Abstract
Repeated infections and experimental prime-boost regimens frequently result in the generation of secondary (2°) CD8+ T-cell responses. In contrast to primary (1°) CD8+ T cells, the parameters that influence the abundance and phenotype of 2° effector and memory CD8+ T-cell populations are largely unknown. Here, we analyze the impact of different booster infections, Ag curtailment, and systemic inflammation on the quality and quantity of secondary CD8+ T-cell responses. We show that similar to 1° CD8+ T-cell responses, the phenotype of 2° effector and memory CD8+ T-cell populations is critically dependent on the nature of the infectious pathogen and the inflammatory milieu early after infection. In addition, systemic inflammation increases the number of 2° effector and memory CD8+ T cells after booster infections and immunizations. Therefore, our data reveal new means to boost the number of 2° effector and memory CD8+ T cells in prime-boost regimens and show a surprisingly high degree of plasticity in 2° memory CD8+ T-cell phenotype that is controlled by systemic inflammation.
Keywords: Inflammation, Memory, Secondary responses
Introduction
Memory CD8+ T cells have evolved to optimally protect from recurrent infections with pathogens [1–3]. Following an acute infection, this specialized CD8+ T-cell subset is generated from a low number of naïve pathogen-specific CD8+ T cells that expand exponentially in numbers and then contract by 90–95% [4–6]. Compared with the naïve CD8+ T cells they derive from, the resulting pathogen-specific memory CD8+ T-cell population is increased in frequency which enables them to respond more efficiently to future infections with the same or related pathogens. In addition, memory CD8+ T cells express a variety of unique phenotypic markers which endow them with special trafficking properties and enhanced cytolytic effector functions compared with their naïve counterparts [7].
The importance of primary (1°) memory CD8+ T-cell frequency and phenotype for the protective capacity of memory CD8+ T-cell populations is well recognized. For many infections, a clear correlation between the absolute numbers of memory CD8+ T cells and the protection against infections has been demonstrated [2]. Phenotypic markers have been used extensively to identify memory CD8+ T-cell subsets that differ with regard to their localization in tissues, their proliferative potential, or their protective capacity against specific pathogens [8–10]. It is therefore not surprising that much effort has been devoted to the identification of the mechanisms that control 1° memory CD8+ T-cell numbers, phenotype, or both. These studies have shown that both the quantity and the quality of 1° memory CD8+ T-cell populations depend on a complex interplay of signals that CD8+ T cells receive during the priming, effector, and contraction phase [2, 11, 12]. These signals are usually referred to as signal I (Ag presentation), signal II (costimulation), and signal III (inflammatory cytokine signalling). In conjunction, these signals regulate critical aspects of T-cell functions like expansion, survival, effector functions, and the rate of memory phenotype acquisition [13–18].
Most of the studies that have identified regulatory mechanisms of T-cell function stem from the analysis of 1° memory CD8+ T cells which are generated after a single encounter of naïve T cells with their cognate Ag. However, many pathogens infect human hosts more than once and thus lead to repeated Ag stimulations of memory CD8+ T-cell populations [19, 20]. In addition, many experimental vaccinations employ prime-boost regimens to increase the absolute number of memory CD8+ T cells [21]. Finally, we recently showed that each additional Ag challenge of memory CD8+ T-cell populations resulted in the differential regulation of several hundred new genes in the ensuing memory CD8+ T-cell populations and, therefore, in stepwise diversification of CD8+ T-cell transcriptomes [22]. Whether the abundance and phenotype of these repeatedly stimulated memory CD8+ T cells is regulated by the same parameters that control 1° memory CD8+ T-cell quantity and quality is largely unknown. While some studies have shown that 1° and secondary (2°) immune responses both require DC-mediated Ag presentation and costimulation [23], others have revealed significant discordances regarding the role of Ag presentation [24]. Most importantly, the role of inflammatory cytokines which influence expansion and survival of 1° effector CD8+ T cells and phenotype of 1° effector and memory CD8+ T cells [2, 11, 25] has not been studied in 2° immune responses.
Here, we show that, similar to the 1° CD8+ T-cell responses, the kinetics and phenotype of CD8+ T cells in 2° immune responses are dependent on the type of pathogen used, the duration of infection and the inflammatory milieu. Furthermore, we demonstrate that differences in the levels of systemic inflammation are the main cause for the observed differences in the number and phenotype of 2° memory CD8+ T cells.
Results
Infectious pathogens differentially modulate 2° effector and memory CD8+ T cells
To determine how infection with different pathogens influences 2° immune responses, we cotransferred, in the first set of experiments, Thy1-disparate 1° memory OT-I T cells together with naïve OT-I cells into naïve Thy1.2/1.2 hosts. Naïve OT-I cells were cotransferred as a positive control since the impact of infections with different pathogens on the phenotype and frequency of 1° CD8+ T-cell populations is well known [2, 11, 25]. We recently showed that a low and physiological number of naïve OT-I TCR-Tg cells (5 × 102 per mouse used in cotransfer studies here) are not able to provide any measurable changes in protection after infection [26], suggesting that a modest increase in naïve CD8+ T-cell precursor frequency in vivo will not have a substantial influence on 2° CD8+ T-cell responses when analyzed in the same hosts.
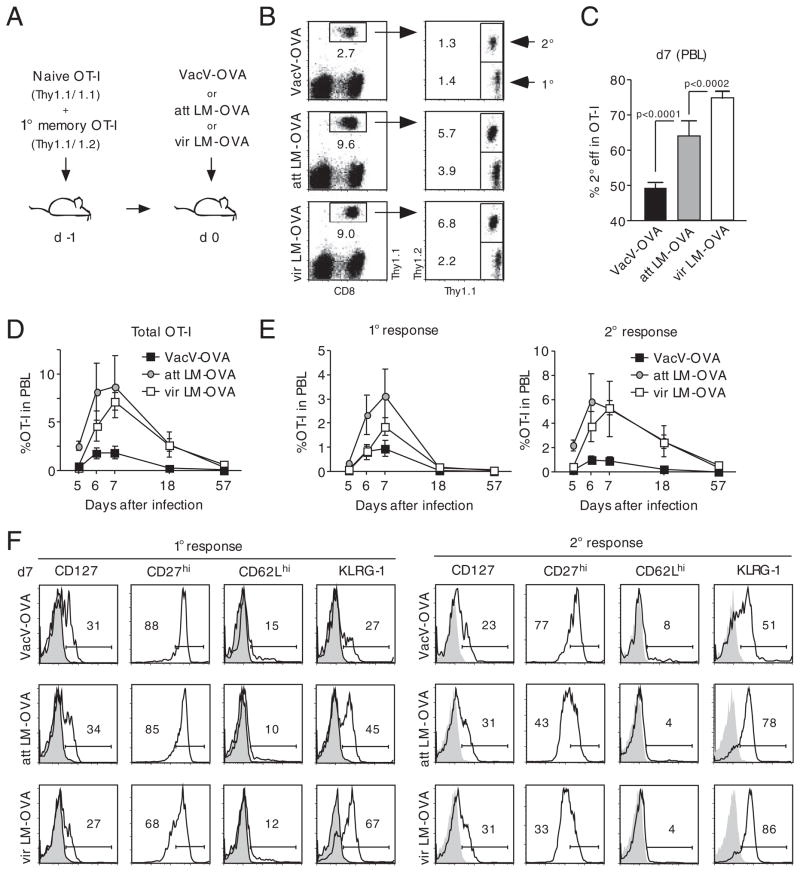
Upon adoptive transfer, groups of mice were infected with SIINFEKL (OVA)-peptide expressing Vaccinia virus (VacV-OVA), actA-Listeria monocytogenes (att LM-OVA) or virulent L. monocytogenes (vir LM-OVA, Fig. 1A). Expansion in numbers of both OT-I cell populations was detectable on day 7 after infection in peripheral blood samples (PBL, Fig. 1B) yet the frequency of the two populations differed between the three groups. Infection with VacV-OVA resulted in equal frequencies of 1° and 2° effector OT-I cells but a lower magnitude of overall expansion compared with the groups infected with att LM-OVA or vir LM-OVA. In contrast to the similar frequencies of 1° and 2° effector CD8+ T cells after VacV-OVA infection, 2° effector OT-I cell frequency was 50% higher than 1° effector OT-I cell frequency after att LM-OVA infection and approximately 200% higher after vir LM-OVA infection. This difference in frequency was reflected in the percentage of 2° effector OT-I T cells in the total OT-I cell population which ranged from 50% for VacV-OVA infection to 75% after vir LM-OVA infection (Fig. 1C). Thus, challenge with different pathogens changes the magnitude of the proliferative expansion of 1° and 2° CD8+ T cells in the same host as well as the composition of effector CD8+ T cells (ratio of 2° versus 1°) in the ensuing immune response.
Figure 1.
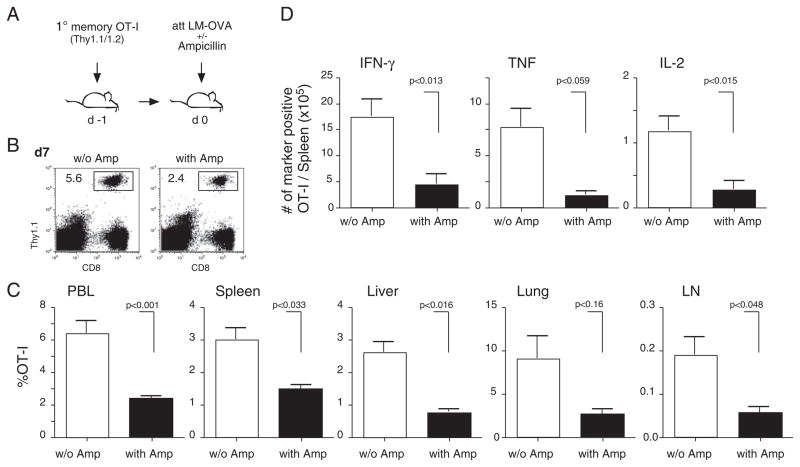
Pathogens differentially impact 2° effector CD8+ T-cell expansion and phenotype. (A) Experimental setup: 5 × 102 Thy1.1 naïve and 1 × 104 Thy1.1/1.2 1° memory OT-I cells were mixed and injected into naïve Thy1.2 hosts (n = 8/group). Mice were infected with either 3 × 106 PFU VacV-OVA i.p., 5 × 106 CFU att LM-OVA or 5 × 104 vir LM-OVA i.v. (B) Thy1.1+ OT-I cells were identified in PBL samples 7 days p.i. and 1° and 2° effector OT-I cells were distinguished by Thy1.1/1.2 costaining. Numbers show the percentage of the OT-I cell populations in the PBL population of representative mice. (C) Percentage (mean+SEM; n = 8 mice/group) of 2° effector CD8+ T cells in the total OT-I cell population in PBL 7 days p.i. (D) Kinetics of the combined 1° and 2° effector OT-I cell responses in PBL. (E) Kinetics of the 1° (left graph) and 2° (right graph) OT-I cell response in PBL. Numbers show mean±SEM (n = 8). (F) PBLs of all mice (n = 8/group) 7 days p.i. were pooled and the phenotype of 1° (left panel) and 2° (right panel) effector OT-I cells was analyzed. Numbers show the percentage of marker-positive OT-I cells, and shaded histograms represent isotype controls. Data are representative of two independent experiments.
For a more detailed analysis of the kinetics of 1° and 2° immune responses, expansion and contraction of the two OT-I cell populations were monitored in peripheral blood samples of individual mice. For all three groups, the combined OT-I T-cell response of 1° and 2° effectors (Fig. 1D) and the 1° immune response alone (Fig. 1E, left graph) peaked on day 7 and contracted afterwards. Interestingly, the 2° immune response peaked on day 6 in both VacV-OVA and att LM-OVA infected mice but on day 7 in mice infected with vir LM-OVA (Fig. 1E, right graph). Phenotypic differences in 1° effector OT-I cells were most pronounced for KLRG-1 with low expression in the group infected with VacV-OVA, intermediate expression in the group infected with att LM-OVA and highest expression in the group receiving vir LM-OVA (Fig. 1F, left panel). Surprisingly, similar differences in phenotype were observed in 2° effector OT-I cells (e.g. KLRG-1, right panel). For the costimulatory molecule CD27, the changes in gene expression were even more pronounced than in 1° memory CD8+ T cells.
These results demonstrate that pathogens differ in their ability to generate 1° and 2° effector CD8+ T cells and that each pathogen evokes characteristic changes in the phenotype of not only 1°, but also 2° effector CD8+ T cells.
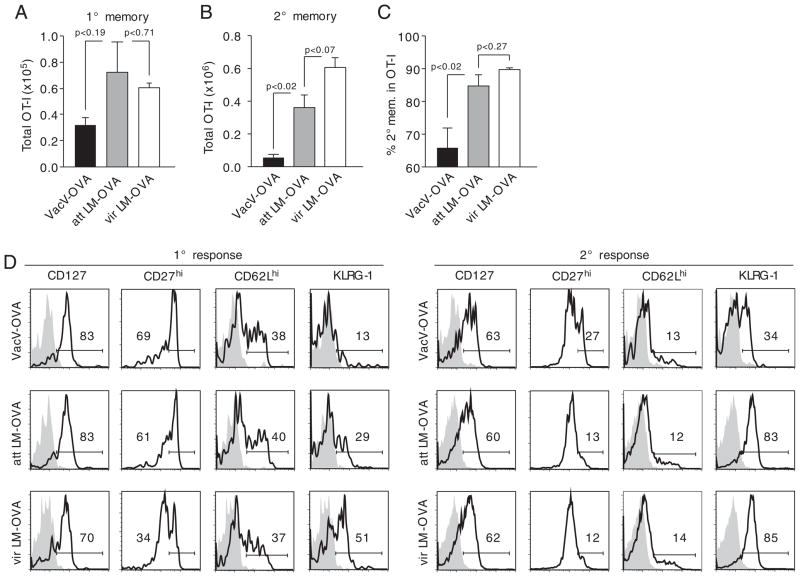
To determine whether infection with different pathogens also leads to changes in the absolute number of 2° memory CD8+ T cells, the content of 1° and 2° memory OT-I cells in spleens of mice from the experiment shown in Fig. 1 was analyzed at the memory stage. Infection with att and vir LM-OVA resulted in slightly higher numbers of 1° memory OT-I cells than infection with VacV-OVA and no differences between the two Listeria strains (all changes not significant, Fig. 2A). Similar differences between VacV-OVA and att Listeria-infected mice (statistically significant, p = 0.02) were observed for 2° memory CD8+ T cells as well as slightly higher absolute numbers of 2° memory CD8+ T cells after vir LM-OVA compared with att LM-OVA infection (changes not significant, Fig. 2B). This was confirmed by an increased frequency of 2° memory OT-I cells in the total OT-I cell population in att Listeria-infected mice compared with VacV-OVA-infected mice (Fig. 2C) with the highest frequency in vir LM-OVA immune mice. When 1° memory OT-I cell phenotype was assessed, marker expression was found to resemble the expression patterns found on 1° effector CD8+ T cells (Fig. 1F, left panel) with differences in CD27 and KLRG-1 expression but only subtle changes in the expression of CD127 and CD62L. Again, a similar regulation of marker expression was found on 2° memory CD8+ T cells with the most pronounced differences in KLRG-1 expression between VacV-OVA- and Listeria-infected mice (Fig. 2D).
Figure 2.
Pathogens differentially impact the number and phenotype of 2° memory CD8+ T cells. (A) Mice from the experiment described in Fig. 1 were sacrificed on day 57 p.i. and total 1° memory OT-I cell numbers were analyzed in the spleen of up to five mice from each group. (B) Absolute numbers of 2° memory OT-I cells in the spleen. (C) Percentage of 2° memory OT-I cells in the total OT-I cell population. Bars show mean + SEM for each group. (D) Spleens of individual mice (n = 5/group) were pooled and the phenotype of 1° (left panel) and 2° (right panel) memory OT-I cells was analyzed. Numbers show the percentage of marker-positive OT-I cells, and shaded histograms represent isotype controls. Data are representative of two independent experiments.
These data indicate that the absolute number and phenotype of memory CD8+ T cells that can be generated in 2° immune responses is dependent on the pathogen used for the booster infection.
The tissue distribution and function of 2° effector CD8+ T cells is pathogen dependent
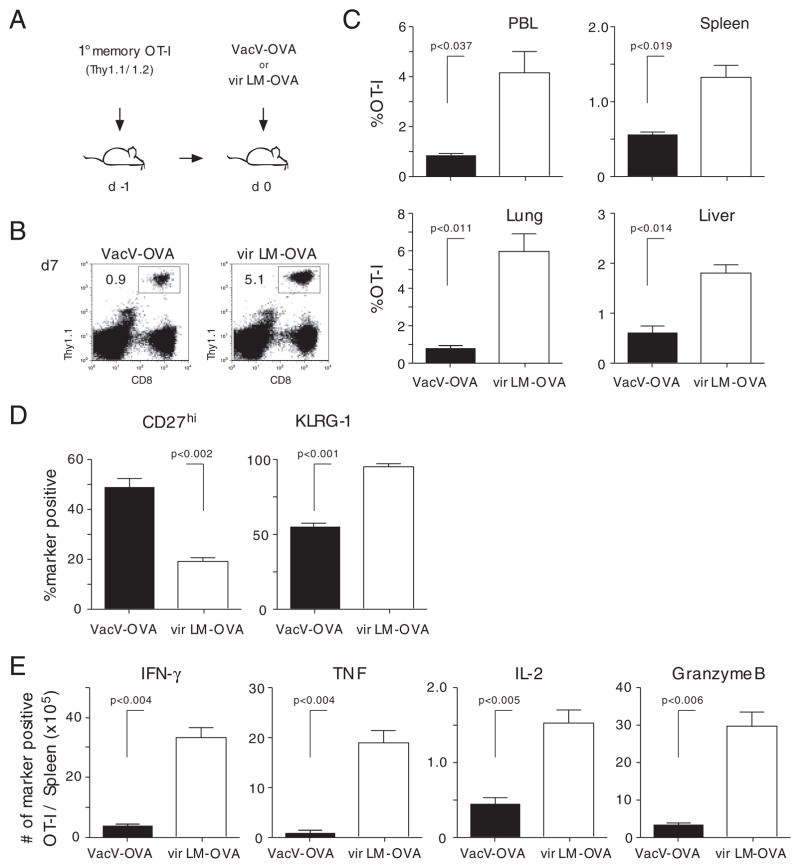
To determine if the differences in the expansion of 1° memory CD8+ T cells observed in PBL are also detected in 2° lymphoid and tertiary tissues (e.g. spleen, lung, and liver) and if an increase in numbers correlates with the ability of 2° effector cells to function (e.g. produce cytokines after Ag-restimulation), 1° memory OT-I cells were transferred into naïve B6 mice 1 day before infection with VacV-OVA or vir LM-OVA (Fig. 3A). However, naïve OT-Is were not cotransferred in this experiment. Similar to the previous experiment (Fig. 1), the magnitude of the 2° expansion was significantly lower in the PBL after VacV-Ova compared with vir LM-OVA infection (Fig. 3B and C). Importantly, the magnitude of expansion was significantly lower in various organs of the VacV-OVA-infected mice (Fig. 3C), suggesting that the pathogen biology dictates the differences in overall expansion of 1° memory CD8+ T cells after 2° Ag-encounter. In addition to the numerical differences, the phenotypic differences (CD27hi, KLRG-1) of the responding cells in the spleen (Fig. 3D) and lungs (data not shown) were also observed. Finally, direct ex vivo peptide stimulation of splenocytes at day 7 p.i. revealed significantly higher numbers of Granzyme B-positive 2° effector CD8+ T cells capable of producing IFN-γ, TNF, and IL-2 in mice boosted with vir LM-OVA (Fig. 3E). Thus, the quantity and quality of 2° CD8+ T-cell responses, including the ability to provide effector functions such as cytokine production, can be influenced by the choice of the boosting agent.
Figure 3.
Tissue distribution and function of 2° effector CD8+ T cells is pathogen dependent. (A) Experimental setup: 1° memory OT-I cells (1 × 104) were injected into naïve Thy1.2 hosts. Mice were infected with 3 × 106 PFU VacV-OVA i.p or 5 × 104 vir LM-OVA i.v. (B) Thy1.1+ OT-I cells were identified in PBL 7 days p.i. Numbers show the percentage of the OT-I T-cell populations in the PBL population of representative mice. (C) Percentage of 2° effector CD8+ T cells in indicated organs 7 days p.i. Numbers show mean+SD (n = 4 mice/group). (D) The phenotype (CD27 and KLRG-1) of OT-I cells in the spleen. Bars show mean+SD. (E) Total number of cytokine- (IFN-γ, TNF, and IL-2) and Granzyme B-producing OT-I cells in the spleen after direct ex vivo OVA257–264-peptide stimulation. Bars show mean+SD.
Curtailment of infection alters 2° CD8+ T-cell responses
Since antibiotic treatment early after Listeria infection and subsequent termination of infection and Ag display has been shown to regulate 1° effector and memory CD8+ T-cell phenotype [13, 27], we chose to assess the effect of ampicillin treatment on 2° immune responses.
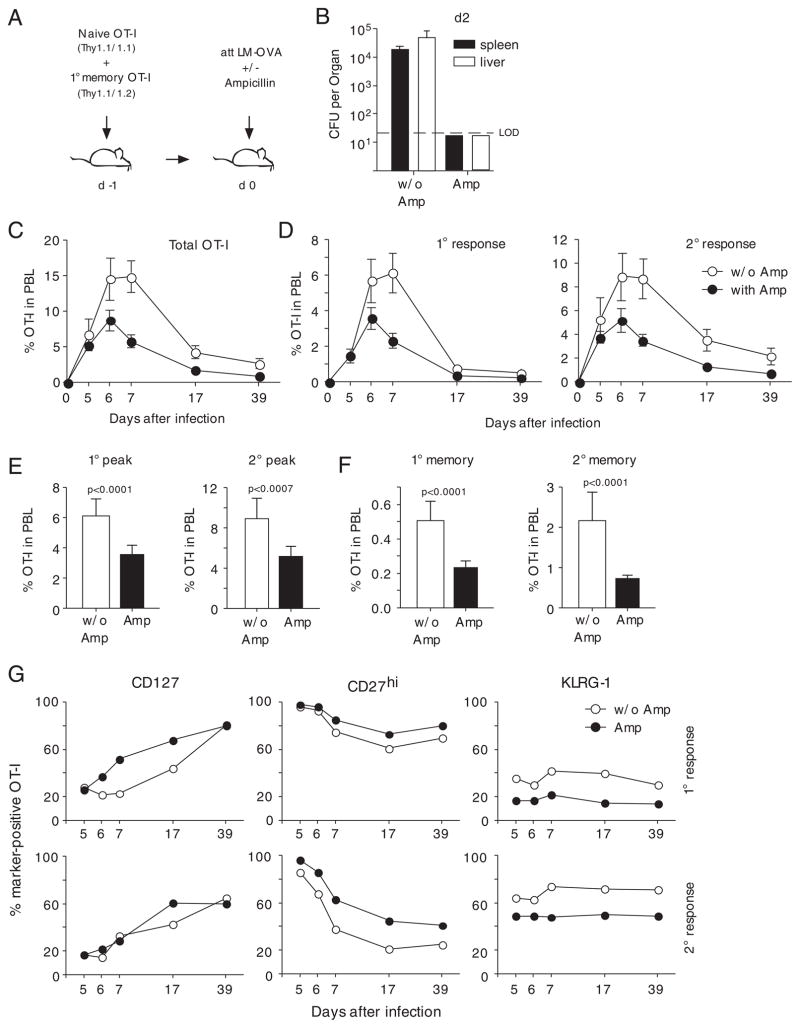
Naïve and 1° memory OT-I cells were adoptively transferred into naïve hosts prior to infection with att LM-OVA (Fig. 4A). A second group was put on ampicillin treatment 16 h after LM-OVA infection. Antibiotic treatment efficiently eliminated Listeria from both liver and spleen by day 2 after infection (Fig. 4B). Analysis of the kinetics of the OT-I cell response in peripheral blood showed a similar expansion of total OT-I cells on day 5 after infection but lower expansion of the OT-I cell population in ampicillin-treated mice at later stages of the effector phase (Fig. 4C). Interestingly, the kinetics of 1° and 2° OT-I cell responses were similar until day 7 (Fig. 4D). In the ensuing contraction phase, however, 2° effector CD8+ T cells contracted less than 1° effector CD8+ T cells as described previously [28–31]. Analysis of the peak expansion of 1 and 2° effector OT-I cells revealed that antibiotic treatment reduced peak frequencies by approximately 50% in both groups (Fig. 4E). This difference was preserved in both 1° and 2° immune responses at the memory stage (Fig. 4F). Therefore, curtailment of infection via antibiotic treatment reduces the expansion of 1° and 2° effector CD8+ T cells in a similar manner and decreases the frequency of both 1° and 2° memory CD8+ T cells.
Figure 4.
Ag curtailment decreases 2° memory CD8+ T cell numbers and changes 2° memory CD8+ T-cell phenotype. (A) Briefly, 5 × 102 Thy1.1 naïve and 1 × 104 1° memory OT-I cells were mixed and injected into naïve Thy1.2 hosts (n = 8/group) prior to infection with 1 × 107 CFU att LM-OVA. In total, 16 h later, one group of mice was given ampicillin in the drinking water for 2 days. (B) Bacterial titers in spleen (CFU/total organ) and liver (CFU/g) were determined 2 days p.i. (n = 3). Kinetics of (C) the total OT-I cell response, (D) the 1° OT-I T-cell response (left panel), and the 2° OT-I T-cell response (right panel) in PBL. Numbers show mean±SEM (n = 5). (E) Peak frequencies of the 1° OT-I cell response (left graph) and the 2° OT-I T-cell response (right graph) in PBL (mean±SEM). (F) 1° (left graph) and 2° (right graph) memory OT-I cell frequency in PBL (mean+SEM). (G) PBLs of individual mice (n = 5) were pooled and phenotype of 1° (upper panel) and 2° (lower panel) OT-I cells was analyzed. The experiment was repeated twice with similar results.
As described earlier, antibiotic treatment also affected 1° effector CD8+ T-cell phenotype and led to accelerated acquisition of a memory phenotype [13] as indicated by higher expression of CD127 and CD27 and lower expression of KLRG-1 (Fig. 4G). Similar to the previous experiment, changes in phenotype were not restricted to 1° immune responses but affected 2° immune responses as well. Interestingly, changes in the expression of CD27 and KLRG-1 reflected the changes in the 1° immune response, whereas CD127 expression did not differ substantially after antibiotic treatment.
In a repeated experiment where 1° memory CD8+ T cells were transferred in the absence of naïve OT-I cells (Fig. 5A), a similar decrease in 2° effector CD8+ T-cell expansion after antibiotic treatment was observed not only in the blood (Fig. 5B and C) but also in the spleen, liver, lung, and inguinal lymph nodes of Listeria-infected mice (Fig. 5C). Similarly, the curtailment of infection leads to a substantial decrease in the number of 2° effector cells able to respond by cytokine production (IFN-γ, TNF, and IL-2) after Ag restimulation (Fig. 5D).
Figure 5.
Tissue distribution and function of 2° effector CD8+ T cells is influenced by the duration of infection. (A) Experimental setup: 1.5 × 104 1° memory OT-I cells were transferred into naïve Thy1.2 hosts prior to infection with 1 × 107 CFU att LM-OVA. One group of mice was given ampicillin in the drinking water for 2 days. (B) Thy1.1+ OT-I cells were identified in PBL 7 days p.i. Numbers show the percentage of the OT-I cell populations in the PBL. (C) Percentage of 2° effector CD8+ T cells in indicated organs 7 days p.i. Numbers show mean+SD (n = 3 mice/group). (D) Total number of cytokine-(IFN-γ, TNF, and IL-2) producing OT-I cells in the spleen after direct ex vivo OVA257–264-peptide stimulation. Bars show mean+SD.
Taken together, these findings suggest that the duration of infection (or Ag display) might influence the kinetics, phenotype, and function of responding memory CD8+ T-cell populations.
Inflammation controls 2° effector and memory CD8+ T cells
To dissect whether pathogen load or systemic inflammation was the cause of the observed changes in the 2° immune responses, we used an immunization protocol that employs the injection of mature DCs in the presence or absence of pro-inflammatory stimuli.
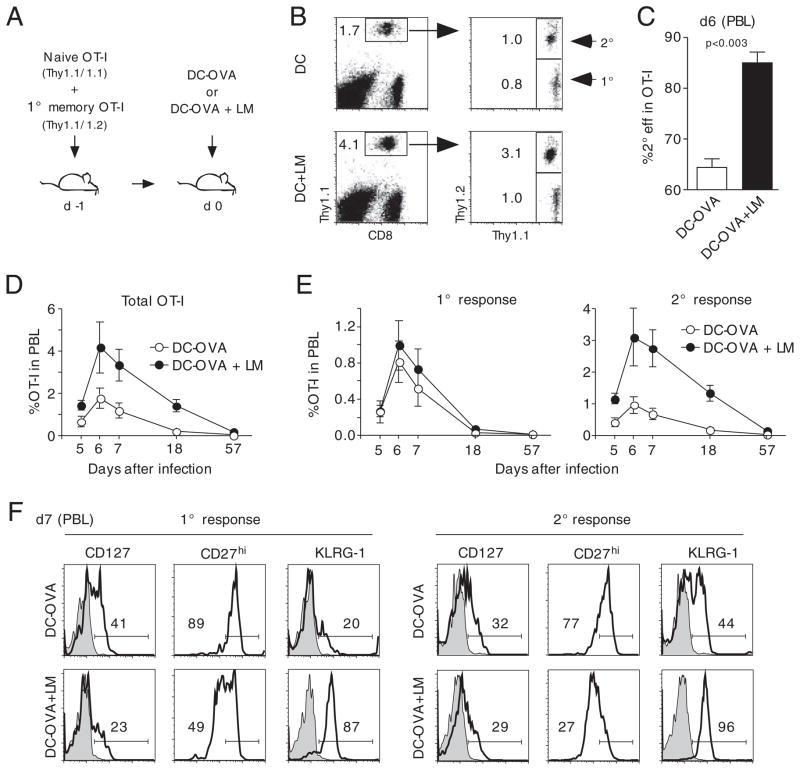
Naïve and memory OT-I cells were adoptively transferred into the same host and 1 day later mice were injected with mature DCs that were pulsed with the OVA257–264 peptide (Fig. 6A). A second group of mice received the same number of DCs as well as an i.v. injection of att L. monocytogenes that lacked the OVA peptide. In this scenario, OT-I cells are primed by the Ag presented on the injected DCs and are exposed to inflammation that is induced by Listeria infection [14]. Analysis of OT-I cell expansion showed similar frequencies of 1° and 2° effector OT-I cells in the group immunized with DCs only (Fig. 6B, top panels). In mice that were coinfected with Listeria, 1° effector OT-I cells reached similar frequencies as the group that had received DCs only (Fig. 6B, bottom panels). The frequency of 2° effector OT-I cells, however, was three-fold higher in mice that had been infected with Listeria at the time of the DC immunization. As a consequence, the percentage of 2° effector OT-I cells in the total OT-I response was much higher in Listeria-infected mice (Fig. 6C). In the longitudinal analysis, the total OT-I cell response to DC immunization was consistently higher in Listeria-infected mice throughout the effector and contraction phase (Fig. 6D). 1° effector OT-I frequency was slightly higher in the group that was subjected to Listeria coinfection compared with the group that had received DCs only (Fig. 6E, left panel). The differences between Listeria-infected and noninfected mice, however, were much more pronounced in 2° immune responses where the kinetic analyses revealed differences as early as day 5 after infection that were maintained during effector and contraction phase. These findings indicate that, similar to the previous studies that analyzed 1° immune responses, 2° effector OT-I cell expansion is controlled by inflammation. The fact that 1° effector CD8+ T-cell expansion was only slightly altered in our experiments could indicate that 2° effector CD8+ T cells outcompete 1° effector CD8+ T cells for the proliferative and antiapoptotic stimuli provided by systemic inflammation when the two populations are simultaneously present in a host.
Figure 6.
Systemic inflammation increases 2° effector CD8+ T-cell numbers and induces a sustained effector phenotype. (A) Experimental setup: 5 × 102 Thy1.1 naïve and 1 × 104 1° memory OT-I cells were mixed and injected into naïve Thy1.2 hosts (n = 10) prior to immunization with OVA-coated DCs. One group of mice was coinfected with att LM. (B) Detection of Thy1.1+ OT-I cells (left dot plots) and discrimination between 2° (Thy1.1/1.2) and 1° (Thy1.1) OT-I cell responses (right dot plots). Numbers show the frequency of OT-I T cells in PBL in representative mice. (C) Percentage of 2° effector OT-I cells in the total OT-I cell population (mean+SEM). (D) Kinetics of the total OT-I cell response in PBL (mean±SEM). (E) Kinetics of the 1° (left graph) and 2° (right graph) OT-I cell response in PBL (mean±SEM). (F) Phenotype of 1° (left side) and 2° (right side) effector OT-I cells was analyzed in pooled PBL from up to ten mice. Shaded histograms represent isotype controls. The experiment was repeated once with similar results. Data are representative of two independent experiments.
Despite the negligible effect of systemic inflammation on 1° effector OT-I cell expansion, there was a profound impact of Listeria coinfection on the phenotype of these cells (Fig. 6F). In concordance with the previous studies, DC-immunized 1° effector OT-I cells showed phenotypic characteristics of memory CD8+ T cells in the absence of systemic inflammation [17]. Thus, the cells had higher expression of CD127 and CD27 but lower expression of KLRG-1 compared with mice that were coinfected with Listeria. Again, similar differences in the expression of phenotypic markers were found on 2° effector OT-I cells (Fig. 6F, right panels), particularly in the surface expression of CD27 and KLRG-1. These data show that inflammation prevents the acquisition of an early memory phenotype of both 1 and 2° effector CD8+ T cells.
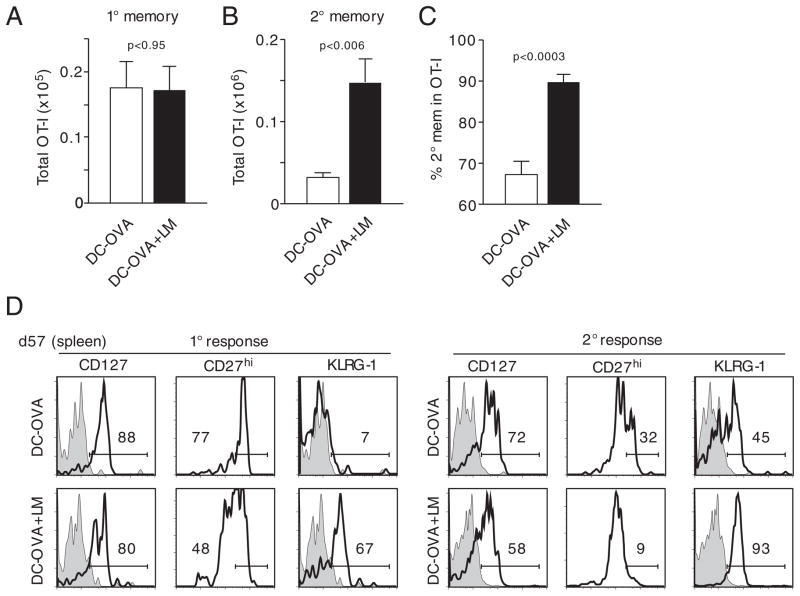
To analyze the influence of inflammation on the generation of 2° memory CD8+ T cells, DC-immunized mice (in the presence or absence of Listeria coinfection [see Fig. 6A]) were analyzed ~2 months after infection. Similar to the results obtained at the effector stage, 1° memory OT-I cell numbers in the spleens were similar between the two groups (Fig. 7A). The number of 2° memory CD8+ T cells, however, was increased fourfold in the group that was subjected to systemic Listeria-induced inflammation (Fig. 7B). In these mice, nearly 90% of all OT-I cells were found to be 2° memory CD8+ T cells (Fig. 7C). Therefore, systemic inflammation increases the number of 2° memory CD8+ T cells substantially more than the number of 1° memory CD8+ T cells when both naïve and 1° memory CD8+ T cells are present in the same host at the time of infection. Surprisingly, both 2° effector and memory OT-I cell numbers were increased fourfold after Listeria coinfection. This finding could indicate that, in contrast to what has been shown for 1° effector CD8+ T cells [32], increased expansion of 2° effector OT-I cells is not followed by increased contraction.
Figure 7.
Inflammation increases memory CD8+ T-cell numbers and decreases the rate of memory phenotype acquisition. (A) Total 1° memory OT-I cell numbers (mean+SD) were assessed in spleens of mice (n = 3/group) 57 days p.i. (B) Total 2° memory OT-I cell numbers in the spleen of mice (n = 3) in the same experiment (mean+SD). (C) Briefly, 57 days p.i., spleens of mice were analyzed for the percentage of 2° memory OT-I cells in the total memory OT-I cell population. Mean+SD are shown. (D) Phenotype of 1° (left histograms) and 2° (right histograms) memory OT-I cells in pooled spleen samples (three spleens/group). Shaded histograms show isotype controls. Data are representative of two independent experiments.
While expression of CD127 was high in both groups of 1° memory OT-I cells, expression of CD27 and KLRG-1 was still markedly different (Fig. 7D). The same difference in the regulation of CD27 and KLRG-1 was evident on 2° memory OT-I cells although the absolute percentage of CD27hi cells was lower and that of KLRG-1+ cells was higher compared with 1° memory CD8+ T cells. Interestingly, the phenotypic differences between 1° and 2° memory CD8+ T cells were only minor when 1° memory CD8+ T cells generated under highly inflammatory conditions and 2° memory CD8+ T cells generated under low-inflammatory conditions were compared. These data suggest that the inflammation-induced changes in 1° and 2° effector CD8+ T-cell phenotype are preserved at the memory stage.
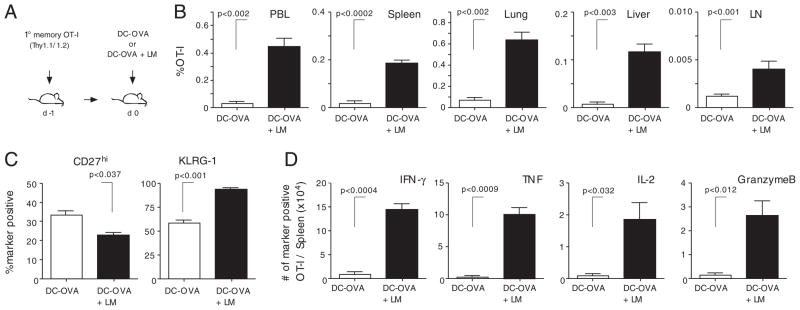
Finally, we repeated the experiment in the absence of naïve OT-I and analyzed the number and tissue distribution of 2° memory CD8+ T cells together with their phenotype and function (Fig. 8A). As shown in Fig. 8B, systemic inflammation induces a statistically significant increase in 2° memory CD8+ T-cell numbers in the lymphoid organs and tertiary tissues analyzed. As expected, the phenotype of 2° memory CD8+ T cells was also influenced by Listeria coinfection (Fig. 8C). Importantly, direct ex vivo OVA257–264 peptide stimulation of splenocytes 1 month after immunization revealed a statistically significant higher number of 2° memory CD8+ T cells that are capable of producing effector cytokines and/or cytolytic molecules (IFN-γ, TNF, IL-2, and Granzyme B) when DC-OVA immunization was coupled with Listeria-induced systemic inflammation (Fig. 8D).
Figure 8.
Systemic inflammation influences the quantity and quality of 2° memory CD8+ T cells. (A) Experimental setup: 1° memory OT-I cells (1 × 104) were injected into naïve Thy1.2 mice prior to immunization with OVA-peptide pulsed DCs. One group of mice was coinfected with att LM. (B) Percentage of 2° memory OT-I cells in indicated lymphoid organs and tertiary tissues 28 days post-immunization. (C) The phenotype (CD27 and KLRG-1) of OT-I cells in the spleen. (D) Total number of cytokine- (IFN-γ, TNF, and IL-2) and granzyme B-producing OT-I cells in the spleen after direct ex vivo OVA257–264-peptide stimulation. (B–D) Data show mean+SD (n = 5 mice/group).
Taken together, these data suggest that systemic inflammation controls the quantity and quality of 2° effector and memory CD8+ T-cell populations.
Discussion
The protective capacity of memory CD8+ T-cell populations is dependent on their abundance in 2° lymphoid organs and tertiary tissues as well as their phenotype at the time of rechallenge [7, 9]. Our results reveal a surprising plasticity of 2° effector and memory CD8+ T-cell phenotype that can be influenced by the choice of the boosting pathogen, the pathogen load, and the levels of systemic inflammation. The direct comparison of 1° and 2° immune responses in the same host demonstrates that for some phenotypic markers 2° memory CD8+ T cells are at least as sensitive as 1° memory CD8+ T cells to inflammation-induced changes. These phenotypic markers include CD27 and KLRG-1, two markers which have been used to characterize the recall capacity and the proliferative potential of memory CD8+ T-cell populations [8, 33]. Currently, KLRG-1 expression is believed to mark senescent cells that have undergone extensive proliferation [31]. In support of this notion, KLRG-1+ memory CD8+ T cells have been shown to possess low proliferative potential following Ag challenge [31, 33, 34]. Our data show that KLRG-1 expression does not always correlate with the number of cell divisions but that the expression of this molecule on 1° and 2° memory CD8+ T cells can be modulated by systemic inflammation as well. It will be of interest to analyze the impact of the observed changes in phenotype on the ability of 2° memory CD8+ T cell to expand in numbers and kill invading pathogens upon rechallenge.
Perhaps even more important than the observed impact on memory CD8+ T-cell phenotype is the influence of inflammation on 2° memory CD8+ T-cell numbers. Protection against recurrent infections is closely related to the frequency of circulating memory CD8+ T cells and for some infections, thresholds have been identified above which memory CD8+ T cells provide reliable protection [35]. Our analysis of 2° effector and memory CD8+ T-cell numbers suggests that systemic inflammation could increase both 2° effector and memory CD8+ T-cell numbers in lymphoid organs as well as in tertiary tissues. The differential susceptibility of 1° and 2° effector CD8+ T cells to inflammation-induced apoptotic stimuli may seem surprising, but in fact differences in the regulation of apoptosis between 1° and 2° effector CD8+ T cells have been shown earlier [29].
It will require further studies to analyze the molecular basis for this differential susceptibility to inflammation and the role of molecules such as IL-12 [12], type I IFNs [36], IFN-γ [37], Bim [38], and T-bet [15] in this process. Even more importantly, it will be important to determine whether these findings can be harnessed to increase the efficacy of prime-boost regimens. As one example, our data predict that it would be more beneficial to use adjuvants in booster infections than in 1° infections because systemic inflammation would increase 2° memory CD8+ T-cell numbers more efficiently than 1° memory CD8+ T-cell numbers. The underlying mechanism(s) and inflammatory mediators that might be involved in controlling the development of 2° effector and memory CD8+ T cells will be the subject of future studies.
The differential susceptibility of 1° and 2° memory CD8+ T cells to inflammation opens up new avenues to alter the composition and phenotype of the memory CD8+ T-cell compartment in prime-boost regimens. In natural settings, many individuals will harbor both naïve and memory CD8+ T cells specific for a certain pathogen due to incomplete recruitment of naïve CD8+ T cells, continuous output of naïve precursors from the thymus or accumulation of CD8+ T cells with a memory phenotype in the absence of infection [39, 40]. For naturally occurring infections, our data predict that infections that are accompanied by systemic pathogen spread and high levels of inflammation lead to the preferential expansion of 2° effector CD8+ T cells, whereas local infections and low levels of inflammation would preferentially generate 1° effector CD8+ T cells. This could be part of a regulatory system that employs memory CD8+ T cells to combat more severe, systemic infections but allows for the recruitment of new naïve precursors in the case of local and less harmful infections. Interestingly, a similar concept has been proposed recently by a study that found that naïve T cells can be efficiently primed by DCs in peripheral tissues, whereas memory CD8+ T cells are more potently stimulated by CD8+ DCs that reside in lymph nodes and spleen [41].
Our data also identify new means to alter the composition of memory CD8+ T-cell populations in hosts that are repeatedly challenged with the same or related pathogens/immunizations. Several studies have shown that 1° and 2° memory CD8+ T-cell populations differ in their lineage commitment and their protective capacity [10, 30, 42]. Therefore, memory CD8+ T-cell populations can potentially be tailored to contain the memory CD8+ T-cell subset that provides optimal protection. According to our data, the choice of the booster pathogen and modulations in the inflammatory milieu offer the possibility to modulate the expansion of 2° effector CD8+ T cells and thus control the number of 2° memory CD8+ T cells in the ensuing memory population. As an example, the use of L. monocytogenes and boosting agents that induce high levels of systemic inflammation would result in more efficient expansion of 2° effector CD8+ T cells compared with Vaccinia vectors and booster infections that induce less systemic inflammation. Vice versa, Vaccinia vectors would allow for a more effective priming of naïve T cells and the generation of memory populations with higher percentages of 1° memory CD8+ T cells. Therefore, our data indicate new possibilities to modify 2° memory CD8+ T-cell numbers and phenotype which could help to increase the efficacy of prime-boost regimens that target acute and chronic infections.
Materials and methods
Mice, infections
C57Bl/6 mice (6–8 wk) were obtained from the NCI. OT-I mice were bred to Thy1.1 mice to obtain Thy1.1/1.1 and Thy1.1/1.2 OT-I donors. Vaccinia virus expressing OVA (VacV-OVA) was grown and injected as described previously [43]. Attenuated actA-deficient L. monocytogenes (att LM-OVA) and virulent L. monocytogenes (vir LM-OVA) expressing OVA were grown and quantified as described previously [43]. All animal experiments followed approved Institutional ACURF protocols.
DC immunizations
Splenic DCs were isolated after subcutaneous injection of C57Bl/6 mice with 5 × 106 B16 cells expressing Flt3L as described previously [17]. To induce systemic inflammation, mice immunized with DC were infected with actA-deficient Listeria (DP-L1942) 1 day after DC immunization.
OT-I cells transfer and isolation of lymphocytes from tissues
For cotransfer of naïve and 1° memory cells, naïve Thy1.1 OT-I T cells were obtained from peripheral blood samples of 2- to 3-month-old Thy1.1 OT-I mice. The percentage of CD44hi/CD11ahi Vα2+Vβ5+ OT-I cells was <5%. To generate Thy1.1/1.2 memory OT-I T cells for adoptive transfer experiments, 1 × 103 naïve Thy1.1/1.2 OT-I T cells were transferred into Thy1.2 recipients and mice were immunized with either VacV-OVA (3 × 106 PFU/mouse; i.p.) or att LM-OVA (5 × 106 CFU/mouse; i.v.). In total, 40–60 days after infection, memory OT-I T cells were isolated by positive selection for Thy1.1. Memory OT-I T cells (1–1.5 × 104/mouse) were either transferred alone or in combination with 5 × 102 naïve OT-I i.v. into naïve hosts. Mice were immunized 24 h after transfer. For some experiments, animals received 2 mg/mL of ampicillin in their drinking water 12–16 h after infection for a period of 2 days. For quantification of CD8+ T-cell responses and assessment of their tissue distribution, mice were sacrificed and perfused with PBS before harvesting the organs and making single-cell suspensions [44].
Antibodies
For FACS analysis, the following antibodies were used: Thy1.1 (OX-7) and CD62L (MEL-14, both BD Pharmingen), KLRG-1 (2F1, Southern Biotech), CD8 (53–6.7), CD127 (A7R34), CD27 (LG.7F9), IFN-γ (XMG1.2), TNF (MP6-XT22), IL-2 (JES6-5HG, all eBioscience), Granzyme B (Caltag), and appropriate isotype controls.
Quantification of CD8+ T cells
OT-I T-cell responses in PBL samples and spleen were monitored by FACS analysis for Thy1.1 marker. Thy1.2 expression was used to discriminate between 1° and 2° OT-I T-cell responses in the same host. The ability of effector and memory OT-I T-cell populations to produce cytokines (IFN-γ, TNF, and IL-2) was determined by peptide (OVA257–264) stimulated intracellular cytokine staining as described previously [44]. In the same stimulated samples, the expression of granzyme B was also determined.
Clearance of infection
To validate eradication of Listeria after ampicillin treatment, spleens from infected mice were harvested 2 days after infection and analyzed for bacterial content as described previously [13].
Statistical analysis
Statistical significance was assessed by using the two-tailed t-test with a confidence interval of >95%. Data are presented as mean (±SD or SEM). All experiments were repeated at least once to ensure reproducibility.
Acknowledgments
This work was supported by startup funds from the Department of Pathology (VPB), NIH grants AI83286 (V. P. B.), AI42767, AI46653, AI50073, AI59752 (J. T. H.), and DFG-fellowship WI 3308/1-1 (T. C. W.).
Abbreviations
- 1°
primary
- 2°
secondary
- att LM-OVA
actA-Listeria monocytogenes expressing OVA peptide
- VacV-OVA
Vaccinia virus expressing OVA peptide
- vir LM-OVA
virulent Listeria monocytogenes expressing OVA peptide
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 3.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 4.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 5.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 8.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 10.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 11.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 13.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 14.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 15.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol. 2002;169:6842–6849. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 19.Koch S, Larbi A, Ozcelik D, Solana R, Gouttefangeas C, Attig S, Wikby A, et al. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann NY Acad Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- 20.Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 21.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravkov EV, Williams MA. The magnitude of CD4+ T cell recall responses is controlled by the duration of the secondary stimulus. J Immunol. 2009;183:2382–2389. doi: 10.4049/jimmunol.0900319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Wirth TC, Pham NL, Harty JT, Badovinac VP. High initial frequency of TCR-transgenic CD8 T cells alters inflammation and pathogen clearance without affecting memory T cell function. Mol Immunol. 2009;47:71–78. doi: 10.1016/j.molimm.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 28.Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- 29.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 30.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 32.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 34.Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, Plunkett FJ, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+T cells. Blood. 2009;113:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci USA. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 37.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 38.Prlic M, Bevan MJ. Exploring regulatory mechanisms of CD8+ T cell contraction. Proc Natl Acad Sci USA. 2008;105:16689–16694. doi: 10.1073/pnas.0808997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 40.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat Immunol. 2007;8:1060–1066. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- 42.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wirth TC, Harty JT, Badovinac VP. Modulating numbers and phenotype of CD8+ T cells in secondary immune responses. Eur J Immunol. 2010;40:1916–1926. doi: 10.1002/eji.201040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rai D, Pham NL, Harty JT, Badovinac VP. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol. 2009;183:7672–7681. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]