Abstract
Objective
There have been no prospective large-scale studies to evaluate the prevalence and determinants of Barrett’s esophagus (BE) in children who are free from neurodevelopmental disorders and tracheoesophageal abnormalities.
Design
A prospective cross-sectional study
Setting
Three pediatric GI Centers in Houston, TX, Phoenix AZ, and Portland ME between February, 2006 and December, 2007.
Patients
Children and adolescents consecutively presenting for elective upper endoscopy. Patients with neurodevelopmental and tracheoesophageal disorders were excluded.
Interventions
Endoscopic pictures of all cases with suspected BE were independently reviewed and verified by 2 experienced investigators. Esophageal biopsies were obtained in all patients, and targeted biopsies were also obtained from suspected BE.
Main Outcome Measurements
Endoscopically suspected BE and histologically confirmed BE.
Results
A total of 840 patients (mean age, 9.5 years) were enrolled and had complete questionnaire and endoscopic data. Twelve patients were suspected of having BE (prevalence of 1.43% (95% CI: 0.73–2.45)) and only 1 patient has intestinal metaplasia for a prevalence of 0.12% (95% CI 0–0.65), while the rest had gastric (n=6) or squamous (n=5). Patients with suspected BE had higher mean BMI (23.0 vs. 19.1, p=0.05) and more chest pain (50% vs. 13%, p<0.01) than patients without BE or reflux esophagitis. There was a trend of higher frequency of dysphagia, heartburn and regurgitation in patients with suspected BE.
Limitations
The accuracy of BE prevalence estimates is limited by the small number of cases.
Conclusions
BE is rare in children without neurodevelopmental delay or tracheoesophageal anomalies presenting for elective upper endoscopy.
Introduction
Gastroesophageal reflux (GER) is considered physiologic in infants and usually spontaneously resolves by 18–24 months of age.1, 2 GER in older children is considered pathologic and consistent with gastroesophageal reflux disease (GERD) .3–6 In particular, reflux disorders in older children may become chronic, and may be associated with esophageal and extraesophageal disorders. GERD symptoms occur in an estimated 2% to 8% of children aged 3–18 years.7 The endoscopic manifestations of GERD include reflux esophagitis (RE) and Barrett’s esophagus (BE).1 There is little information on the endoscopic manifestations of pediatric GERD based on prospective data with standardized of endoscopic recordings and biopsies.
BE in children has been examined in retrospective studies, consisting of case series and cross-sectional studies. Hassall et al. conducted a meta review of case reports and small case series described a total of 119 reported cases of BE in children, of whom only 36% had intestinal metaplasia.8 This review did not evaluate prevalence of BE because the appropriate denominator was missing from most reports. We have conducted several studies geared towards estimating the prevalence of BE in children. In a single center cross-sectional study examining the prevalence of endoscopic manifestations of GERD in 402 neurologically normal children without esophageal congenital abnormalities, the prevalence of suspected BE was 2.7% and none of these cases was confirmed by histologic examination. In the same study, endoscopically visible RE was found in 34.6%.5 In another retrospective multicenter cross-sectional study using Pediatric Clinical Outcomes Research Initiative (PEDS-CORI) database, only 17 of 6731 (0.25%) children had suspected BE.9 Using the same data source, Gilger et al. found 888 of 7188 patients (12.4%) who underwent endoscopy were reported to have RE.10 The limitations of these studies included the variations of endoscopic interpretation and the absence of histologic findings for RE. In addition, the PEDS-CORI studies contained an undefined number of children with congenital disorders and tracheoesophageal disorders thus limiting its generalizability to the vast majority of children with GERD who do not have these comorbidities. Furthermore, given the retrospective nature of these studies, there was a lack of standardization of endoscopic recordings and biopsies.11
In this study, we examine the prevalence of BE as well as RE as defined by endoscopic and histologic criteria in a prospective, multicenter setting in normal children without neurodevelopmental or tracheoesophageal disorders.
Methods
Study Design and Study Population
We conducted a prospective cross-sectional study among children and adolescents presenting during 2006–2007 for upper endoscopy in three pediatric centers (Texas Children’s Hospital, Houston, Texas; Phoenix Children’s Hospital, Phoenix, Arizona; and Barbara Bush Children’s Hospital, Portland, Maine). Consecutive patients less than 18 years of age who were referred to endoscopy for non-urgent indications were recruited for the study. We excluded patients with cerebral palsy, congenital or post operative esophageal stricture, tracheoesophageal fistula, and mental retardation. Before endoscopy, patients or their legal guardians completed a standardized questionnaire that assessed for the presence, duration, and frequency of upper gastrointestinal symptoms (aspiration, chest pain, dysphagia, epigastric pain, heartburn, regurgitation, vomiting, and poor weight gain) and possible risk factors for GERD or BE (tube feeding, cerebral palsy, esophageal stricture, fundoplication, mental retardation, or tracheoesophageal fistula). We obtained same-day weight (kilograms) and height (meters) before endoscopy using a calibrated scale and stadiometer.
Endoscopy
Suspected BE was defined by any length of visible pink mucosa in the tubular esophagus above the proximal end of the gastric folds. For these cases, the length of circumferential areas of BE and the number and length of BE tongues were measured according to the Prague C and M grading system.12, 13 The presence and severity of erosive esophagitis seen on endoscopy were graded according to the Los Angeles (LA) Classification (grade A–D).14 The presence of hiatal hernia, nodularity, and ulceration in the esophagus were also systematically recorded.
To ensure standardization of study endoscopy report, the principal investigator (MG) conducted orientation sessions with all endoscopists involved in the study. These involved both live as well as picture demonstration and agreement on landmarks of gastroesophageal endoscopic findings. All endoscopists were required to provide endoscopic photograph of the gastroesophageal junction (GEJ) area including the squamocolumnar junction in all procedures. The endoscopists involved were not limited to members of the investigative team. Every 4 months, a sample of 10 photographs , including all suspected BE, of the GEJ area from each endoscopist were reviewed by the PI/co PI, both of whom were blinded to the findings of the endoscopic report (presence/absence of suspected BE). If a discrepancy between the endoscopist and the PI/coPI exceeded 20%, another training session was conducted with that endoscopist.
Esophageal Biopsies and Histopathology
Esophageal biopsies were obtained in all cases at 2–3 cm above the squamocolumnar junction and in suspected BE cases, four-quadrant biopsies for every 1 cm of suspected circumferential BE tissue and biopsies from any tongues were also obtained. We did not require taking a biopsy of the squamocolumnar junction. All biopsies were reviewed by local pathologist at each of the 3 institutions. All mucosal biopsies from suspected BE were subsequently examined and graded by one gastrointestinal pathologist blinded to the endoscopic finding and risk factors. Cases of definite BE were defined as endoscopic presence of any visible length of pink mucosa in the esophagus combined with histological presence of goblet cells in biopsies taken from these areas. Cases of RE were defined as endoscopic erosion according to the LA classification or reflux esophagitis on histopathology, which is defined as biopsy showing reactive epithelial changes with hyperplasia of the basal cell layer, elongation of vascular papillae, and increase infiltration of inflammatory cells.15 We excluded 19 patients with possible eosinophilic esophagitis as defined by the histological presence of greater than 15 eosinophils per high power field irrespective of symptoms.
The study protocol was approved by the Institutional Review Board (IRB) at the three institutions and informed consent was obtained from parents of study subjects.
Statistical Analysis
The prevalence rate (and 95% confidence intervals) of suspected BE, definite BE, or RE were calculated by dividing the number of patients with these conditions by the total number of patients in the study with complete historical, endoscopic, and histological data. Potential risk factors or associated symptoms were analyzed for suspected BE (vs. no BE or RE) and RE (vs. no BE or RE) using chi-square tests or Fisher’s exact test for categorical variables and ANOVA for continuous variables. Logistic regressions were used to estimate odds ratios and their accompanying 95% confidence intervals.
Results
Between February 2006 and December 2007, a total of 840 patients were enrolled with complete questionnaire and endoscopic data. Of those patients, 570 were from Texas Children’s Hospital, 146 were from Phoenix Children’s Hospital, and 124 were from Barbara Bush Children’s Hospital. The mean age of enrolled patients was 9.5 (SD: 5.3) years. Twelve patients were suspected of having BE thus giving a prevalence of 1.41% (95% CI: 0.73–2.45). Six of the suspected BE were C0M1, two C0M1.5, two C0M1.5, one C0M2 and one C0.5M1.5 on the Prague classification where C refers to circumferential length and M refers to maximum length in centimeters 13. The level of agreement on suspected BE among the endoscopist, PI and co PI was 100%. Only 1 patient (14 years old) with suspected BE had intestinal metaplasia in biopsy obtained from suspected BE areas (Figure 1) thus giving a prevalence of 0.12% (95% CI: 0–0.65); the indication for endoscopy in that child was nausea and vomiting unresponsive to PPI. The rest of the patients with suspected BE had gastric oxyntic glands (n=6) or squamous (n=5) esophageal epithelium and no goblet cells on histologic examinations of biopsies obtained from suspected BE.
Figure 1.
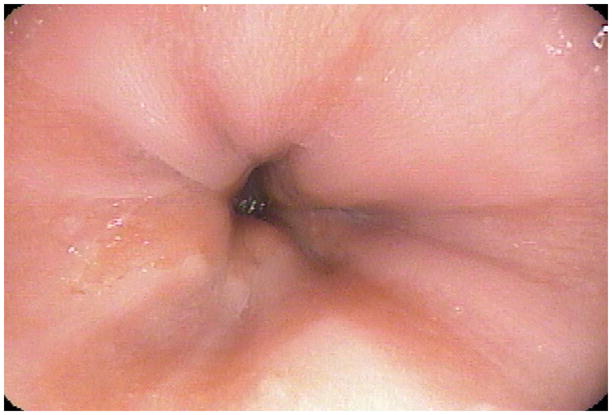
Endoscopic picture of the one patient with confirmed Barrett's esophagus based on endoscopic finding (C0M1) and histologic finding of specialized intestinal epithelium.
The mean age of patients with suspected BE was significantly different than those without BE or RE (11.3 vs. 9.2 years, p=0.03) (Table 1). Patients with suspected BE had higher mean BMI (23.0 vs. 19.1, p=0.05) and reported significantly more chest pain (50% vs. 15%, p<0.01) than patients without BE or RE. While dysphagia, epigastric pain heartburn, and regurgitation were more frequent in patients with suspected BE than patients without BE or RE, the differences were not statistically significant. Multivariate analysis was not conducted given the low number of suspected BE cases.
Table 1.
Characteristics of the Cohort by Disease Category (Suspected Barrett’s esophagus (BE), reflux esophagitis (RE), or neither)
| Suspected BE N=12 # (%) | RE N=216 # (%) | Neither N=612 # (%) | p-value* (general association) | |
|---|---|---|---|---|
| Age | ||||
| Mean (sd), yrs | 11.3 (4.5) | 10.1 (5.0) | 9.2 (5.3) | 0.03 |
| Median | 12.5 | 11.0 | 10.0 | |
| 0–5 yrs | 2 (17) | 45 (21) | 175 (29) | |
| 6–12 yrs | 4 (33) | 83 (38) | 228 (37) | |
| 13+ yrs | 6 (50) | 88 (41) | 209 (34) | |
| BMI | ||||
| Mean (sd) | 23.0 (7.2) | 19.0 (5.6) | 19.1 (5.4) | 0.05 |
| Median | 22.8 | 18.0 | 17.6 | |
| Tobacco Use | 1 (8) | 1 (0) | 0 (0) | <0.01 |
| Endoscopic Signs | ||||
| Erosions | 2 (17) | 10 (5) | 0 (0) | <0.01 |
| Hiatal Hernia | 1 (8) | 6 (3) | 18 (3) | 0.54 |
| Nodularity | 0 (0) | 13 (6) | 18 (3) | 0.09 |
| Ulceration | 0 (0) | 6 (3) | 0 (0) | <0.01 |
p-values for general associations were calculated as 2-sided using Chi-square tests, Fisher’s exact test, or ANOVA with an asterisk indicating significance at the 0.05 level.
A total of 216 patients (25.7%, 95% CI: 18.0–22.4) had RE. Of those, only 16 patients had erosions seen endoscopically while the rest had histological esophagitis. The mean age of patients with RE was 10.1 (SD 5.0) years. Patients with RE reported significantly more chest pain (20% vs. 13%, p<0.01), dysphagia (19% vs. 10%, p<0.01), epigastric pain (79% vs. 66%, p<0.01), heartburn (38% vs. 28%, p=0.01), and regurgitation (38% vs. 26%, p<0.01) than patients without BE or RE (Table 2). The association between RE and dysphagia and epigastric pain remained statistically significant in a multivariable analysis that adjusted for age, BMI, fundoplication, and the other upper gastrointestinal symptoms (aspiration, chest pain, heartburn, regurgitation, vomiting, or poor weight gain).
Table 2.
The association between several symptoms and suspected Barrett’s esophagus (BE), reflux esophagitis (RE), or neither condition.
| Suspected BE N=12 # (%) | RE N=216 # (%) | Neither BE or RE N=612 # (%) | p-value (BE vs. neither) | p-value (RE vs. neither) | Adjusted OR* (95% CI) for RE vs. neither | |
|---|---|---|---|---|---|---|
| Aspiration | 2 (17) | 21 (10) | 39 (6) | 0.15 | 0.10 | 1.18 (0.61–2.26) |
| Chest Pain | 6 (50) | 43 (20) | 77 (13) | <0.01 | <0.01 | 1.08 (0.67–1.74) |
| Dysphagia | 2 (17) | 40 (19) | 68 (10) | 0.49 | <0.01 | 1.67 (1.03–2.70) |
| Epigastric Pain | 9 (75) | 171 (79) | 401 (66) | 0.49 | <0.01 | 1.67 (1.13–2.46) |
| Decreased appetite | 2 (17) | 59 (27) | 130 (21) | 0.70 | 0.06 | 1.29 (0.83–2.00) |
| Heartburn | 6 (50) | 81 (38) | 172 (28) | 0.10 | 0.01 | 1.10 (0.74–1.63) |
| Poor weight gain | 3 (25) | 60 (28) | 151 (25) | 1.00 | 0.37 | 1.04 (0.68–1.59) |
| Regurgitation | 4 (33) | 83 (38) | 162 (26) | 0.59 | <0.01 | 1.35 (0.92–1.96) |
| Vomiting | 4 (33) | 81 (38) | 199 (33) | 1.00 | 0.18 | 1.23 (0.87–1.75) |
| Fundoplication | 0 (0) | 8 (4) | 5 (1) | 0.75 | <0.01 | 3.07 (0.93–10.10) |
Odds ratios adjusted for BMI, age, fundoplication, and the other upper gastrointestinal symptoms (aspiration, chest pain, dysphagia, epigastric pain, feeding, heartburn, poor weight gain, regurgitation, and vomiting).
Discussion
In this large, prospective, multicenter study of children and adolescents presenting for upper endoscopy, we found that BE is rare in children without neurodevelopmental delay or tracheoesophageal anomalies. The prevalence of endoscopically suspected BE was 1.41%, and was reduced to 0.12% if histologic finding of intestinal metaplasia was also considered. The prevalence of RE (25.7%), on the other hand, is much higher and approaches the prevalence of RE in the adult population.16
The very low prevalence of BE in children without neurodevelopmental delay or tracheoesophageal anomalies affirms previous retrospective studies. Collectively, these studies also confirm that congenital BE is very rare or nonexistent. In two separate studies among different patient populations, we reported the prevalence of endoscopically suspected BE in children without neurodevelopmental delay or tracheoesophageal anomalies to be between 0.25% and 2.7%.5, 9 While different explanations (to the reported low BE prevalence in children) related to endoscopic recognition of land marks have been posited,11, 17 this study with its prospective design, standard definition of landmarks, and quality control measures should allay these concerns. Larger prospective studies, with stricter or different definition of landmarks and more extensive biopsy protocols may result in slightly different BE prevalence estimates, however, we believe that the question has been answered for this type of population. The findings however cannot be generalized to general pediatric population or to patients with neurodevelopmental delay.
Despite the high prevalence of GERD symptoms as evidenced by approximately 42.7% of the study population reporting heartburn or regurgitation and 88.6% reporting at least some upper GI symptom, the prevalence of endoscopic BE was very low, much lower than the expected rates in adults with similar symptoms.18–20 This suggests that duration of symptoms and or age related effects are important risk factors for BE. Studies in adults indicate that long duration of GERD symptoms is a significant risk factor for BE.21, 22
There were several cases in our current study where endoscopic BE was reported but intestinal metaplasia was not found on histological examination of biopsies obtained from these areas. This finding is consistent with previous studies.5, 8, 9 While sampling error may be a possible explanation, another explanation may be that these areas of endoscopic BE become populated with intestinal metaplasia at a later time. At this time, it is difficult to make a firm recommendation regarding the prognosis or follow up of these patients.
The power to examine potential risk factors for BE was very limited for the very few cases of suspected BE and virtually non existent for the only one confirmed BE case. Nevertheless, obesity was a weak risk factor for suspected BE. While we cannot exclude type 1 error, this might be a finding worth pursuing. Given the obesity epidemic in children, it is possible the prevalence of BE would be higher in the future in children. Given the rare occurrence of BE in the pediatric population any study of the characteristics of pediatric BE would require a large multicenter study over many years.
Our study showed that the prevalence of RE was 25.7%, but only 16 of 840 patients (0.02%) had erosions seen endoscopically while the rest had histological esophagitis. While we were careful not to classify patients in whom eosinophil count was greater than 15 per high power field as eosinophilic esophagitis rather than RE, we could have misclassified cases of eosinophilic esophagitis that were well controlled with treatment at that time. The low prevalence of erosions on endoscopy is different from two previous studies that examined the prevalence of erosive esophagitis in children, which report the prevalence of endoscopic erosive esophagitis to be 12.4% and 34.6%.5, 10 The difference in prevalence of endoscopic erosive esophagitis could be due to the lack of standard scoring system for esophagitis or it could be due to the real changes in the prevalence of endoscopic esophagitis secondary to the changes in prescribing practice of proton pump inhibitors (PPIs) and histamine H2-receptor antagonist (H2RA). The prior studies by El-Serag et al. and Gilger et al. examined patients who underwent upper endoscopy during 1996–2000 and 1999–2001, instead the current study examines patients during 2006–2007 where PPIs may more readily available. We did not systematically collect information on the use of medications and therefore could not ascertain the use of PPI, H2RA, or other medications that could be used to treat other esophageal disorders such as eosinophilic esophagitis.
In summary, we have shown in a large multicenter prospective study that suspected BE is rare (and confirmed BE is very rare) in children and adolescent without neurodevelopmental delay or tracheoesophageal anomalies.
Acknowledgments
Drs. Gilger and El-Serag are supported by R03 (NIH 1-R03-DK068148-01). Dr. El-Serag is also supported in part by The Texas Gulf Coast Digestive Diseases Center (NIH P50 DK56338), and Houston VA HSR&D Center of Excellence (HFP90-020).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Shepherd RW, Wren J, Evans S, Lander M, Ong TH. Gastroesophageal reflux in children. Clinical profile, course and outcome with active therapy in 126 cases. Clin Pediatr (Phila) 1987;26:55–60. doi: 10.1177/000992288702600201. [DOI] [PubMed] [Google Scholar]
- 2.Campanozzi A, Boccia G, Pensabene L, Panetta F, Marseglia A, Strisciuglio P, Barbera C, Magazzu G, Pettoello-Mantovani M, Staiano A. Prevalence and natural history of gastroesophageal reflux: pediatric prospective survey. Pediatrics. 2009;123:779–783. doi: 10.1542/peds.2007-3569. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Gilger M, Carter J, Genta RM, Rabeneck L. Childhood GERD is a risk factor for GERD in adolescents and young adults. Am J Gastroenterol. 2004;99:806–812. doi: 10.1111/j.1572-0241.2004.30098.x. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Gilger M, Kuebeler M, Rabeneck L. Extraesophageal associations of gastroesophageal reflux disease in children without neurologic defects. Gastroenterology. 2001;121:1294–1299. doi: 10.1053/gast.2001.29545. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Bailey NR, Gilger M, Rabeneck L. Endoscopic manifestations of gastroesophageal reflux disease in patients between 18 months and 25 years without neurological deficits. Am J Gastroenterol. 2002;97:1635–1639. doi: 10.1111/j.1572-0241.2002.05820.x. [DOI] [PubMed] [Google Scholar]
- 6.Orenstein SR, Shalaby TM, Kelsey SF, Frankel E. Natural history of infant reflux esophagitis: symptoms and morphometric histology during one year without pharmacotherapy. Am J Gastroenterol. 2006;101:628–640. doi: 10.1111/j.1572-0241.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 7.Nelson SP, Chen EH, Syniar GM, Christoffel KK. Prevalence of symptoms of gastroesophageal reflux during childhood: a pediatric practice-based survey. Pediatric Practice Research Group. Arch Pediatr Adolesc Med. 2000;154:150–154. doi: 10.1001/archpedi.154.2.150. [DOI] [PubMed] [Google Scholar]
- 8.Hassall E. Columnar-lined esophagus in children. Gastroenterol Clin North Am. 1997;26:533–548. doi: 10.1016/s0889-8553(05)70312-5. [DOI] [PubMed] [Google Scholar]
- 9.El-Serag HB, Gilger MA, Shub MD, Richardson P, Bancroft J. The prevalence of suspected Barrett's esophagus in children and adolescents: a multicenter endoscopic study. Gastrointest Endosc. 2006;64:671–675. doi: 10.1016/j.gie.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Gilger MA, El-Serag HB, Gold BD, Dietrich CL, Tsou V, McDuffie A, Shub MD. Prevalence of endoscopic findings of erosive esophagitis in children: a population-based study. J Pediatr Gastroenterol Nutr. 2008;47:141–146. doi: 10.1097/MPG.0b013e31815eeabe. [DOI] [PubMed] [Google Scholar]
- 11.Hassall E. Endoscopy in children with GERD: “the way we were” and the way we should be. Am J Gastroenterol. 2002;97:1583–1586. doi: 10.1111/j.1572-0241.2002.05853.x. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong D. Review article: towards consistency in the endoscopic diagnosis of Barrett's oesophagus and columnar metaplasia. Aliment Pharmacol Ther. 2004;20 (Suppl 5):40–47. doi: 10.1111/j.1365-2036.2004.02132.x. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Dent J, Armstrong D, Bergman JJ, Gossner L, Hoshihara Y, Jankowski JA, Junghard O, Lundell L, Tytgat GN, Vieth M. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392–1399. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 14.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherman PM, Hassall E, Fagundes-Neto U, Gold BD, Kato S, Koletzko S, Orenstein S, Rudolph C, Vakil N, Vandenplas Y. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. Am J Gastroenterol. 2009;104:1278–1295. doi: 10.1038/ajg.2009.129. [DOI] [PubMed] [Google Scholar]
- 16.Nandurkar S, Talley NJ. Epidemiology and natural history of reflux disease. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:743–757. doi: 10.1053/bega.2000.0122. [DOI] [PubMed] [Google Scholar]
- 17.Hassall E. Esophageal metaplasia: definition and prevalence in childhood. Gastrointest Endosc. 2006;64:676–677. doi: 10.1016/j.gie.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Winters C, Jr, Spurling TJ, Chobanian SJ, Curtis DJ, Esposito RL, Hacker JF, III, Johnson DA, Cruess DF, Cotelingam JD, Gurney MS. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987;92:118–124. [PubMed] [Google Scholar]
- 19.Sarr MG, Hamilton SR, Marrone GC, Cameron JL. Barrett's esophagus: its prevalence and association with adenocarcinoma in patients with symptoms of gastroesophageal reflux. Am J Surg. 1985;149:187–193. doi: 10.1016/s0002-9610(85)80031-3. [DOI] [PubMed] [Google Scholar]
- 20.Falk GW. Barrett's esophagus. Gastroenterology. 2002;122:1569–1591. doi: 10.1053/gast.2002.33427. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman DA, Oehlke M, Helfand M. Risk factors for Barrett's esophagus in community-based practice. GORGE consortium. Gastroenterology Outcomes Research Group in Endoscopy. Am J Gastroenterol. 1997;92:1293–1297. [PubMed] [Google Scholar]
- 22.Eisen GM, Sandler RS, Murray S, Gottfried M. The relationship between gastroesophageal reflux disease and its complications with Barrett's esophagus. Am J Gastroenterol. 1997;92:27–31. [PubMed] [Google Scholar]


