Abstract
Background
Gelsolin is an actin-binding protein found in the cytoplasm and in extracellular fluids including blood plasma. Plasma gelsolin concentration decreases after a wide range of traumatic injuries. We hypothesized that the repletion of exogenous gelsolin would limit inflammation and tissue injury in a rat model of sepsis using cecal ligation and double puncture (2CLP).
Methods
Exogenous human plasma gelsolin (pGSN, 10 mg in 1 ml saline) was administered once immediately following surgery, and control 2CLP (2CLP Alb) and sham animals were injected with 1 ml saline containing equimolar albumin. Treatments were administered intraperitoneally (IP), intravenously (IV), or subcutaneously (SC).
Results
Gelsolin levels in the 2CLP Alb group were lower than in sham animals compared to sham animals. Administration of pGSN increased levels when administered IV and SC, but not IP. Morbidity scores were significantly less severe in the 2CLP pGSN group than in the 2CLP Alb group when pGSN was administered IV and SC, but not IP. Furthermore, enzymatic activity indicative of tissue damage (lactate dehydrogenase and alanine transaminase) was significantly lower in 2CLP pGSN group when treated SC compared to 2CLP Alb group.
Conclusion
These data provide further evidence that exogenous gelsolin can reduce morbidity from sepsis.
Keywords: Sepsis, tissue injury
Introduction
Sepsis can precipitate an overly exuberant inflammatory response to invading microorganisms, which may lead to organ failure and even death (1). Numerous studies have attempted to identify a therapeutic target to limit injurious inflammation, but as of yet no consistently safe and efficacious therapy has been developed (2).
Recent work has suggested that the blood plasma isoform of the actin-scavenging protein gelsolin (pGSN (3)) could temper host inflammatory responses during sepsis (4). However, pGSN is depleted following injuries such as major trauma, burns, surgery, and sepsis (5). Recovery of pGSN levels has been correlated with improved patient outcomes (4, 6–8).
Exogenous pGSN has been used as treatment for LPS-induced endotoxic shock and cecal ligation and puncture (CLP)-induced sepsis in the mouse. Lee et al. (4) subcutaneously injected mice with a dose of pGSN approximately 20 times the total typical (if this is the normal mouse amount, not septic) endogenous amount in the mouse, and observed decreases in the mortality rate of both LPS and CLP treated animals. Furthermore, they found pGSN treatment significantly altered inflammatory cytokine expression 24 hours after LPS administration, but had no evident effect on cytokine expression in the CLP animals.
In this communication, we confirm and extend the findings of Lee et al. Using the CLP model of sepsis in rats, we found that exogenous pGSN administered either intravenously or subcutaneously improved clinical outcomes, modulated cytokine expression, and reduced tissue injury.
Methods
Cecal Ligation and Double Puncture (CLP) and Gelsolin Administration
Under sterile conditions and isoflurane anesthesia, male Sprague-Dawley rats (Charles River, Boston, MA) weighing 240–260 grams were randomly assigned to undergo CLP or sham as previously described (9). Immediately following the CLP surgery, the animals were injected intravenously (IV), intraperitoneally (IP), or subcutaneously (SC), with 1 ml of saline containing either 10 mg of human pGSN (2CLP pGSN) or an albumin control of equivalent molarity (2CLP Alb). Sham animals were treated with either pGSN (Sham pGSN) or albumin (Sham Alb) via matched routes of administration. The number of animals per group is shown in the figures.
Twenty-four hours following the initial surgery, the animals were anesthetized with sodium pentobarbital, 55 mg/kg IP. Blood samples were obtained via the descending aorta with a heparin-coated 21 gauge needle for analysis of cytokine expression or pGSN concentration. Expression of 19 cytokines was analyzed with a fluorescent glass slide array per the manufacturer’s instructions (Rat Cytokine Array G, RayBiotech, Norcross, GA). Blood plasma enzyme activity was analyzed using a commercially available kit for lactate dehydrogenase (LDH) and alanine transaminase (ALT) (BioVision, Mountain View, CA, and Pointe Scientific, Canton, MI, respectively). An abdominal aortotomy was then performed to exsanguinate the rat. Randomly selected lungs were removed, fixed in 4% paraformaldehyde, paraffin embedded, sectioned and stained with hematoxylin and eosin. The protocol was approved by the University of Pennsylvania animal care and use committee.
Clinical Scoring
Prior to sacrifice, animal heath was quantified by scoring 7 clinical readouts on a scale of 0–3 (0-negative, 3-floridly positive): porphyrin staining of the eyes and nose, piloerection, lethargy, pallor of the ears, tissue dehydration, intestinal hyperemia, and cecal necrosis. Additionally, the overall clinical impression was rated on a 0–3 scale for each animal. A final score was calculated by taking the sum of all individual scores. Scoring was done on all animals by the original surgeon, who was not blinded. A second set of scoring was done by a blinded observer on a subset of the n animals, and these values did not differ from those of the surgeon.
Gelsolin Concentration Assay
Gelsolin concentrations were determined from G-actin nucleation activity measured by the initial slope of the near-linear fluorescence increase with actin polymerization (7). Gelsolin concentrations were determined in triplicate.
Statistics
All statistical comparisons were made using a Student’s t-test corrected for multiple comparisons by a Bonferroni correction factor (β) indicated in the figure legends.
Results
Blood Plasma pGSN Levels
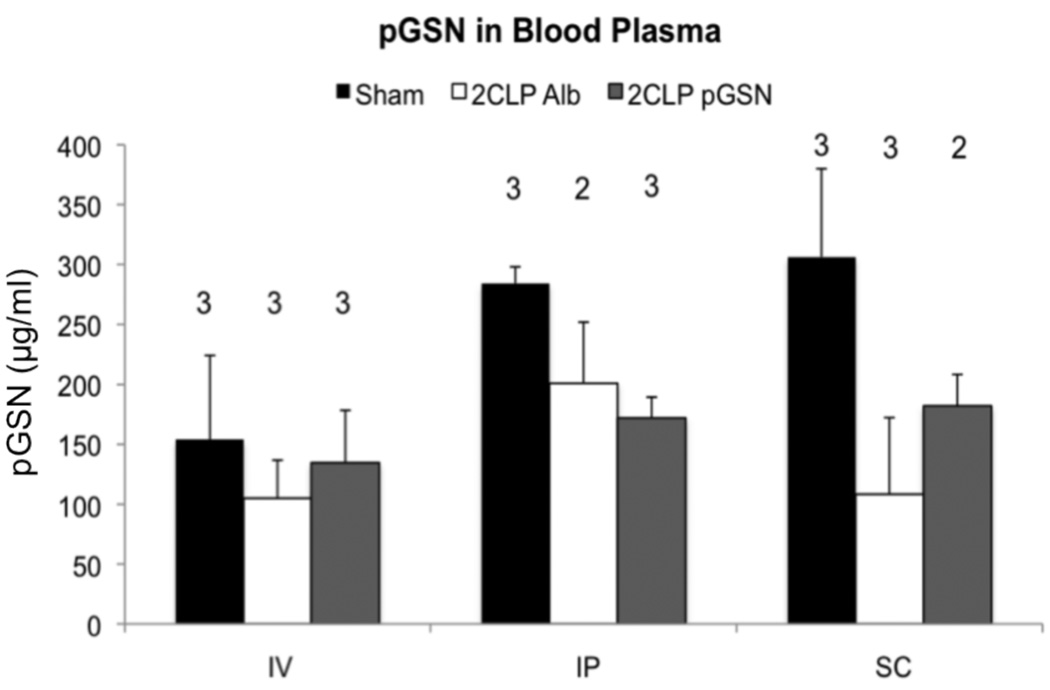
In pilot studies, mean plasma pGSN levels in 10 2CLP animals were significantly lower than levels in 12 sham animals (120 versus 190 µg/ml, p= 0.023). pGSN levels in IV sham animals were often lower than in the SC or IP sham groups, potentially due to clotting around the vein following injection. That said, administration of pGSN to 2CLP animals via IV or SC routes, but not IP, partially reduced the decrement in plasma pGSN (Figure 1), indicating that administration of pGSN at a site distant from the injury site could limit decreases in systemic levels of pGSN 24 hours following the initial insult.
Figure 1.
pGSN levels (µg/ml) in sham, 2CLP Alb, and 2CLP pGSN animals 24 hours postoperatively. 2CLP Alb decreased pGSN levels compared to sham, significant in the SC group (bar, p < 0.025, β =2). Gelsolin administration partially prevented these reductions, except when given IP. Data are presented as mean ± SD, N shown in figure.
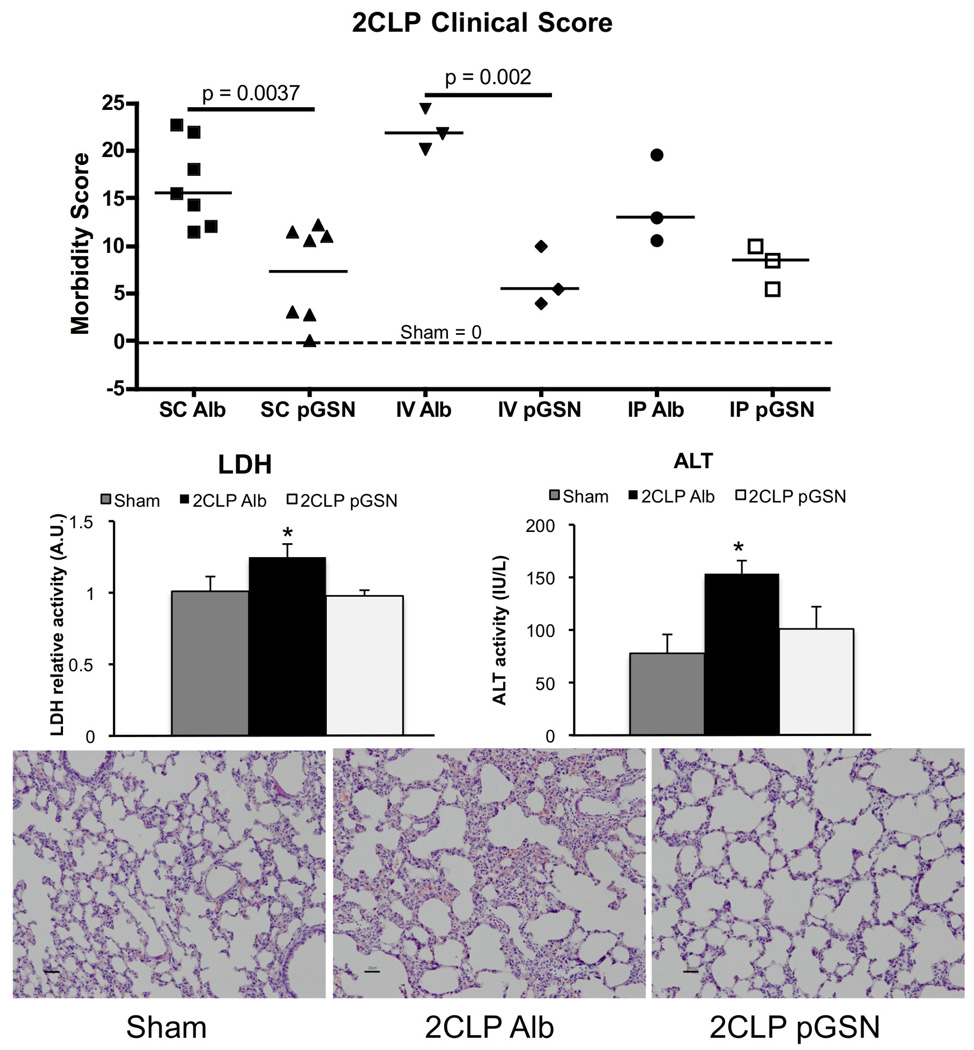
Clinical Scoring and Enzyme Concentration
All 2CLP animals scored significantly worse than surgical shams (all of which scored 0 out of 3 for all injury criteria), regardless of treatment. Administration of exogenous pGSN by IV or SC routes significantly lowered clinical scores compared to Alb-treated 2CLP animals (Figure 2 top). Blood plasma concentrations of LDH and ALT in SC treated animals were significantly increased in 2CLP Alb compared to sham, and pGSN reversed these increases (Figure 2 middle). Representative lung sections showed that the interstitial thickening in 2CLP Alb animals was largely prevented with pGSN treatment (Figure 2 bottom).
Figure 2.
Injury score and expression of markers of tissue injury in the blood for 2CLP animals following either albumin (Alb) or pGSN treatment. All scores after 2CLP were significantly greater than sham (dotted line, value = 0). pGSN administration via IV or SC routes significantly improved the clinical score. Furthermore, SC-administered pGSN largely prevented the significant (* p < 0.05, β = 2) increases in LDH and ALT levels observed in the blood of animals treated with 2CLP Alb compared to sham (N=3 per group). Thickening of interstitial spaces was observed in 2CLP Alb animals compared to sham, and this was reduced in pGSN treated animals.
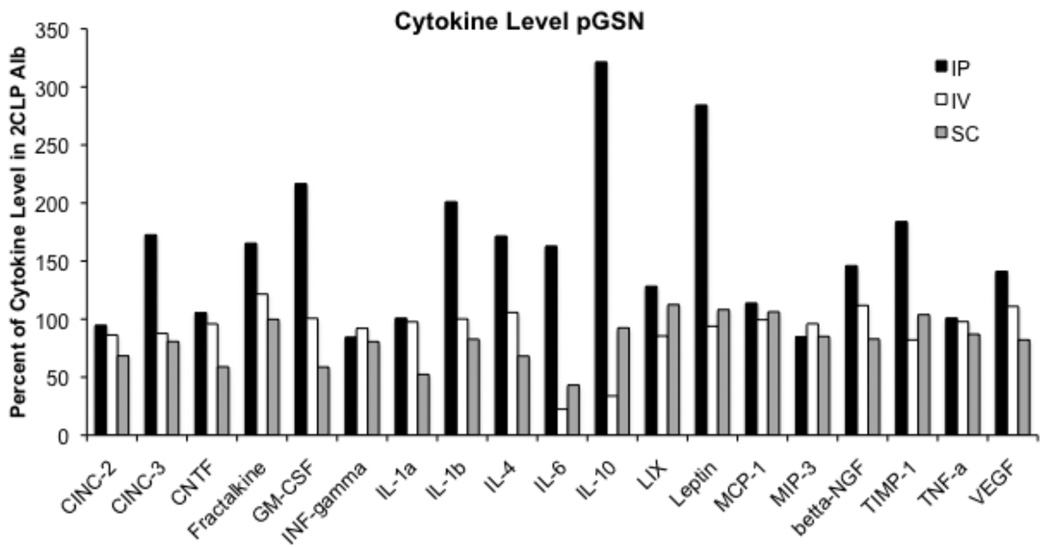
Blood Plasma Cytokine Expression
Blood plasma cytokine expression in 2CLP pGSN and 2CLP Alb animals differed from that measured in sham animals (data not shown), with the largest increases observed in IL-6, IL-10, and LIX levels. Treatment with pGSN did not alter expression of IL-10 or LIX compared to Alb treatment. IL-6 levels were decreased in pGSN treated animals by 22.4% and 43.1% compared to Alb treated animals in the IV (22.4% Alb level) and SC (43.1% Alb level) groups, respectively, although these differences did not reach statistical significance. In contrast, IP injection of pGSN actually increased expression of numerous cytokines, including IL-6. Western blot analysis of ascitic fluid demonstrated that pGSN injected SC or IV entered the abdomen of 2CLP animals.
Discussion
Plasma gelsolin levels decrease following various injuries, including sepsis (5). Consequently, pGSN concentration has received attention as a marker of sepsis severity, and its levels have been inversely correlated with mortality rates and expression of the inflammatory cytokine IL-6 (but not IL-10 or TNFα) (6, 10). Using a rat model of sepsis, we confirmed that pGSN levels were significantly lower in 2CLP animals compared to sham animals 24 hours following surgery (4). Furthermore, morbidity scores were reduced by administration of exogenous pGSN at a site distant from the injury. Although not statistically significant, we also found decreased expression of IL-6, consistent with reports of decreased IL-6 correlating with increased survival in humans (11).
Lee et al. had examined the role of pGSN in LPS and 2CLP models of sepsis in mice, and observed reduced mortality with administration of 10 mg of pGSN. In the current study, with a significantly smaller dose by body weight, we still observed beneficial effects with systemic pGSN administration.
The site of pGSN injection was shown to be a factor in its effectiveness. Significant reductions in systemic pGSN concentration and injury score were prevented by injection of pGSN either SC or IV, but not IP, despite the abdomen being the site of injury. These data suggest that to be effective, pGSN must act systemically as opposed to simply being concentrated at the site of injury. Administration of pGSN by SC and IV routes reduced LDH and ALT concentrations in the blood, indicating that tissue injury was prevented.
We conclude that administration of exogenous pGSN can lessen the overall severity of illness precipitated by intra-abdominal sepsis. Although the exact mechanism of action has yet to be elucidated, gelsolin repletion may ameliorate injury by altering expression of proinflammatory cytokines such as IL-6. Despite the small size and other limitations of our hypothesis-generating study, these findings support larger, blinded, dose-ranging, rigorously designed studies to treat pGSN as a potential therapy for sepsis.
Figure 3.
Cytokine expression in 2CLP pGSN (N = 2-IP, 3-IV, 3-SC) treated animals as compared to those in 2CLP Alb (N = 2-IP, 3-IV, 5-SC) treated. Cytokine levels are expressed as a percentage of the levels observed in the blood plasma of 2CLP Alb treated animals. pGSN treatment via IP injection increased expression of numerous cytokines while administration via IV or SC routes lowered (IL-6) or did not alter cytokine expression.
Acknowledgments
Funding: Support was provided by NIH RO1-HL57204, HL067286 and the Cystic Fibrosis Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics Statement: All animal use was done in accordance with, and with the approval of, the IACUC in the Office of Regulatory Affairs of the University of Pennsylvania.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen D, Corina K, Chow EP, et al. The plasma and cytoplasmic forms of human gelsolin differ in disulfide structure. Biochemistry. 1996;35:9700–9709. doi: 10.1021/bi960920n. [DOI] [PubMed] [Google Scholar]
- 4.Lee PS, Waxman AB, Cotich KL, et al. Plasma gelsolin is a marker and therapeutic agent in animal sepsis. Crit Care Med. 2007;35:849–855. doi: 10.1097/01.CCM.0000253815.26311.24. [DOI] [PubMed] [Google Scholar]
- 5.Bucki R, Levental I, Kulakowska A, et al. Plasma gelsolin: function, prognostic value, and potential therapeutic use. Curr Protein Pept Sci. 2008;9:541–551. doi: 10.2174/138920308786733912. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Cheng B, Chen Q, et al. Time course of plasma gelsolin concentrations during severe sepsis in critically ill surgical patients. Crit Care. 2008;12:R106. doi: 10.1186/cc6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothenbach PA, Dahl B, Schwartz JJ, et al. Recombinant plasma gelsolin infusion attenuates burn-induced pulmonary microvascular dysfunction. J Appl Physiol. 2004;96:25–31. doi: 10.1152/japplphysiol.01074.2002. [DOI] [PubMed] [Google Scholar]
- 8.Christofidou-Solomidou M, Scherpereel A, Solomides CC, et al. Changes in plasma gelsolin concentration during acute oxidant lung injury in mice. Lung. 2002;180:91–104. doi: 10.1007/s004080000084. [DOI] [PubMed] [Google Scholar]
- 9.Levine GK, Deutschman CS, Helfaer MA, et al. Sepsis-induced lung injury in rats increases alveolar epithelial vulnerability to stretch. Crit Care Med. 2006;34:1746–1751. doi: 10.1097/01.CCM.0000218813.77367.E2. [DOI] [PubMed] [Google Scholar]
- 10.Lee PS, Patel SR, Christiani DC, et al. Plasma gelsolin depletion and circulating actin in sepsis: a pilot study. PLoS ONE. 2008;3:e3712. doi: 10.1371/journal.pone.0003712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frink M, van Griensven M, Kobbe P, et al. IL-6 predicts organ dysfunction and mortality in patients with multiple injuries. Scand J Trauma Resusc Emerg Med. 2009;17:49. doi: 10.1186/1757-7241-17-49. [DOI] [PMC free article] [PubMed] [Google Scholar]