Abstract
Proteolytic processing of transmembrane receptors and ligands can have a dramatic impact on cell signaling processes and subsequent cellular responses, including activation and differentiation. A member of the disintegrin and metalloproteinase family, ADAM10, has emerged as a prominent regulator of numerous receptors and ligands, including Notch and CD23. Here, we review studies resulting from the recent generation of ADAM10 conditional knockout mice which revealed a critical role for ADAM10 in Notch-dependent lymphocyte development. Additionally, we discuss results of numerous in vitro and ex vivo studies indicating that ADAM10 regulates the production of multiple secreted factors that contribute to autoimmune reactions.
Keywords: a disintegrin and metalloproteinase 10, Notch, CD23, Kuzbanian, lymphocyte development, autoimmunity
1. Introduction
Given that receptor:ligand binding events are critical for intercellular signaling, proteolytic processing of transmembrane receptors and ligands can have a dramatic impact on signaling-mediated cellular responses. Proteolysis of eukaryotic proteins is performed by members of the metzincin superfamily, including matrix metalloproteinases (MMPs), disintegrin and metalloproteinases (ADAMs), and ADAM-thromospondins (ADAM-TS). Although all active metzincin proteases contain zinc-binding motifs within their protease domains, the presence of a transmembrane domain is characteristic of the ADAMs family. Thus, studies examining the processing of receptors and ligands expressed on cell surfaces have focused on ADAMs, which perform ectodomain shedding as well as regulated intramembrane proteolysis (RIP) of transmembrane proteins. Shedding extracellular domains of membrane-anchored proteins releases soluble fragments into extracellular space. This event can down-regulate signaling events that require transmembrane receptor expression or activate paracrine signaling by soluble products derived from ADAM substrates, such as soluble CD23 (sCD23) and tumor necrosis factor-α (TNF- α). Although ectodomain shedding is thought to occur constitutively, RIP requires the binding of ligands expressed on adjacent cells. Numerous receptors including Notch and CD44 require regulated proteolysis of the receptor:ligand complex to initiate the release of receptor intracellular domains (ICD) that translocate to the nucleus and alter gene expression. Mutations in the negative regulatory region (NRR) of ADAM substrates can cause ligand-independent intramembrane proteolysis, resulting in excessive ICD signaling and disease states, discussed below (Blobel, 2005; Crawford et al., 2009; Seals and Courtneidge, 2003).
The prototypical ADAM contains an inhibitory pro-domain, a highly conserved metalloprotease domain, a disintegrin domain conferring substrate specificity, a cysteine-rich region, a transmembrane portion, and a cytoplasmic tail capable of binding SH3 domains(Seals and Courtneidge, 2003). Although 38 ADAMs have been identified to date, only a subset of members, including ADAMs 8, 9, 10, 12, 15, 17, and 33, contain the conserved zinc-binding consensus motif that confers proteolytic activity to the protease domain. As a result of in vitro-based assays utilizing pharmacologic inhibitors and various ADAM-deficient murine embryonic fibroblasts (MEFs), ADAM10 has emerged as an important mediator of ectodomain shedding and RIP of numerous substrates, including amyloid plaque precursor (APP), Ephrins, cadherins, chemokines, Notch receptors, Delta-1, CD23, Lag-3, FasL, and CD44. Proteolytic processing of many of these substrates contributes to the pathogenesis of multiple disease states, including cancer and inflammation(Blobel, 2005; Crawford et al., 2009). In particular, the role of ADAM10-mediated APP processing and its effect on Alzheimer's disease have been extensively studied and are reviewed elsewhere(Postina, 2008). Here, we discuss recent studies that highlight the physiologic role of ADAM10-mediated cleavage events in lymphocyte development and inflammation related to autoimmunity. Additionally, we review reports describing novel functions of ADAM10, and the regulation of proteolytic activity.
2. The rate-limiting protease in Notch RIP
2.1 A historical perspective
Since its discovery, it was evident that ADAM10 and its drosophila homolog, Kuzbanian (kuz), perform critical roles in developmental pathways. Kuz was initially identified by Rooke et al. in 1996, who generated kuz– embryos that revealed its essential role in lateral inhibition required for development of peripheral and central nervous systems. Cloning and sequence analysis of kuz demonstrated the presence of disintegrin and metalloproteinase domains(Rooke et al., 1996). Database searches identified a mammalian homolog (43% amino acid identity), bovine metalloprotease (BMP, later named ADAM10(Wolfsberg et al., 1995)) that was isolated from brain myelin and shown to cleave myelin basic protein in vitro(Howard and Glynn, 1995). By overexpressing dominant negative (DN) mutants of kuz that lack the protease domain in drosophila and Xenopus, Pan et al. demonstrated that the defect in lateral inhibition is due to the requirement for kuz to initiate RIP-mediated signaling through the Notch receptor (Pan and Rubin, 1997). This finding was supported by Sotillos et al. However, the authors acknowledged phenotypic differences between kuz and Notch mutant flies and concluded that Notch could also be processed in a kuz-independent manner(Sotillos et al., 1997).
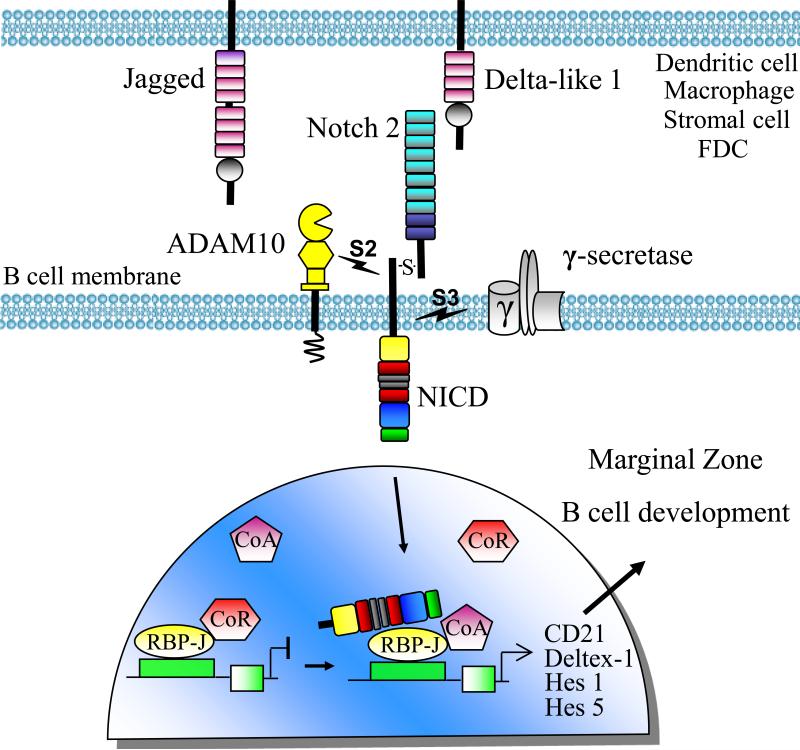
In mammalian cells, signaling through Notch receptors critically regulates cell fate processes of numerous cell types, including murine embryos. Following receptor ligation of Notch ligands, including Delta-like (Dll)1-4 and Jagged 1-2, expressed on adjacent cells, Notch signaling is initiated by ADAM-mediated proteolysis of the extracellular domain of the Notch receptor that is endocytosed by the ligand-expressing cell. This cleavage event, termed the S2 cleavage, produces a substrate for the γ-secretase complex to perform S3 cleavage and release the Notch intracellular domain (NICD) from the cell membrane. This results in NICD translocation to the nucleus, where it complexes with the transcription factor RBP-Jκ, and induces transcription of Notch target genes, including Hairy enhancer of split (Hes1), Hes5, and Deltex-1 (Figure 1).
Figure 1. ADAM10-mediated RIP of Notch2 is required for Marginal Zone B cell development.
Signaling through the Notch2 receptor expressed on transitional B cells directs marginal zone B cell differentiation(Saito et al., 2003). The Notch2 heterodimer on B cells binds ligands, Jagged 1-2 and Delta-like 1, present on stromal and antigen presenting cells. Binding initiates sequential cleavage events by ADAM10 (S2 cleavage) and a γ-secretase complex (S3 cleavage). Cleavage releases the Notch2 intracellular domain (N2ICD), containing RAM  , nuclear localization
, nuclear localization  , transactivating (TAD)
, transactivating (TAD)  , EGF-repeat
, EGF-repeat  ,and PEST domains
,and PEST domains  . Transport of the N2ICD to the nucleus followed by binding to the transcription factor, RBP-Jĸ, allows the release of co-repressors (CoR), and attraction of co-activators (CoA) to the transcriptional complex. The activated complex transcribes Notch target genes, including CD21/35, Deltex-1, Hes 1, and Hes 5, that promote development of marginal zone B cells. Deletion of ADAM10, Notch2, and RBP-Jĸ from B cells, or Delta-like 1 from stromal cells prevents marginal zone B cell development.
. Transport of the N2ICD to the nucleus followed by binding to the transcription factor, RBP-Jĸ, allows the release of co-repressors (CoR), and attraction of co-activators (CoA) to the transcriptional complex. The activated complex transcribes Notch target genes, including CD21/35, Deltex-1, Hes 1, and Hes 5, that promote development of marginal zone B cells. Deletion of ADAM10, Notch2, and RBP-Jĸ from B cells, or Delta-like 1 from stromal cells prevents marginal zone B cell development.
Although numerous regulators of Notch signaling have been identified, the identity of the metalloprotease that initiates RIP-mediated Notch signaling in mammalian cells was highly controversial(Kopan and Ilagan, 2009). Initially, two independent groups concluded that ADAM17 (TNF-α converting enzyme, TACE) initiates signaling by performing S2 cleavage of the Notch1 receptor(Brou et al., 2000; Mumm et al., 2000). Loading cell membrane fragments from Notch-transfected HeLa cells onto RED-TSK columns resulted in co-elution of Notch receptor cleavage products with ADAM17, rather than ADAM10. Additionally, results of an in vitro cleavage assay and a monocytic cell differentiation experiment demonstrated ADAM17's ability to cleave truncated forms of Notch1 and direct Notch-dependent monocyte differentiation(Brou et al., 2000). Mumm et al. supported this finding by demonstrating Notch cleavage in transfected MEFs derived from ADAM10-null embryos(Mumm et al., 2000). As a result, ADAM17 is often referenced in the literature as the relevant proteinase that initiates Notch signaling(Radtke et al., 2004). Although these findings were in direct contrast to studies of kuz in drosophila, the authors could not rule out a role for ADAM10 in Notch cleavage, and suggested that ADAM10 and ADAM17 may be functionally redundant in vivo. This conclusion was supported by later in vitro studies demonstrating that ADAM10 can cleave many ADAM17 substrates from ADAM17–/– MEFs(Le Gall et al., 2009), while ADAM17 can also cleave numerous ADAM10 substrates(Hinkle et al., 2004; Sahin et al., 2004). However, a critical role for ADAM10 in Notch activation re-emerged following the generation of ADAM10-deficient mouse embryos that displayed many features also observed in nonviable Notch1–/– embryos (Hartmann et al., 2002; Swiatek et al., 1994) In contrast, embryonic loss of ADAM17 did not result in a Notch phenotype(Peschon et al., 1998). Following these observations, two groups utilizing ADAM10–/– MEFs recently reported that while multiple proteases can perform ligand-independent proteolysis of Notch1, ADAM10 is required for ligand-dependent cleavage of Notch (Bozkulak E.C. and Weinmaster, 2009; van Tetering G. et al., 2009). Thus, ADAM10 may play a more critical role in Notch signaling than earlier in vitro studies predicted. However, in utero lethality of ADAM10-null embryos at day E9.5 had limited the examination of ADAM10-mediated cleavage events in the development of other cell types, including lymphocytes.
2.2 T cell development
The impact of Notch signaling in T cell development has been thoroughly examined, and is reviewed elsewhere(Tanigaki and Honjo, 2007). Briefly, Notch1 signaling is essential for the development of thymocyte precursors. This is best illustrated by the presence of thymic B cells in mice that lack Notch1 expression in common lymphoid progenitors (CLPs)(Wilson et al., 2001). Additionally, enforced Notch1 signaling in bone marrow progenitors expressing the constitutively active NICD promotes T cell fate(Pui et al., 1999). In fact, human mutations in the NRR surrounding the S2 cleavage site of Notch1 results in ligand-independent proteolysis and excessive Notch1 activation, ultimately causing T cell acute lymphocytic leukemia (T-ALL). This mutation accounts for approximately 50 percent of all T-ALL cases(Kopan and Ilagan, 2009). Multiple groups have reported effective use of γ-secretase inhibitors (GSIs) in limiting T cell development in vitro and in mouse models of T-ALL(Real et al., 2009; Sanda et al., 2010). However, GSIs cause gastrointestinal disease and must be coupled with potent anti-inflammatory drugs, such as dexamethasone, in T-ALL mouse models(Real et al., 2009). Thus, there has been great interest in elucidating the role of ADAMs in Notch1-mediated thymocyte development.
Manilay et al. circumvented the limitation of ADAM10–/– embryo lethality by generating transgenic mice that overexpress the dominant negative form of ADAM10 (dnKuz) under control of the T cell-specific promoter, lck(Manilay et al., 2005). dnKuz expression caused a partial block in thymocyte development between the double negative (DN) and double positive (DP) stages. This corresponded to decreased TCRβ expression and pre-mature down-regulation of CD25. Although these findings were also observed in Notch1-deficient thymocytes(Wolfer et al., 2002), there were discrete differences in thymocyte development and gene expression between these mice. dnKuz mice have reduced levels of DN thymocytes and γδ T cells, whereas conditional Notch1-deficient mice do not, suggesting that ADAM10 may regulate early thymocyte development by processing other substrates in addition to Notch1. Furthermore, expression of Notch target genes, Hes1 and Deltex-1, was only moderately decreased compared to non-transgenic mice. However, this could be due to the persistence of endogenous ADAM10 expression in dnKuz mice. Interestingly, enforced expression of the predominant Notch ligand, Dll1, rescued thymocyte development in dnKuz mice in a non-cell autonomous manner. This indicated that ADAM10 may regulate thymocyte development by processing Dll1 expressed on adjacent cells. This possibility is supported by studies demonstrating that ADAM10 also cleaves Dll1 in transfected MEFs(Six et al., 2003). However, another study demonstrated that Dll1 is a substrate of multiple other ADAMs(Dyczynska et al., 2007). Nevertheless, the impaired development of dnKuz thymocytes is also consistent with the conclusion that dnKuz impairs ADAM10-dependent processing of the Notch1 receptor.
Further examination of ADAM10's role in thymocyte development awaited the production of ADAM10-floxed mice, which has resulted in significant progress toward elucidating the physiologic impacts of ADAM10-mediated cleavage events. By utilizing lck-cre transgenic mice, Tian et al. reported impaired development and suppressed Notch1 signaling in ADAM10-deficient thymocytes(Tian et al., 2008). ADAM10-deficient thymocyte development resembled that of dnKuz and lck-directed Notch1-deficient mice. Additionally, Tian et al. demonstrated that production of the NICD was not detectable in ADAM10-deficient thymocytes. However, in contrast to the previous reports, TCRβ expression was not altered by ADAM10 deletion. Thus, the authors concluded that ADAM17 may perform a compensatory role in Notch activation during early stages of DN thymocyte development. However, generation of ADAM17 mutant and conditional knockout mice has not resulted in Notch related phenotypes in lymphocytes (Le Gall et al., 2009; Li et al., 2007a). Interestingly, although the effects of dnKuz and ADAM10 deletion indicate an important Notch-mediated role for ADAM10 in thymocyte development, neither demonstrated altered absolute numbers of T cells in the periphery. Moreover, authors of both reports acknowledged that the modest ADAM10-mediated alterations of thymocyte development do not approach the complete loss of T cell development in mice lacking Notch1 expression in CLPs. They hypothesize that this is dependent on the promoter driving dnKuz or cre expression. Lck-cre did not completely prevent ADAM10 expression in early DN thymocytes. Thus, examining the role of ADAM10 in CLP-commitment to the T cell lineage and the development of early thymocyte precursors must await the generation of other conditional knockout or transgenic mice.
Notch signaling has also been implicated in multiple T cell-mediated autoimmune diseases, including autoimmune lymphoproliferative syndrome (ALPS) and systemic lupus erythematosus (SLE)(Teachey et al., 2008). Additionally, very recent studies have demonstrated a role for Notch ligands, including Dll4, in regulating the differentiation of Th2 and Th17 cells(Mukherjee et al., 2009; Schaller et al., 2007). However, the role of ADAM10 in mature T cell differentiation and activation has not been examined, and may require the use of conditional knockout mice with unaltered thymocyte development.
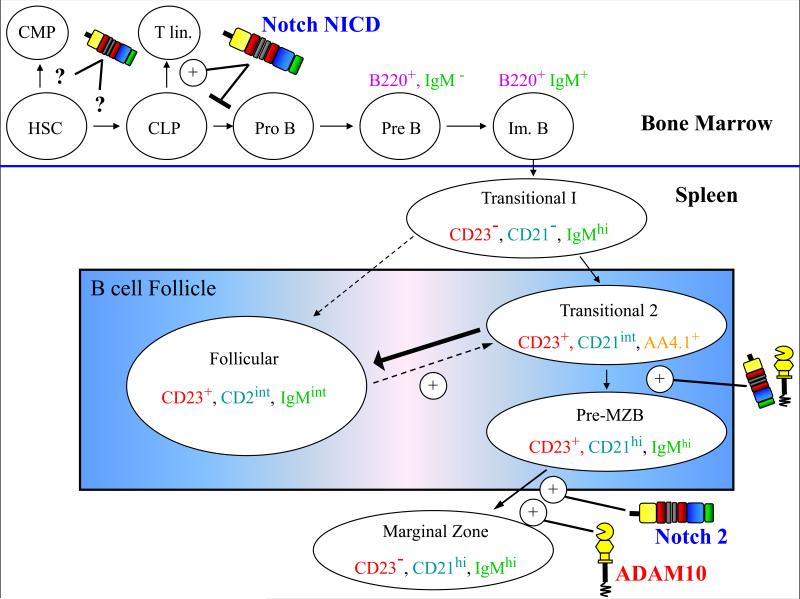
2.3 B cell development is ‘marginal’
B2 lymphocytes, which comprise the majority of circulating B cells, develop from CLPs and differentiate into pro-, then pre-, and ultimately immature B cells prior to exiting the bone marrow (Figure 2). Just as Notch signaling promotes CLP commitment to the T cell lineage, it prevents B lineage fate. Thus, multiple studies have demonstrated that enforced expression of active NICD in bone marrow progenitors completely abrogates B2 cell development(Kawamata et al., 2002; Pui et al., 1999). Following exit from the bone marrow, B2 cells enter a transitional stage and undergo further maturation in the spleen, where the majority differentiate into follicular B cells, while a subset develop into cells of the marginal zone B (MZB) cell lineage, including pre-MZBs and MZB cells. In contrast to T cells, which express Notch1 during development, B lymphocytes preferentially express Notch2, and express minimal levels of Notch1, 3, and 4(Moriyamaet al., 2008; Saito et al., 2003; Santos et al., 2007). By generating B-cell specific Notch2 knockout mice, Saito et al. demonstrated that Notch2 signaling is required for development of the MZB cell lineage(Saito et al., 2003), which initiates immune responses to blood-borne infections and transports antigen into the spleen follicles (Pillai et al., 2005). By deleting ADAM10 in a mature B cell-specific manner with CD19-cre knockin mice, Gibb et al. also demonstrated an absolute requirement for ADAM10 in MZB development(Gibb et al., 2010). Analysis of Notch target gene expression revealed a dramatic defect in Notch2 signaling. Furthermore, in contrast to wildtype B cells, ADAM10-null cells were completely unresponsive to Dll1-induced Notch stimulation. These findings not only revealed the importance of ADAM10 in B cell development, but also demonstrated that ADAM10 is also responsible for activating RIP-mediated signaling through Notch2. Although S2 cleavage of Notch3 and Notch4 has not been examined, this result suggests that ADAM10 may contribute to their activation as well. In contrast to reports of ADAM10 deletion in thymocytes, deletion in mature B cells did not result in any compensatory Notch2 cleavage by other proteases, including ADAM17. Thus, ADAM10 may be the only protease that can recognize the Notch2 cleavage site, which is distinct from the Notch1 site(Brou et al., 2000).
Figure 2. ADAM10 and Notch similarly regulate B cell development.
Precursors to the B cell lineage develop from common lymphoid progenitors (CLPs) in the bone marrow. Constitutive expression of the Notch intracellular domain (NICD), Hes 1 or Hes 5 in CLPs prevents B lineage commitment, while promoting T cell development. Development of the marginal zone B cell (MZB) lineage is regulated by Notch2 signaling. The majority of transitional cells differentiate into follicular B cells, which constitute the majority of circulating B cells. However, ADAM10-mediated Notch2 signaling promotes MZB lineage development. HSC-hematopoietic stem cell, CMP-common myeloid progenitor, Im-Immature. Solid arrows and dashed arrows indicate confirmed and hypothetical differentiation events, respectively.
Interestingly, enhanced Notch2 signaling is a defining characteristic of B cell chronic lymphocytic leukemia (B-CLL), diffuse large B cell lymphoma, and marginal zone lymphoma (Lee et al., 2009; Rosati et al., 2009; Troen et al., 2008).Thus, ADAM10 is a potential target for the treatment of B cell proliferative disease. However the role of ADAM10 in early B cell development in the bone marrow has not been explored, and warrants further study.
2.4 B cell activation, germinal center formation, and antibody production
Recent studies have addressed the role of Notch signaling in mature B cell activation and function. Thomas et al. demonstrated that Notch signaling synergizes with B-cell receptor (BCR) and CD40 signaling to enhance murine B cell activation. Upon BCR and CD40 engagement, follicular B cells cultured on OP9 stromal cells expressing Dll1 showed enhanced proliferation and generation of IgG1+ cells, when compared to cells cultured on control stroma. Furthermore, in vivo experiments showed that Notch signaling contributed to the production of IgG+ cells during a T-dependent immune response. Moreover, Dll1-mediated B cell activation was determined to be dependent upon RBP-Jĸ transcriptional regulation. Follicular B cells isolated from mice expressing a dominant negative form of MAML1, a positive regulator of RBP-Jĸ, failed to show enhanced proliferation and production of IgG1+ cells upon stimulation with anti-CD40 or anti-IgM in the presence of Dll1(Thomas et al., 2007).
A later study by Santos et al. showed that Notch signaling contributes to B cell differentiation to antibody secreting cells (ASC) and also, under some conditions, promotes class switching. Experiments showed that Dll1-expressing cells enhanced LPS-induced ASC differentiation. Disruption of Notch1 or RBP-Jĸ reduced ASC recovery, thus, demonstrating that these results are dependent on Notch signaling. Interestingly, the authors also demonstrated that when MZB and B1 cells are cultured in the presence of Dll1, a significant percentage of the cells become ASCs. The authors concluded that Notch signaling by Dll1 could release an inhibitory signal that otherwise maintains B cells in a non-secreting state. This paper also addressed the differential role of Dll1 and Jagged1 (Jg1). Immunohistochemical analysis of murine spleens revealed that Dll1 and Jg1 are both expressed in the marginal zone. However, Dll1 is also expressed in the FDC area within primary and secondary follicles. Results of ex vivo cultures suggest that Dll1 enhances Ig secretion, while Jg1 has an inhibitory role(Santos et al., 2007).
In addition to B-cell activation and differentiation to ASCs, Notch signaling is also involved in germinal center formation. Yoon et al. recently demonstrated that Notch signaling protects germinal center (GC) B cells from apoptosis. Co-culturing GC B cells with FDC-like cells, HK, which express Dll1 and Jg1, enhanced GC B cell survival. Conversely, blockade of Notch signaling with GSIs increased GC B cell apoptosis. More studies are needed to determine the role of Notch signaling in GC formation in vivo. Furthermore, it has yet to be determined whether Notch1 or Notch2 is responsible for promoting GC B cell survival(Yoon et al., 2009).
Although these studies do not address the role of ADAM10, they demonstrate the importance of Notch signaling in regulating B cell survival, activation and differentiation into ASCs. Given that ADAM10 is a critical regulator of Notch2 signaling in B cells, they have generated intriguing questions about the role of ADAM10 in mature B cell differentiation and function.
2.5 Myeloid Development
In the classic model of hematopoiesis, hematopoietic stem cells (HSCs) differentiate to yield common myeloid progenitors (CMPs) or CLPs(Kawamoto and Katsura, 2009). CMPs undergo further differentiation into more mature myeloid cells, including the recently identified myeloid-derived suppressor cells (MDSCs). Because MDSCs suppress anti-tumor responses, myeloid differentiation has been the subject of many recent investigations(Gabrilovich and Nagaraj, 2009). To date, numerous hematopoietic pathways, including Notch signaling, have been implicated but remain controversial. Several investigators have reported that alterations in Notch signaling have minimal effects on the myeloid compartment(Carlesso et al., 1999; Pui et al., 1999; Stier et al., 2002).Yet, Kawamata et al. reported that constitutive Notch signaling promotes myeloid differentiation in a non-cell autonomous manner(Kawamata et al., 2002).This is supported by a report of abrogated B cell and myeloid cell development in mice deficient in downstream targets of Notch(Schroeder et al., 2003). However, other studies have indicated that Notch signaling inhibits myeloid differentiation (Bigas et al., 1998; Qyang et al., 2004). Qyang et al. demonstrated that blockade of Notch signaling at the γ-secretase cleavage site induces myeloid accumulation (Qyang et al., 2004).
Many of these alterations were observed in mice with altered lymphocyte development. This suggests that Notch signaling may modulate the differentiation pathway of CLPs and CMPs from a common upstream progenitor. Two groups have recently described common myelo-lymphoid progenitors (CMLPs) that are indistinguishable from HSCs(Chi et al., 2009; Kawamoto and Katsura, 2009). Although these multipotent cells often commit to the B or T cell lineage, they retain the potential for myeloid development (Kawamoto and Katsura, 2009). Therefore, alterations in Notch signaling during lymphocyte development could also affect myelopoiesis. Collectively, these findings indicate that myeloid differentiation may be regulated by the signal strength and temporal stage of Notch signaling. Therefore, examination of other Notch regulators, including ADAM10, could clarify the role of Notch signaling in myeloid differentiation.
3. ADAM10 shedding and autoimmunity
Although the role of ADAM10 in autoimmune disease has not been directly examined to date, numerous putative ADAM10-substrates are known contributors to autoimmune reactions and inflammation.
3.1 CD23
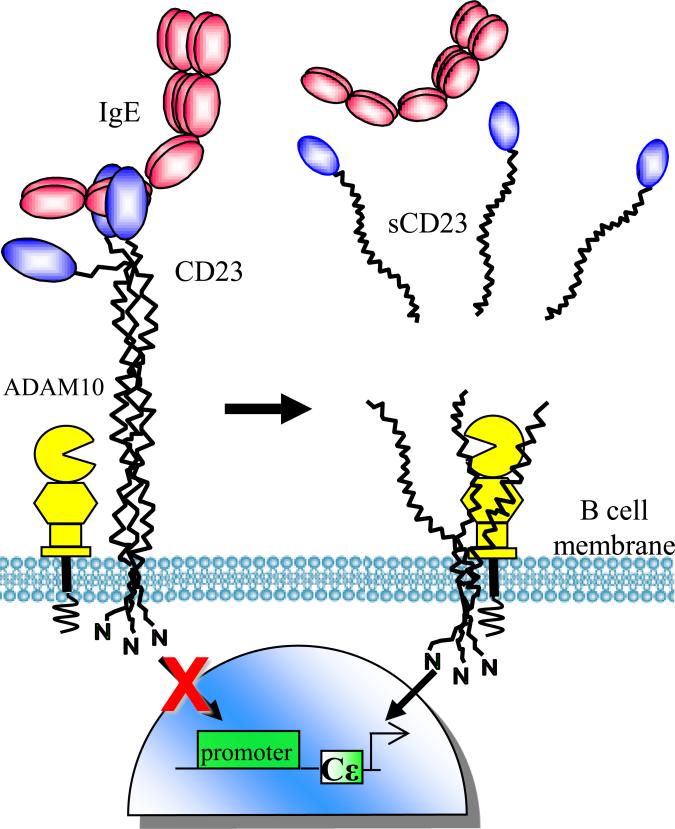
The low affinity IgE receptor, FcεRII or CD23, is a type II transmembrane Fc receptor expressed by B cells, follicular dendritic cells (FDCs) and some human myeloid cells. Numerous studies have implicated CD23 as a natural regulator of IgE production and IgE-mediated Type I hypersensitivity reactions, including allergic airway inflammation. Binding of IgE to CD23 on B cells is thought to transduce an inhibitory signal that suppresses further IgE synthesis (Figure 3). However, ADAM10 cleavage of CD23 may interrupt this negative feedback mechanism, resulting in elevated IgE levels (Conrad et al., 2007). In fact, treatment of immunized mice with the metalloprotease inhibitor, batimistat, not only inhibited CD23 cleavage, but also markedly reduced IgE production (Christie et al., 1997). We have recently shown that this cleavage occurs primarily intracellularly and both the CD23 and the ADAM10 are incorporated into exosomes (Mathews et al., 2010).
Figure 3. Ectodomain shedding of CD23, a regulator of IgE-mediated inflammation.
CD23 monomers, consisting of a short cytoplasmic tail, a transmembrane portion, an extracellular stalk region forming a coiled-coil structure, and a lectin head domain that binds IgE, form a homo-trimeric tertiary structure, which is stabilized by IgE binding. In the absence of IgE or the presence of anti-stalk antibodies, ADAM10 constitutively cleaves CD23 ectodomains and releases soluble CD23 (sCD23). Cleavage prevents IgE:CD23 interaction at the B cell surface, and results in elevated IgE synthesis. This figure was modified from Conrad et al.(Conrad et al., 2007).
Additionally, the soluble cleaved product, sCD23, is a contributing mediator in rheumatoid arthritis. CD23–/– mice have a delayed onset and reduced severity of collagen-induced arthritis (CIA), and treatment of wildtype mice with anti-CD23 antibodies markedly attenuates disease signs(Kleinau et al., 1999; Plater-Zyberk and Bonnefoy, 1995). Moreover, sCD23 is elevated in synovial fluids of patients with rheumatoid arthritis, and is associated with erosive status(Ribbens et al., 2000). sCD23 may contribute to rheumatic disease by activating monocytes via interactions with CD11c-CD18(Lecoanet-Henchoz et al., 1995). Because of these findings, inhibiting cleavage by the endogenous CD23 sheddase has been proposed as a novel therapy for controlling allergic and rheumatic disease (Conrad et al., 2007).
CD23 monomers, consisting of a short cytoplasmic tail, a transmembrane portion, an extracellular stalk region forming a coiled-coil structure, and a lectin head domain that binds IgE, form a homo-trimeric tertiary structure, which is stabilized by IgE binding. This stabilization can be mimicked by antibody binding to the lectin heads. Anti-lectin antibodies have been shown to inhibit CD23 cleavage and airway inflammation in a mouse asthma model(Dasic et al., 1999; Munoz et al., 1998). However, in the absence of ligand, monomers are highly susceptible to proteolytic cleavage within the stalk domain. Demonstration that CD23 cleavage is sensitive to hydroxamic acid inhibitors, which inhibit the proteolytic activity of ADAMs, stimulated significant progress toward identifying the sheddase(Conrad et al., 2007). Although early studies implicated the proteolytic activity of ADAMs 8 and 15(Fourie et al., 2003), subsequent studies ruled out a role for ADAMs 8, 9, 12, 15, and 17 in vivo (Le Gall et al., 2009; Weskamp et al., 2006). Two independent groups utilized ADAM10-null MEFs and pharmacologic inhibitors to demonstrate that ADAM10 activity is responsible for CD23 cleavage (Lemieux et al., 2007; Weskamp et al., 2006). However, (Jackson et al., 2009) later reported that MMP-9 is the principal sheddase of CD23 in LPS-treated mice, calling into question the physiologic relevance of ADAM10-mediated CD23 cleavage. Thus, Gibb et al. examined CD23 cleavage in B cell-specific ADAM10-deficient mice. Flow cytometric and immunohistochemical analysis revealed a remarkable increase in CD23 levels expressed on ADAM10-null B cells, while ELISAs illustrated a significant decrease in serum sCD23. Additionally, destabilization of CD23 trimers with anti-stalk CD23 antibodies only stimulated cleavage from control B cells without influencing cleavage from ADAM10-deficient cells (Gibb et al., 2010). The seemingly contradictory reports on MMP-9 and ADAM10-mediated cleavage may be explained by the use of LPS-treated mice by Jackson et. al (Jackson et al., 2009). Although analysis of ADAM10-deficient B cells demonstrated a clear role for ADAM10 in constitutive cleavage of CD23 from naïve B cells, LPS-induced activation may allow ADAM10-independent cleavage.
The impact of ADAM10-mediated CD23 cleavage on allergic and rheumatic disease remains the focus of current studies. Because ADAM10 has multiple substrates, including Notch, the response of ADAM10-null B cells in disease states might not be attributed to CD23. Thus, the generation of novel in vivo models is required to further elucidate the role of ADAM10-mediated cleavage events in allergic and rheumatic inflammation.
3.2 CD44
CD44 constitutes numerous cell adhesion proteins that are generated by alternative splicing of CD44 mRNA. Many CD44 transmembrane proteins, expressed by most inflammatory leukocytes, bind hyaluronan (HA) in the extracellular matrix. Inflammation-induced upregulation of CD44 by endothelial cells can cause leukocyte adhesion to inflamed tissue via CD44-HA-CD44 bridged binding. Cleavage of CD44 is thought to allow detachment of leukocytes, including T cells, from the endothelium and transmigration into inflamed tissue. Anti-CD44 antibodies have reduced the severity of numerous models of autoimmunity in mice, including CIA, type 1 diabetes in NOD mice, and experimental autoimmune encephalomyelitis (EAE)(Naor et al., 2007). Conversely, levels of the cleaved CD44 ectodomain are elevated in patients with rheumatoid arthritis. Interestingly, CD44 cleavage not only alters leukocyte adhesion and migration by reducing CD44 surface levels, but also activates RIP-mediated CD44 signaling. The intracellular domain of CD44 can alter gene expression and may result in various cell processes that cause leukocyte activation, proliferation, or apoptosis. Cleavage of CD44 also regulates migration and invasion by tumor cells, and was recently reviewed by Stamenkovic and Yu(Stamenkovic and Yu, 2009). Multiple MMPs, ADAM10, and ADAM17 have all been shown to cleave CD44 in multiple cell types. However, a recent report demonstrated that only ADAM10 can cleave CD44 in melanoma cells(Anderegg et al., 2009). Authors of an additional study utilizing ovarian carcinoma cells concluded that ADAM17 was responsible for CD44 release from the cell surface, while ADAM10 processed CD44 within the endosomal pathway(Stoeck et al., 2006). Additional studies of CD44 cleavage revealed a novel function of ADAM10, discussed below. However, the physiologic consequences of ADAM10-mediated CD44 processing have not been examined in vivo.
3.3 Chemokine shedding
Catabolic shedding of membrane-bound chemokines is an important processing event that regulates inflammatory responses. Results of several studies indicate that ADAM10 is responsible for release of fraktalkine (CX3CL1) and CXCL16 from the cell surface. The transmembrane form of fraktalkine facilitates leukocyte adhesion to endothelial cells by interacting with its receptor CX3CR1, while fraktalkine cleavage allows detachment(Hundhausen et al., 2007). Additionally, soluble fraktalkine is a chemoattractant for T cells and monocytes(Bazan et al., 1997). Elevated levels have been reported in multiple autoimmune states, including autoimmune exocrinopathy, neuropsychiatric lupus, and EAE(Huang et al., 2006; Sato et al., 2006; Tsubota et al., 2009). Additionally, fraktalkine inhibition ameliorates experimental autoimmune myositis and CIA in mice(Nanki et al., 2004; Suzuki et al., 2005).
CXCL16 is thought to regulate atherogenic inflammation, because the membrane-bound form can function as a scavenger for oxidized low density lipoprotein (oxLDL)(Sawamura et al., 1997), and the soluble form can attract NKT cells to inflamed tissue(Johnston et al., 2003). Elevation of soluble CXCL16 is considered a marker for atherosclerosis and coronary disease(Lehrke et al., 2007), while inhibition of CXCL16 can suppress antibody-mediated glomerulonephritis (Garcia et al., 2007). Thus, CXCL16 cleavage could promote arterial inflammation that underlies multiple disease states.
In vitro studies have demonstrated that siRNA or pharmacologic inhibition of ADAM10 can significantly impair cellular release of CXCL16(Abel et al., 2004; Gough et al., 2004). Accordingly, INF-γ and TNF-α induce soluble CXCL16 production from wildtype MEFs but do not influence release by ADAM10-deficient cells(Abel et al., 2004). Two studies extended ADAM10's role in chemokine release to fraktalkine by demonstrating diminished fraktalkine release from ADAM10-null MEFs and transfected COS-7 cells treated with an ADAM10 inhibitor(Ludwig et al., 2005; Schulte et al., 2007a). Schulte et al. also described sequential processing of both chemokines by ADAM10 and γ-secretase, which indicates an additional role for chemokine intracellular domains(Schulte et al., 2007a). Although these results indicate that ADAM10 performs an important role in chemokine processing and subsequent immune responses, this has not been examined in vivo. Thus, elucidating the physiologic consequences of ADAM10-mediated chemokine release awaits analysis of experimental autoimmunity in conditional knockout or transgenic mice.
3.4 FasL and TNF-α
Binding of the Fas receptor, a ubiquitously expressed member of the TNF receptor family, with Fas ligand (FasL) expressed on adjacent activated T cells and NK cells triggers apoptosis(Strasser et al., 2009). Mice lacking Fas or FasL develop lymphadenopathy and SLE-like autoimmune disease(Takahashi et al., 1994; Watanabe-Fukunaga et al., 1992). Additionally, a subset of ALPS patients have an inherited mutation in the Fas gene(Fisher et al., 1995; Rieux-Laucat et al., 1995). Thus, FasL:Fas interactions induce cell death of infected target cells, maintain lymphocyte homeostasis, and regulate lymphocyte expansion by mediating activation-induced cell death (AICD). Cleavage of FasL, a member of the TNF cytokine family, is thought to be an important mechanism regulating apoptosis, since soluble FasL is elevated in serum of patients with dysregulated inflammatory diseases. Although multiple proteases have been implicated in FasL cleavage, Schulte et al. initially demonstrated that ADAM10 inhibition significantly reduces FasL shedding from primary human T cells(Schulte et al., 2007b). The subsequent increase in FasL cell surface expression increased the efficiency of T cell-mediated killing of target cells and AICD. Additionally, Kirkin et al. described ADAM10-mediated RIP of FasL, leading to release of an intracellular domain that inhibits gene transcription(Kirkin et al., 2007). These results indicate that ADAM10-mediated FasL cleavage downregulates Fas-mediated apoptosis. However, other proteases have been implicated in FasL cleavage(Mitsiades et al., 2001; Powell et al., 1999). Thus, elucidating the biologic relevance of ADAM10-mediated FasL processing awaits in vivo examination of ADAM10-deficient activated T cells.
TNF-α is also released from cell surfaces following cleavage of pro-TNF-α. Two early reports described a role for ADAM10 in this process(Lunn et al., 1997; Rosendahlet al., 1997). Additionally, Arduise et al. recently reported that ADAM10 is responsible for TNF-α release in response to antibody binding to tetraspanins(Arduise et al., 2008). However, the vast majority of data demonstrates that most TNF-α secretion is dependent upon ADAM17 activity in vitro and in vivo(Black et al., 1997).
3.5 Lag-3
In early stages of T cell activation, effector molecules are upregulated, while inhibitory proteins are inactivated. Lymphocyte activation gene-3 (Lag-3) is an inhibitory transmembrane protein expressed by activated T cells and NK cells that binds MHC class II molecules with a higher affinity than CD4. It regulates the suppressive activity of regulatory T cells and controls activation-induced effector T cell expansion(Huang et al., 2004; Workman et al., 2004). Following T cell activation in vivo, soluble Lag-3 accumulates in mouse serum. Li et al. reported that Lag-3 cleavage is required for antigen-specific T cell activation, as non-cleavable mutants prevent proliferation and cytokine production(Li et al., 2007b). The authors also demonstrate that cleavage is mediated by ADAM10 and ADAM17. ADAM10 is responsible for constitutive and activation-induced cleavage, while ADAM17 mediates PKCΘ –dependent cleavage. Additionally, ADAM10 siRNA suppressed T cell proliferation in a Lag-3 dependent manner. One report also described Lag-3 expression on activated B cells(Kisielow et al., 2005). Thus, Lag-3 cleavage may also regulate B cell proliferation. Li et al. (Li et al., 2007b) have provided the most direct evidence describing a role for ADAM10 activity in T cell activation, and have raised intriguing questions about ADAM10's role in adaptive immune responses.
4. Regulation of ADAM10 activity
Endogenous regulation of ADAM10 activity has not been thoroughly examined. ADAM10 is expressed as a zymogen. Cleavage of the pro-domain by a furin protease results in ADAM10 activation(Hwang et al., 2006). Following pro-domain removal, regulation of ADAM10-mediated cleavage is dependent on the structural conformation of the substrate. If the cleavage site is exposed, cleavage is thought to occur constitutively. However, several reports have indicated that proteolytic activity is enhanced by retinoic acid receptor signaling, PKC signaling, nardilysin expression, cholesterol depletion, N-glycosylation, and calcium influx(Escrevente et al., 2008; Hiraoka et al., 2007; Kojro et al., 2001; Sanderson et al., 2005; Skovronsky et al., 2000; Tippmann et al., 2009). Thus, ionomycin is routinely used to enhance ADAM10-mediated cleavage(Le Gall et al., 2009). Additionally, inhibition of proteolytic activity by tissue inhibitors of metalloproteinases 1 (TIMP1), TIMP3, and the reversion-inducing cysteine-rich protein with Kazal motifs (RECK) has been reported(Amour et al., 2000; Muraguchi et al., 2007). These regulatory mechanisms may represent opportunities for pharmacologic intervention of autoimmune disease states in which ADAM10 substrates contribute to pathogenesis or disease progression.
5. Novel functions of ADAM10
In addition to ectodomain shedding and RIP, two novel functions of ADAM10 were recently described. Examination of cellular release of CD44 and another ADAM10 substrate, L1, has revealed that ADAM10 contributes to exosomal sorting of substrates. Stoeck et al. demonstrated that L1 and CD44, thought to be cleaved from the cell membrane, were actually cleaved in endosomal vesicles that were subsequently released in the form of exosomes(Stoeck et al., 2006). Additionally, Sharples et al. described the release of another ADAM10 substrate, APP, in exosomes(Sharples et al., 2008), and both reports also demonstrated that ADAM10 itself is sorted into exosomes. Although exosomal transport of many substrates has not been examined, it is possible that ADAM10 is also responsible for the inclusion of other substrates in exosomes. Although the exact role of exosomes in immune reactions may depend on exosomal content, many exosomal proteins maintain their biological function. Exosome release has also been shown to increase upon lymphocyte and dendritic cell activation (Thery et al., 2002). Thus, exosomes may have a role in the enhancement of autoimmune reactions. Conversely, multiple studies have described the ability of dendritic cell-derived exosomes to attenuate the severity of autoimmune disease models in mice(Bianco et al., 2007). Nevertheless, this novel role of ADAM10 in exosomal sorting may modulate autoimmune reactions.
Finally, Tousseyn et al. recently demonstrated that while ADAM10 mediates RIP of numerous other substrates, ADAM10 itself is also susceptible to RIP by ADAMs 9 and 15 and γ-secretase(Tousseyn et al., 2009). The intracellular domain of ADAM10 was observed in the nucleus, localized to nuclear speckles thought to represent sites of gene regulation. Although the biological consequences of this potential signaling cascade have not been examined, this finding illustrates that alterations in ADAM10 expression may have effects independent of ADAM10 substrates. Moreover, it suggests that blockade of ADAM10 activity and ADAM10 expression may have differential effects.
6. Conclusion
Through the development of hydroxamate inhibitors, dominant negative constructs, and protease-deficient MEFs, a multitude of putative ADAM10 substrates with known roles in inflammation and autoimmunity have been identified. The generation of conditional knockout mice has revealed the physiologic relevance of several ADAM10-mediated cleavage events, but most await further analysis. Initiation of Notch-dependent development has been the most striking observation in lymphocyte studies. Although interesting, these developmental phenotypes may present challenges for the examination of ADAM10's role in adaptive immunity and autoimmunity. However, the generation of novel in vivo models should overcome this hurdle and allow further examination of ADAM10 as a potential target for the treatment of lymphoproliferative and autoimmune diseases.
Abbreviations
- Dll
delta-like
- NICD
Notch intracellular domain
- RIP
regulated intramembrane proteolysis
- NRR
negative regulatory region
- CMP
common myeloid progenitor
- HSC
hematopoietic stem cell
- CLP
common lymphoid progenitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel S, et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J. Immunol. 2004;172:6362–6372. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- Amour A, et al. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 2000;473:275–279. doi: 10.1016/s0014-5793(00)01528-3. [DOI] [PubMed] [Google Scholar]
- Anderegg U, et al. ADAM10 is the constitutive functional sheddase of CD44 in human melanoma cells. J. Invest Dermatol. 2009;129:1471–1482. doi: 10.1038/jid.2008.323. [DOI] [PubMed] [Google Scholar]
- Arduise C, et al. Tetraspanins regulate ADAM10-mediated cleavage of TNF-alpha and epidermal growth factor. J. Immunol. 2008;181:7002–7013. doi: 10.4049/jimmunol.181.10.7002. [DOI] [PubMed] [Google Scholar]
- Bazan JF, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- Bianco NR, et al. Modulation of the immune response using dendritic cell-derived exosomes. Methods Mol. Biol. 2007;380:443–55. 443–455. doi: 10.1007/978-1-59745-395-0_28. [DOI] [PubMed] [Google Scholar]
- Bigas A, Martin DI, Milner LA. Notch1 and Notch2 inhibit myeloid differentiation in response to different cytokines. Mol. Cell Biol. 1998;18:2324–2333. doi: 10.1128/mcb.18.4.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RA, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Bozkulak EC, Weinmaster G. Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol. Cell Biol. 2009;29:5679–5695. doi: 10.1128/MCB.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brou C, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- Carlesso N, et al. Notch1-induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood. 1999;93:838–848. [PubMed] [Google Scholar]
- Chi AW, et al. Untangling the T branch of the hematopoiesis tree. Curr. Opin. Immunol. 2009;21:121–126. doi: 10.1016/j.coi.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie G, et al. IgE secretion is attenuated by an inhibitor of proteolytic processing of CD23 (FcεRII). Eur. J. Immunol. 1997;27:3228–3235. doi: 10.1002/eji.1830271221. [DOI] [PubMed] [Google Scholar]
- Conrad DH, et al. CD23: an overlooked regulator of allergic disease. Curr. Allergy Asthma Rep. 2007;7:331–337. doi: 10.1007/s11882-007-0050-y. [DOI] [PubMed] [Google Scholar]
- Crawford HC, et al. ADAM10 as a therapeutic target for cancer and inflammation. Curr. Pharm. Des. 2009;15:2288–2299. doi: 10.2174/138161209788682442. [DOI] [PubMed] [Google Scholar]
- Dasic G, et al. Critical role of CD23 in allergen-induced bronchoconstriction in a murine model of allergic asthma. Eur. J. Immunol. 1999;29:2957–2967. doi: 10.1002/(SICI)1521-4141(199909)29:09<2957::AID-IMMU2957>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dyczynska E, et al. Proteolytic processing of delta-like 1 by ADAM proteases. J. Biol. Chem. 2007;282:436–444. doi: 10.1074/jbc.M605451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrevente C, et al. Functional role of N-glycosylation from ADAM10 in processing, localization and activity of the enzyme. Biochim. Biophys. Acta. 2008;1780:905–913. doi: 10.1016/j.bbagen.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Fisher GH, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Fourie AM, et al. Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J. Biol. Chem. 2003;278:30469–30477. doi: 10.1074/jbc.M213157200. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GE, et al. Inhibition of CXCL16 attenuates inflammatory and progressive phases of anti-glomerular basement membrane antibody-associated glomerulonephritis. Am. J. Pathol. 2007;170:1485–1496. doi: 10.2353/ajpath.2007.060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb DR, et al. ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage in vivo. J. Exp. Med. 2010;207:623–635. doi: 10.1084/jem.20091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough PJ, et al. A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J. Immunol. 2004;172:3678–3685. doi: 10.4049/jimmunol.172.6.3678. [DOI] [PubMed] [Google Scholar]
- Hartmann D, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum. Mol. Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- Hinkle CL, et al. Selective roles for tumor necrosis factor alpha-converting enzyme/ADAM17 in the shedding of the epidermal growth factor receptor ligand family: the juxtamembrane stalk determines cleavage efficiency. J. Biol. Chem. 2004;279:24179–24188. doi: 10.1074/jbc.M312141200. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y, et al. Enhancement of alpha-secretase cleavage of amyloid precursor protein by a metalloendopeptidase nardilysin. J. Neurochem. 2007;102:1595–1605. doi: 10.1111/j.1471-4159.2007.04685.x. [DOI] [PubMed] [Google Scholar]
- Howard L, Glynn P. Membrane-associated metalloproteinase recognized by characteristic cleavage of myelin basic protein: assay and isolation. Methods Enzymol. 1995;248:388–95. 388–395. doi: 10.1016/0076-6879(95)48025-0. [DOI] [PubMed] [Google Scholar]
- Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Huang D, et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- Hundhausen C, et al. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J. Immunol. 2007;178:8064–8072. doi: 10.4049/jimmunol.178.12.8064. [DOI] [PubMed] [Google Scholar]
- Hwang EM, et al. Furin is an endogenous regulator of alpha-secretase associated APP processing. Biochem. Biophys. Res. Commun. 2006;349:654–659. doi: 10.1016/j.bbrc.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Jackson L, Cady CT, Cambier JC. TLR4-Mediated Signaling Induces MMP9-Dependent Cleavage of B Cell Surface CD23. J. Immunol. 2009;183:2585–2592. doi: 10.4049/jimmunol.0803660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B, et al. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J. Immunol. 2003;171:2960–2969. doi: 10.4049/jimmunol.171.6.2960. [DOI] [PubMed] [Google Scholar]
- Kawamata S, et al. Notch1 perturbation of hemopoiesis involves non-cell- autonomous modifications. J. Immunol. 2002;168:1738–1745. doi: 10.4049/jimmunol.168.4.1738. [DOI] [PubMed] [Google Scholar]
- Kawamoto H, Katsura Y. A new paradigm for hematopoietic cell lineages: revision of the classical concept of the myeloid-lymphoid dichotomy. Trends Immunol. 2009;30:193–200. doi: 10.1016/j.it.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Kirkin V, et al. The Fas ligand intracellular domain is released by ADAM10 and SPPL2a cleavage in T-cells. Cell Death. Differ. 2007;14:1678–1687. doi: 10.1038/sj.cdd.4402175. [DOI] [PubMed] [Google Scholar]
- Kisielow M, et al. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur. J. Immunol. 2005;35:2081–2088. doi: 10.1002/eji.200526090. [DOI] [PubMed] [Google Scholar]
- Kleinau S, et al. Importance of CD23 for collagen-induced arthritis: Delayed onset and reduced severity in CD23-deficient mice. J. Immunol. 1999;162:4266–4270. [PubMed] [Google Scholar]
- Kojro E, et al. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha -secretase ADAM 10. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall SM, et al. ADAMs 10 and 17 Represent Differentially Regulated Components of a General Shedding Machinery for Membrane Proteins such as TGF{alpha}, L-Selectin and TNF{alpha}. Mol. Biol. Cell. 2009;20:1785–1794. doi: 10.1091/mbc.E08-11-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoanet-Henchoz S, et al. CD23 regulates monocyte activation through a novel interaction with the adhesion molecules CD11b-CD18 and CD11c-CD18. Imm. 1995;3:119–125. doi: 10.1016/1074-7613(95)90164-7. [DOI] [PubMed] [Google Scholar]
- Lee SY, et al. Gain-of-function mutations and copy number increases of Notch2 in diffuse large B-cell lymphoma. Cancer Sci. 2009;100:920–926. doi: 10.1111/j.1349-7006.2009.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, et al. CXCL16 is a marker of inflammation, atherosclerosis, and acute coronary syndromes in humans. J. Am. Coll. Cardiol. 2007;49:442–449. doi: 10.1016/j.jacc.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Lemieux GA, et al. The low affinity IgE receptor (CD23) is cleaved by the metalloproteinase ADAM10. J Biol. Chem. 2007;282:14836–14844. doi: 10.1074/jbc.M608414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, et al. Non-cell autonomous expression of TNF-alpha-converting enzyme ADAM17 is required for normal lymphocyte development. J. Immunol. 2007a;178:4214–4221. doi: 10.4049/jimmunol.178.7.4214. [DOI] [PubMed] [Google Scholar]
- Li N, et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007b;26:494–504. doi: 10.1038/sj.emboj.7601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, et al. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb. Chem. High Throughput. Screen. 2005;8:161–171. doi: 10.2174/1386207053258488. [DOI] [PubMed] [Google Scholar]
- Lunn CA, et al. Purification of ADAM 10 from bovine spleen as a TNFalpha convertase. FEBS Lett. 1997;400:333–335. doi: 10.1016/s0014-5793(96)01410-x. [DOI] [PubMed] [Google Scholar]
- Manilay JO, et al. Impairment of thymocyte development by dominant-negative Kuzbanian (ADAM-10) is rescued by the Notch ligand, delta-1. J. Immunol. 2005;174:6732–6741. doi: 10.4049/jimmunol.174.11.6732. [DOI] [PubMed] [Google Scholar]
- Mathews J, et al. CD23 sheddase a disintegrin and metallo-proteinase 10 (ADAM10) is also required for CD23 sorting into B cell derived exosomes. J Biol. Chem. 2010 doi: 10.1074/jbc.M110.141556. doi:10.1074/jbc.M110.141556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades N, et al. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001;61:577–581. [PubMed] [Google Scholar]
- Moriyama Y, et al. Delta-like 1 is essential for the maintenance of marginal zone B cells in normal mice but not in autoimmune mice. Int. Immunol. 2008;20:763–773. doi: 10.1093/intimm/dxn034. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, et al. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J. Immunol. 2009;182:7381–7388. doi: 10.4049/jimmunol.0804322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JS, et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol. Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- Munoz O, et al. Binding of anti-CD23 monoclonal antibody to the leucine zipper motif of FcεRII/CD23 on B cell membrane promotes its proteolytic cleavage - Evidence for an effect on the oligomer/monomer equilibrium. J. Biol. Chem. 1998;273:31795–31800. doi: 10.1074/jbc.273.48.31795. [DOI] [PubMed] [Google Scholar]
- Muraguchi T, et al. RECK modulates Notch signaling during cortical neurogenesis by regulating ADAM10 activity. Nat. Neurosci. 2007;10:838–845. doi: 10.1038/nn1922. [DOI] [PubMed] [Google Scholar]
- Nanki T, et al. Inhibition of fractalkine ameliorates murine collagen-induced arthritis. J. Immunol. 2004;173:7010–7016. doi: 10.4049/jimmunol.173.11.7010. [DOI] [PubMed] [Google Scholar]
- Naor D, et al. CD44 involvement in autoimmune inflammations: the lesson to be learned from CD44-targeting by antibody or from knockout mice. Ann. N. Y. Acad. Sci. 2007;1110:233–47. 233–247. doi: 10.1196/annals.1423.025. [DOI] [PubMed] [Google Scholar]
- Pan D, Rubin GM. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90:271–280. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu. Rev. Immunol. 2005;23:161–96. 161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- Plater-Zyberk C, Bonnefoy J-Y. Marked amelioration of established collagen-induced arthritis by treatment with antibodies to CD23 in vivo. Nat. Med. 1995;1:781–785. doi: 10.1038/nm0895-781. [DOI] [PubMed] [Google Scholar]
- Postina R. A closer look at alpha-secretase. Curr. Alzheimer Res. 2008;5:179–186. doi: 10.2174/156720508783954668. [DOI] [PubMed] [Google Scholar]
- Powell WC, et al. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr. Biol. 1999;9:1441–1447. doi: 10.1016/s0960-9822(00)80113-x. [DOI] [PubMed] [Google Scholar]
- Pui JC, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- Qyang Y, et al. Myeloproliferative disease in mice with reduced presenilin gene dosage: effect of gamma-secretase blockage. Biochemistry. 2004;43:5352–5359. doi: 10.1021/bi049826u. [DOI] [PubMed] [Google Scholar]
- Radtke F, et al. Notch regulation of lymphocyte development and function. Nat. Immunol. 2004;5:247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- Real PJ, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat. Med. 2009;15:50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbens C, et al. Increased synovial fluid levels of soluble CD23 are associated with an erosive status in rheumatoid arthritis (RA). Clin. Exp. Immunol. 2000;120:194–199. doi: 10.1046/j.1365-2249.2000.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieux-Laucat F, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- Rooke J, et al. KUZ, a conserved metalloprotease-disintegrin protein with two roles in Drosophila neurogenesis. Science. 1996;273:1227–1231. doi: 10.1126/science.273.5279.1227. [DOI] [PubMed] [Google Scholar]
- Rosati E, et al. Constitutively activated Notch signaling is involved in survival and apoptosis resistance of B-CLL cells. Blood. 2009;113:856–865. doi: 10.1182/blood-2008-02-139725. [DOI] [PubMed] [Google Scholar]
- Rosendahl MS, et al. Identification and characterization of a pro-tumor necrosis factor-alpha-processing enzyme from the ADAM family of zinc metalloproteases. J. Biol. Chem. 1997;272:24588–24593. doi: 10.1074/jbc.272.39.24588. [DOI] [PubMed] [Google Scholar]
- Sahin U, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18:675–685. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- Sanda T, et al. Interconnecting molecular pathways in the pathogenesis and drug sensitivity of T-cell acute lymphoblastic leukemia. Blood. 2010;115:1735–1745. doi: 10.1182/blood-2009-07-235143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson MP, et al. ADAM10 mediates ectodomain shedding of the betacellulin precursor activated by p-aminophenylmercuric acetate and extracellular calcium influx. J Biol. Chem. 2005;280:1826–1837. doi: 10.1074/jbc.M408804200. [DOI] [PubMed] [Google Scholar]
- Santos MA, et al. Notch1 engagement by Delta-like-1 promotes differentiation of B lymphocytes to antibody-secreting cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15454–15459. doi: 10.1073/pnas.0702891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, et al. Soluble fractalkine in the cerebrospinal fluid of patients with neuropsychiatric lupus. Ann. Rheum. Dis. 2006;65:1257–1259. doi: 10.1136/ard.2005.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- Schaller MA, et al. Notch ligand Delta-like 4 regulates disease pathogenesis during respiratory viral infections by modulating Th2 cytokines. J. Exp. Med. 2007;204:2925–2934. doi: 10.1084/jem.20070661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T, et al. Notch signaling induces multilineage myeloid differentiation and up-regulates PU.1 expression. J. Immunol. 2003;170:5538–5548. doi: 10.4049/jimmunol.170.11.5538. [DOI] [PubMed] [Google Scholar]
- Schulte A, et al. Sequential processing of the transmembrane chemokines CX3CL1 and CXCL16 by alpha- and gamma-secretases. Biochem. Biophys. Res. Commun. 2007a;358:233–240. doi: 10.1016/j.bbrc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- Schulte M, et al. ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death. Cell Death. Differ. 2007b;14:1040–1049. doi: 10.1038/sj.cdd.4402101. [DOI] [PubMed] [Google Scholar]
- Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- Sharples RA, et al. Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 2008;22:1469–1478. doi: 10.1096/fj.07-9357com. [DOI] [PubMed] [Google Scholar]
- Six E, et al. The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7638–7643. doi: 10.1073/pnas.1230693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovronsky DM, et al. Protein kinase C-dependent alpha-secretase competes with beta-secretase for cleavage of amyloid-beta precursor protein in the trans-golgi network. J. Biol. Chem. 2000;275:2568–2575. doi: 10.1074/jbc.275.4.2568. [DOI] [PubMed] [Google Scholar]
- Sotillos S, Roch F, Campuzano S. The metalloprotease-disintegrin Kuzbanian participates in Notch activation during growth and patterning of Drosophila imaginal discs. Development. 1997;124:4769–4779. doi: 10.1242/dev.124.23.4769. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I, Yu Q. Shedding light on proteolytic cleavage of CD44: the responsible sheddase and functional significance of shedding. J. Invest Dermatol. 2009;129:1321–1324. doi: 10.1038/jid.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier S, et al. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 2002;99:2369–2378. doi: 10.1182/blood.v99.7.2369. [DOI] [PubMed] [Google Scholar]
- Stoeck A, et al. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem. J. 2006;393:609–618. doi: 10.1042/BJ20051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki F, et al. Inhibition of CX3CL1 (fractalkine) improves experimental autoimmune myositis in SJL/J mice. J. Immunol. 2005;175:6987–6996. doi: 10.4049/jimmunol.175.10.6987. [DOI] [PubMed] [Google Scholar]
- Swiatek PJ, et al. Notch1 is essential for postimplantation development in mice. Genes Dev. 1994;8:707–719. doi: 10.1101/gad.8.6.707. [DOI] [PubMed] [Google Scholar]
- Takahashi T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Honjo T. Regulation of lymphocyte development by Notch signaling. Nat. Immunol. 2007;8:451–456. doi: 10.1038/ni1453. [DOI] [PubMed] [Google Scholar]
- Teachey DT, et al. Targeting Notch signaling in autoimmune and lymphoproliferative disease. Blood. 2008;111:705–714. doi: 10.1182/blood-2007-05-087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Thomas M, et al. Notch activity synergizes with B-cell-receptor and CD40 signaling to enhance B-cell activation. Blood. 2007;109:3342–3350. doi: 10.1182/blood-2006-09-046698. [DOI] [PubMed] [Google Scholar]
- Tian L, et al. ADAM10 is essential for proteolytic activation of Notch during thymocyte development. Int. Immunol. 2008;20:1181–1187. doi: 10.1093/intimm/dxn076. [DOI] [PubMed] [Google Scholar]
- Tippmann F, et al. Up-regulation of the alpha-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J. 2009;23:1643–1654. doi: 10.1096/fj.08-121392. [DOI] [PubMed] [Google Scholar]
- Tousseyn T, et al. ADAM10, the rate-limiting protease of regulated intramembrane proteolysis of Notch and other proteins, is processed by ADAMS-9, ADAMS-15, and the gamma-secretase. J. Biol. Chem. 2009;284:11738–11747. doi: 10.1074/jbc.M805894200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troen G, et al. NOTCH2 mutations in marginal zone lymphoma. Haematologica. 2008;93:1107–1109. doi: 10.3324/haematol.11635. [DOI] [PubMed] [Google Scholar]
- Tsubota K, et al. The role of fractalkine as an accelerating factor on the autoimmune exocrinopathy in mice. Invest Ophthalmol. Vis. Sci. 2009;50:4753–4760. doi: 10.1167/iovs.08-2596. [DOI] [PubMed] [Google Scholar]
- van Tetering G, et al. The metalloprotease ADAM10 is required for notch1 S2 cleavage. J. Biol. Chem. 2009;284:31018–31027. doi: 10.1074/jbc.M109.006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, et al. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Weskamp G, et al. ADAM10 is a principal ‘sheddase’ of the low-affinity immunoglobulin E receptor CD23. Nat. Immunol. 2006;7:1293–1298. doi: 10.1038/ni1399. [DOI] [PubMed] [Google Scholar]
- Wilson A, MacDonald HR, Radtke F. Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus. J. Exp. Med. 2001;194:1003–1012. doi: 10.1084/jem.194.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfer A, et al. Inactivation of Notch1 impairs VDJbeta rearrangement and allows pre-TCR-independent survival of early alpha beta Lineage Thymocytes. Immunity. 2002;16:869–879. doi: 10.1016/s1074-7613(02)00330-8. [DOI] [PubMed] [Google Scholar]
- Wolfsberg TG, et al. ADAM, a novel family of membrane proteins containing A Disintegrin And Metalloprotease domain: multipotential functions in cell-cell and cell-matrix interactions. J. Cell Biol. 1995;131:275–278. doi: 10.1083/jcb.131.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman CJ, et al. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J. Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- Yoon SO, et al. Notch ligands expressed by follicular dendritic cells protect germinal center B cells from apoptosis. J. Immunol. 2009;183:352–358. doi: 10.4049/jimmunol.0803183. [DOI] [PubMed] [Google Scholar]