DGF with pre-transplant related factors during dialysis treatment era such as pre-transplant albumin. In our analyses each 0.2 g/dL higher serum albumin was associated with 4% lower risk of DGF.
Our study should be qualified for several potential limitations. Like all observational studies, ours too cannot prove causality. Repeated post-transplant measures of albumin or other laboratory variables and immunosuppressive and other medical regimens were not available in the SRTR database, but in the full model we did adjust for a number of transplant-related variables. Generalizability may be limited, compared to the US CKD population, given lower proportion of diabetics, lack of peritoneal dialysis patients and re-transplanted patients in our cohort. We did not have detailed co-morbid data, nor did we analyze dialysis treatment time, which might have impact of the associations. We did not have access to data pertaining to death after graft loss, which is another important outcome. Patients who did not have measured serum albumin in the pre-transplant calendar quarter were excluded from the analyses. Although the excluded patients might be different from those under study, their proportion of the former group was relatively small. To our knowledge this is the first study examining the association between pre-transplant serum albumin level, a surrogate of PEW or co-morbidity status, and post-transplant short- and long-term outcomes. Strengths of this study include the high number of patients, the relatively long follow-up time and multi-level adjustment which include several important pre-transplant measures.
Conclusions
In our large and contemporary national database of 8,961 kidney transplant recipients, lower pre-transplant serum albumin concentration during hemodialysis treatment period was associated with worse post-transplant short- and long-term outcomes including higher risk of DGF, increased all-cause and cardiovascular death and higher risk of graft failure. The association between pre-transplant serum albumin and post-transplant mortality was robust in almost all subgroups of patients. Clinical trials to examine interventions to improve nutritional status in transplant-wait-listed hemodialysis patients and their impacts on post-transplant outcomes are indicated.
Acknowledgments
We thank Mr. Robert Lehn at DaVita Laboratories in Deland, FL, Mr. Joe Weldon, from DaVita Informatics, for proving the national database, and Mr. Chris Rucker and Ms. Beth Bennett from DaVita Clinical Research for their continued support.
Funding Source: The study was supported by KKZ's research grant from the American Heart Association grant (0655776Y). KKZ's other funding sources include the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01 DK078106); a research grant from DaVita Clinical Research and a philanthropic grant from Mr. Harold Simmons. MZM received grants from the National Research Fund (NKTH-OTKA-EU 7KP-HUMAN-MB08-A-81231), was also supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (2008-2011), and is recipient of the Hungarian Eötvös Scholarship (MÖB/66-2/2010).
Footnotes
Relevant Potential Conflict of Interest: Drs. Nissenson and Krishnan are an employee of DaVita. Dr. Kalantar-Zadeh is the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA. Other authors have not declared any conflict of interest.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England journal of medicine. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Schnuelle P, Lorenz D, Trede M, Van Der Woude FJ. Impact of renal cadaveric transplantation on survival in end-stage renal failure: evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J Am Soc Nephrol. 1998;9(11):2135–2141. doi: 10.1681/ASN.V9112135. [DOI] [PubMed] [Google Scholar]
- 3.Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. Jama. 1993;270(11):1339–1343. [PubMed] [Google Scholar]
- 4.Ojo AO, Port FK, Wolfe RA, Mauger EA, Williams L, Berling DP. Comparative mortality risks of chronic dialysis and cadaveric transplantation in black end-stage renal disease patients. Am J Kidney Dis. 1994;24(1):59–64. doi: 10.1016/s0272-6386(12)80160-0. [DOI] [PubMed] [Google Scholar]
- 5.Herzog CA, Ma JZ, Collins AJ. Long-term survival of renal transplant recipients in the United States after acute myocardial infarction. Am J Kidney Dis. 2000;36(1):145–152. doi: 10.1053/ajkd.2000.8287. [DOI] [PubMed] [Google Scholar]
- 6.Howard RJ, Patton PR, Reed AI, Hemming AW, Van der Werf WJ, Pfaff WW, et al. The changing causes of graft loss and death after kidney transplantation. Transplantation. 2002;73(12):1923–1928. doi: 10.1097/00007890-200206270-00013. [DOI] [PubMed] [Google Scholar]
- 7.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16(2):496–506. doi: 10.1681/ASN.2004070580. [DOI] [PubMed] [Google Scholar]
- 8.Locatelli F, Pozzoni P, Del Vecchio L. Renal replacement therapy in patients with diabetes and end-stage renal disease. J Am Soc Nephrol. 2004;15 1:S25–29. doi: 10.1097/01.asn.0000093239.32602.04. [DOI] [PubMed] [Google Scholar]
- 9.Varagunam M, Finney H, Trevitt R, Sharples E, McCloskey DJ, Sinnott PJ, et al. Pretransplantation levels of C-reactive protein predict all-cause and cardiovascular mortality, but not graft outcome, in kidney transplant recipients. Am J Kidney Dis. 2004;43(3):502–507. doi: 10.1053/j.ajkd.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Fabrizii V, Winkelmayer WC, Klauser R, Kletzmayr J, Saemann MD, Steininger R, et al. Patient and graft survival in older kidney transplant recipients: does age matter? J Am Soc Nephrol. 2004;15(4):1052–1060. doi: 10.1097/01.asn.0000120370.35927.40. [DOI] [PubMed] [Google Scholar]
- 11.Wu C, Evans I, Joseph R, Shapiro R, Tan H, Basu A, et al. Comorbid conditions in kidney transplantation: association with graft and patient survival. J Am Soc Nephrol. 2005;16(11):3437–3444. doi: 10.1681/ASN.2005040439. [DOI] [PubMed] [Google Scholar]
- 12.Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008;23(9):2995–3003. doi: 10.1093/ndt/gfn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24(3):1039–1047. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 14.Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21(1):153–161. doi: 10.1681/ASN.2009040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almond PS, Matas AJ, Canafax DM. Fixed-rate reimbursement fails to cover costs for patients with delayed graft function. Pharmacotherapy. 1991;11(5):126S–129S. [PubMed] [Google Scholar]
- 16.Almond PS, Troppmann C, Escobar F, Frey DJ, Matas AJ. Economic impact of delayed graft function. Transplant Proc. 1991;23(1 Pt 2):1304. [PubMed] [Google Scholar]
- 17.Rosenthal JT, Danovitch GM, Wilkinson A, Ettenger RB. The high cost of delayed graft function in cadaveric renal transplantation. Transplantation. 1991;51(5):1115–1118. [PubMed] [Google Scholar]
- 18.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H, Jr, Kopple JD, et al. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant. 2005;20(9):1880–1888. doi: 10.1093/ndt/gfh941. [DOI] [PubMed] [Google Scholar]
- 19.Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berglund L, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55(5):1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 20.Kovesdy CP, George SM, Anderson JE, Kalantar-Zadeh K. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr. 2009;90(2):407–414. doi: 10.3945/ajcn.2008.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38(6):1343–1350. doi: 10.1053/ajkd.2001.29250. [DOI] [PubMed] [Google Scholar]
- 22.Molnar MZ, Lukowsky LR, Streja E, Dukkipati R, Jing J, Nissenson AR, et al. Blood pressure and survival in long-term hemodialysis patients with and without polycystic kidney disease. Journal of hypertension. 2010 doi: 10.1097/HJH.0b013e32833e4fd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JE, Kovesdy CP, Norris KC, Mehrotra R, Nissenson AR, Kopple JD, et al. Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patients. American journal of nephrology. 2010;32(5):403–413. doi: 10.1159/000319861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JE, Kovesdy CP, Nissenson AR, Mehrotra R, Streja E, Jing J, et al. Association of Hemodialysis Treatment Time and Dose with Mortality: The Role of Race and Gender. Am J Kidney Dis. 2010;55(1):100–112. doi: 10.1053/j.ajkd.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clinic proceedings. 2010;85(11):991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Miller JE, Kovesdy CP, Mehrotra R, Lukowsky LR, Streja E, et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25(12):2448–2458. doi: 10.1002/jbmr.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? The American journal of clinical nutrition. 2008;88(6):1511–1518. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, Jing J, et al. Associations of Body Mass Index and Weight Loss with Mortality in Transplant-Waitlisted Maintenance Hemodialysis Patients. Am J Transplant. 2010 doi: 10.1111/j.1600-6143.2011.03468.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Ree RM, Gross S, Zelle DM, van der Heide JJ, Schouten JP, van Son WJ, et al. Influence of C-reactive protein and urinary protein excretion on prediction of graft failure and mortality by serum albumin in renal transplant recipients. Transplantation. 2010;89(10):1247–1254. doi: 10.1097/TP.0b013e3181d720e3. [DOI] [PubMed] [Google Scholar]
- 30.Yelken BM, Gorgulu N, Caliskan Y, Yazici H, Turkmen A, Yildiz A, et al. Comparison of nutritional status in hemodialysis patients with and without failed renal allografts. Clin Transplant. 2010;24(4):481–487. doi: 10.1111/j.1399-0012.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- 31.Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, et al. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney international. 2004;66(1):329–337. doi: 10.1111/j.1523-1755.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim JM, Kim SJ, Joh JW, Shin M, Moon JI, Jung GO, et al. The risk factors for cytomegalovirus syndrome and tissue-invasive cytomegalovirus disease in liver transplant recipients who have cytomegalovirus antigenemia. Transplantation proceedings. 2010;42(3):890–894. doi: 10.1016/j.transproceed.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 33.Jassal SV, Schaubel DE, Fenton SS. Baseline comorbidity in kidney transplant recipients: a comparison of comorbidity indices. Am J Kidney Dis. 2005;46(1):136–142. doi: 10.1053/j.ajkd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Chinen J, Shearer WT. 6. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. 2008;121(2 Suppl):S388–392. doi: 10.1016/j.jaci.2007.06.003. quiz S417. [DOI] [PubMed] [Google Scholar]
- 35.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 36.Malyszko J, Malyszko JS, Pawlak K, Wolczynski S, Mysliwiec M. Apelin, a novel adipocytokine, in relation to endothelial function and inflammation in kidney allograft recipients. Transplant Proc. 2008;40(10):3466–3469. doi: 10.1016/j.transproceed.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 37.van Ree RM, Oterdoom LH, de Vries AP, Homan van der Heide JJ, van Son WJ, Navis G, et al. Circulating markers of endothelial dysfunction interact with proteinuria in predicting mortality in renal transplant recipients. Transplantation. 2008;86(12):1713–1719. doi: 10.1097/TP.0b013e3181903d25. [DOI] [PubMed] [Google Scholar]
- 38.Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364(9447):1814–1827. doi: 10.1016/S0140-6736(04)17406-0. [DOI] [PubMed] [Google Scholar]
- 39.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–974. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 40.Koning OH, van Bockel JH, van der Woude FJ, Persijn GG, Hermans J, Ploeg RJ. Risk factors for delayed graft function in University of Wisconsin solution preserved kidneys from multiorgan donors. European Multicenter Study Group on Organ Preservation. Transplant Proc. 1995;27(1):752–753. [PubMed] [Google Scholar]
- 41.Sellers MT, Gallichio MH, Hudson SL, Young CJ, Bynon JS, Eckhoff DE, et al. Improved outcomes in cadaveric renal allografts with pulsatile preservation. Clin Transplant. 2000;14(6):543–549. doi: 10.1034/j.1399-0012.2000.140605.x. [DOI] [PubMed] [Google Scholar]
- 42.Gjertson DW. Impact of delayed graft function and acute rejection on graft survival. Transplant Proc. 2002;34(6):2432. doi: 10.1016/s0041-1345(02)03167-6. [DOI] [PubMed] [Google Scholar]
- 43.Hassanain M, Tchervenkov J, Cantarovich M, Metrakos P, Paraskevas S, Keith D, et al. Delayed graft function has an equally bad impact on deceased donor renal graft survival in both standard criteria donors and expanded criteria donors. Transplant Proc. 2009;41(1):133–134. doi: 10.1016/j.transproceed.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 44.Gentil MA, Alcaide MP, Algarra GR, Pereira P, Toro J, Gonzalez-Roncero F, et al. Impact of delayed graft function on cadaveric kidney transplant outcome. Transplant Proc. 2003;35(2):689–691. doi: 10.1016/s0041-1345(03)00048-4. [DOI] [PubMed] [Google Scholar]
- 45.Arias M. Impact of the delayed graft function in hypersensitized kidney transplant patients. Transplant Proc. 2003;35(5):1655–1657. doi: 10.1016/s0041-1345(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 46.Boom H, Mallat MJ, de Fijter JW, Zwinderman AH, Paul LC. Delayed graft function influences renal function, but not survival. Kidney Int. 2000;58(2):859–866. doi: 10.1046/j.1523-1755.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 47.Marcen R, Orofino L, Pascual J, de la Cal MA, Teruel JL, Villafruela JJ, et al. Delayed graft function does not reduce the survival of renal transplant allografts. Transplantation. 1998;66(4):461–466. doi: 10.1097/00007890-199808270-00008. [DOI] [PubMed] [Google Scholar]
- 48.Narayanan R, Cardella CJ, Cattran DC, Cole EH, Tinckam KJ, Schiff J, et al. Delayed Graft Function and the Risk of Death With Graft Function in Living Donor Kidney Transplant Recipients. Am J Kidney Dis. 2010;56(5):961–970. doi: 10.1053/j.ajkd.2010.06.024. [DOI] [PubMed] [Google Scholar]