Abstract
Coding variants in the apolipoprotein L1 gene (APOL1) are strongly associated with nephropathy in African Americans (AAs). The effect of transplanting kidneys from AA donors with two APOL1 nephropathy risk variants is unknown. APOL1 risk variants were genotyped in 106 AA deceased organ donors and graft survival assessed in 136 resultant kidney transplants. Cox proportional-hazard models tested for association between time to graft failure and donor APOL1 genotypes. Mean follow-up was 26.4 ± 21.8 months. Twenty-two of 136 transplanted kidneys (16%) were from donors with two APOL1 nephropathy risk variants. Twenty five grafts failed; eight (32%) had two APOL1 risk variants. A multivariate model accounting for donor APOL1 genotype, overall African ancestry, expanded criteria donation, recipient age and gender, HLA mismatch, CIT, and PRA revealed that graft survival was significantly shorter in donor kidneys with two APOL1 risk variants (hazard ratio [HR] 3.84; p=0.008) and higher HLA mismatch (HR 1.52; p=0.03), but not for overall African ancestry excluding APOL1. Kidneys from AA deceased donors harboring two APOL1 risk variants failed more rapidly after renal transplantation than those with zero or one risk variants. If replicated, APOL1 genotyping could improve the donor selection process and maximize long term renal allograft survival.
Keywords: African Americans, APOL1, focal segmental glomerulosclerosis, graft survival, kidney donor, kidney transplantation
Introduction
Two coding variants in the apolipoprotein L1 gene (APOL1) are strongly associated with non-diabetic forms of nephropathy in African Americans in an autosomal recessive pattern of inheritance.1, 2 APOL1 nephropathy risk variants include G1, a non-synonymous coding variant (342G:384M), and G2, a six base pair deletion. The APOL1 gene variants are strongly associated with hypertension-attributed end-stage renal disease (HA-ESRD, typically focal global glomerulosclerosis [FGGS] with interstitial scarring and arteriolar changes), idiopathic focal segmental glomerulosclerosis (FSGS), and HIV-associated collapsing glomerulosclerosis in African Americans, with odds ratios of 7.3 and 10.5 (recessive), respectively, for HA-ESRD and FSGS.1–3 APOL1 risk variants likely rose to high frequency in sub-Saharan Africa due to the protection they afford from Trypanosoma brucei rhodesiense infection, the parasite causing African sleeping sickness.1
Variation in APOL1 accounts for the higher rate of non-diabetic kidney disease in African Americans relative to European Americans.1 Thirty percent of African American chromosomes possess either a G1 or a G2 APOL1 allele (alleles are mutually exclusive on single chromosomes); approximately 10–12% of African Americans possess two APOL1 risk variants and 49% lack risk variants.1 In comparison, APOL1 risk variants are rare in European Americans, approximately 0.3% carry G1 and 0.1% G2 alleles.4
Kidneys donated by African Americans are known to function for shorter periods of time than kidneys donated by European Americans.5–8 This effect is observed whether kidneys are transplanted into African American or non-African American recipients. We hypothesized that this difference in renal allograft survival rates according to donor race could be secondary to the presence of APOL1 risk variants. Therefore, we tested for differences in renal allograft survival in kidneys donated by African Americans with two APOL1 nephropathy risk variants versus those with zero or one APOL1 risk variants.
Methods
Study Design
From March 24, 1998 through September 12, 2009, 234 African American deceased organ donors had kidneys recovered and typing material procured or delivered to the Wake Forest University Baptist Medical Center (WFUBMC) for transplantation purposes. DNA from whole blood was available in 106 individuals whose kidneys were subsequently transplanted at the same medical center into 136 unique transplant recipients. These locally performed kidney transplant patients were followed longitudinally at our center for at least one year and form the study sample. This study was approved by the WFUBMC Institutional Review Board.
Transplant Immunosuppression
Prior to 2001, transplant recipients were managed with cyclosporine, mycophenolate mofetil (MMF) and prednisone immunosuppression with selective use of non-depleting antibody induction (basiliximab or daclizumab). From October 2001 to February 2005 patients received T-cell depleting antibody induction with rabbit anti-thymocyte globulin (rATG) in combination with tacrolimus, MMF and prednisone. From February 2005 to September 2007 patients received rATG or alemtuzumab in combination with tacrolimus, MMF and early steroid elimination. Patients with delayed graft function (DGF) underwent a surveillance kidney biopsy at two weeks post-transplant. Tacrolimus target levels and decisions whether to withdraw steroids (after 2005) depended on immunologic risk stratification and initial kidney graft function. Patients considered at higher immunologic risk (retransplants, panel reactive antibody [PRA] levels >20%, African Americans <40 years of age) or those with DGF remained on steroids but were tapered to 5 mg/day by two months after transplant. High immunologic risk patients were managed with target tacrolimus levels 10–12 ng/ml for the first 3 months, then 8–10 ng/ml. Low immunologic risk patients were tapered off steroids by post-operative day 6, target tacrolimus levels were 8–10 ng/ml for the first 3 months, then 6–8 ng/ml.
Patient Follow-up
All transplant recipients were followed closely in the WFUBMC transplant clinic for at least 3 months with a minimum of twice weekly monitoring for the first 6 weeks and then weekly monitoring for the next 6 weeks. Stable patients were referred to their community nephrologist and seen back in the WFUBMC transplant clinic at 6–12 month intervals thereafter. Routine laboratory testing at the WFUBMC transplant clinic is performed in the North Carolina Baptist Hospital Central Laboratory. HLA typing, crossmatching and PRA testing were performed in the Wake Forest University Health Sciences HLA/Immunogenetics Laboratory. All evaluations in this report were based on results from these laboratories.
Tissue Processing and Genotyping
DNA extraction from whole blood was performed using either QIAamp DNA Blood Mini Kits (Qiagen; Venlo, Netherlands) or an AutoPure LS automated DNA extraction robot (Gentra Systems; Valencia, California). Three APOL1 single nucleotide polymorphisms (SNPs) were genotyped on the Sequenom Mass Array (www.sequenom.com); rs73885319 and rs60910145 in G1 (SNPs in perfect linkage disequilibrium; r2=1.0) and rs71785313 in G2. Ancestry estimates were provided by genotyping seventy bi-allelic ancestry informative markers, as previously reported.9
Statistical Analyses
The distribution of demographic variables for African American kidney donors by APOL1 risk status was compared using Wilcoxon two-sample tests for continuous variables and chi-square tests for binary variables. A series of Cox proportional hazard models was then fitted. The 106 deceased donors kidneys transplanted into 136 recipients resulted in correlated but unordered outcomes for 30 kidneys donated by one individual and transplanted into two recipients. We assumed no significant time difference within these 30 clusters, since the transplants were performed within hours of each other and the duration of graft function was measured in years.
The outcome of interest was time to graft failure computed as the difference between the transplant date and the graft loss date (date of return to dialysis). The date of final observation was censored in the event of death or at the final clinical follow-up with a functioning graft. Several marginal10 Cox proportional hazard models were fitted starting with an unadjusted model testing for association between the presence of two copies of APOL1 G1 or G2 nephropathy risk variants (G1 homozygotes, G2 homozygotes, or G1/G2 compound heterozygotes) as defined in Genovese et al.1 and time to graft loss; ending with a fully adjusted model that tested for the same effect after accounting for the proportion of African ancestry in kidney donors, recipient age and gender, HLA match, cold ischemia time, PRA (0% versus > 0%) and standard (versus expanded) criteria donation. Expanded criteria donors were defined as age 60 years or older; or meeting two of three criteria if age 50 to 59: history of hypertension, death from stroke, or terminal serum creatinine concentration greater than 1.5 mg/dl. The marginal model is akin to the well known generalized estimating equations,11 where unbiased parameter estimates are obtained using pseudo-likelihoods and the sandwich estimate of the covariance is used to account for the within cluster correlation. The marginal model works well for unordered outcomes but may yield biased estimates for ordered outcomes. For completeness, we compared the results of the marginal model to frailty models assuming a Gamma distribution, which yielded the best fit for the data. Both models yielded the same inference in each case (results not shown). Diagnostic tests based on the Schoenfeld residuals showed the proportional odds assumption holds. The minimum P-value associated with the interaction effect between all covariates in the final model and log (time to graft loss) was 0.08.
Results
Donor and recipient characteristics in the 136 African American deceased donor kidney transplants are shown in Table 1, contrasting donors having two (N=22) versus less than two (N=114) APOL1 nephropathy risk alleles. Although kidneys from donors with two APOL1 risk alleles were more often transplanted into male recipients (77.3 vs. 53.5%, p=0.04), other characteristics were similar between the donor groups. Four kidney transplants were performed before 2001 (1 from a donor kidney with two APOL1 risk variants), 26 between 2001 and 2005 (3 from donors with two APOL1 risk variants), and 106 after 2005 (18 from donors with two APOL1 risk variants). Donor age, terminal serum creatinine concentration, percentage of recipients who were African American, and percentage of recipients who diabetes-associated ESRD did not differ significantly between recipients of kidneys from donors with two APOL1 nephropathy risk variants versus zero or one risk variants (Table 1). A urinalysis at organ procurement was available in 82 of the 106 deceased donors (14 APOL1 risk donors and 68 non-risk donors). Trace or greater proteinuria was detected in 5 of 14 APOL1 risk donors (36%) and 22 of 68 (32%) non-risk donors.
Table 1.
Demographic characteristics of renal allograft donors and recipients
| Variable | Number of APOL1 G1-G2 nephropathy risk variants from kidney donor | ||
|---|---|---|---|
| Two | Zero or One | P-value | |
| N=22 | N=114 | ||
| Donor age (years) | 43.7 ± 16.8 | 47.7 ± 15.8 | 0.27 |
| Donor gender (% male) | 66.3 | 60.0 | 0.26 |
| Terminal serum creatinine (mg/dl) | 1.34 ± 0.8 | 1.19 ± 0.7 | 0.51 |
| PRA* at transplant (%) | 13.4 ± 27.6 | 19.7 ± 31.3 | 0.48 |
| PRA * > 0 (%) | 31.6 | 38.7 | 0.56 |
| Donor African ancestry (%) | 0.77 ± 0.10 | 0.72 ± 0.21 | 0.53 |
| Cold ischemia time (hours) | 22.5 ± 7.9 | 23.2 ± 8.0 | 0.82 |
| HLA mismatch (N) | 4.2 ± 1.4 | 3.8 ± 1.5 | 0.29 |
| Recipient gender (% male) | 77.3 | 53.5 | 0.04 |
| Recipient age (years) | 45.2 ± 16.9 | 47.1 ± 16.2 | 0.72 |
| Standard criteria donor (%) | 81.8 | 79.8 | 0.83 |
| Recipient race (% African American) | 50.0 | 50.9 | 0.94 |
| Diabetic ESRD in recipients (%) | 27.3 | 39.5 | 0.28 |
PRA - panel reactive antibody percentage
Renal allograft recipients were followed from 0.03 to 127.2 months after transplantation; all patients with less than one year of follow-up had graft failure prior to one year. The mean follow-up was 27.5 ± 22.2 months in the 126 recipients followed beyond January 1st, 2009. The mean duration of follow-up for the 10 recipients lost to follow-up prior to this date was 12.5 ± 10.3 months and 2 (20%) of them received kidneys with two APOL1 risk variants. Ten deaths were recorded, 1 (4.5%) in a recipient of a kidney with two APOL1 risk variants and nine (7.9%) in recipients with either none or one APOL1 risk variant. Twenty five renal allograft failures were recorded, eight in the 22 recipients (36.4%) of a kidney with two APOL1 risk variants, and 17 (14.9%) in the 114 recipients of a kidney with none or one APOL1 risk variants. No transplant recipient had a doubling of serum creatinine concentration without ultimate graft loss during the study.
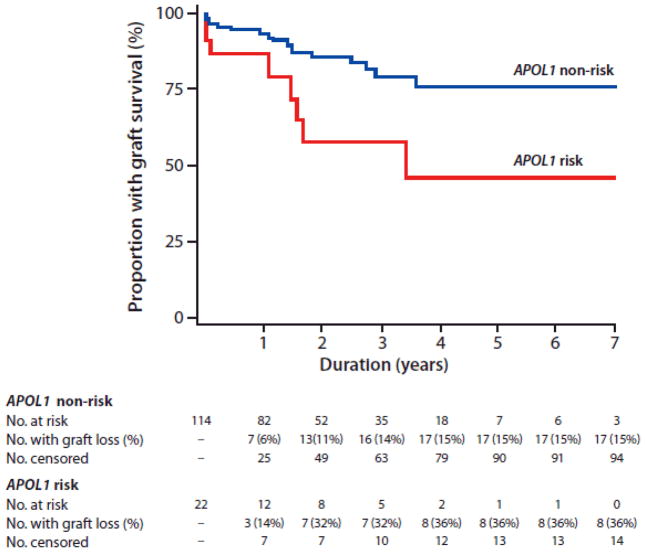
In a model adjusting for genome-wide African ancestry, significantly shorter graft survivals were observed in recipients of donor kidneys with two APOL1 risk variants (hazard ratio [HR] 2.95, p=0.01). In the fully adjusted model, the presence of two APOL1 risk alleles in donor kidneys independently predicted graft failure (HR 3.84, p=0.008), after accounting for global donor African ancestry, recipient age and gender, cold ischemia time, PRA, number of HLA mismatches, and expanded criteria donation (Table 2). Excluding APOL1, donor African ancestry was not independently associated with graft survival. Higher numbers of HLA mismatches (HR 1.52, p=0.030) also predicted shorter graft survival, with a trend towards an effect from increased cold ischemia time (HR 1.06, p=0.057). Figure 1 depicts the Kaplan-Meier curves illustrating renal allograft survival based on the APOL1 genotypes of kidney donors. Recipients of kidneys from donors with one APOL1 nephropathy risk variant had similar renal allograft survivals as those who received kidneys with zero risk variants (p=0.65).
Table 2.
Fully Adjusted Model for Renal Allograft Survival
| Parameter | Parameter Estimate | Standard Error | P-value | Hazard Ratio |
|---|---|---|---|---|
| APOL1 G1/G2 (2 vs. 0/1 copies) | 1.35 | 0.51 | 0.008 | 3.84 |
| African ancestry | −0.76 | 1.66 | 0.65 | 0.47 |
| Recipient age | 0.01 | 0.02 | 0.40 | 1.02 |
| Gender (Female) | −0.01 | 0.52 | 0.98 | 0.99 |
| Donor criteria (Standard) + | −0.38 | 0.56 | 0.50 | 0.69 |
| HLA Mismatch | 0.42 | 0.19 | 0.030 | 1.52 |
| Cold ischemia time | 0.06 | 0.03 | 0.057 | 1.06 |
| PRA* > 0% | 0.33 | 0.56 | 0.56 | 1.39 |
Standard vs. Expanded criteria donor
PRA - panel reactive antibody
Figure 1. Renal allograft survival according to APOL1 genotype.
Kaplan-Meier renal allograft survival curve for recipients of donor kidneys with (red line) and without (blue line) two APOL1 risk variant alleles.
All cases with graft failure had a kidney biopsy. In the 8 cases losing graft function after receiving kidneys from donors with two APOL1 risk variants, potential APOL1-associated lesions were present in 75% (one FSGS, two FSGS collapsing variant, and three donor-acquired nephron scarring with arteriosclerosis). These 6 biopsies were performed after a mean of 74.7 (range 7–183) days, none immediately pre- or post-implantation. The two remaining recipients of APOL1 risk kidneys that failed underwent early nephrectomy (hyperacute rejection day 5; vascular compromise/multiple infarcts day 2). In the 17 cases losing graft function after receiving kidneys from donors with less than two APOL1 risk variants, FSGS was seen in 11.8% (one case each of FSGS and FSGS collapsing variant). Of the remainder, 9 had chronic changes associated with rejection, 2 vascular compromise/multiple infarcts, 2 donor-acquired diabetic glomerulosclerosis (both on post-implantation biopsy), 1 thrombotic microangiopathy, and 1 hyperacute rejection. Excluding 5 of 17 with post-implantation biopsies, the other 12 biopsies were performed after a mean of 446 (range 8–1248) days.
Multivariate analysis was repeated excluding graft failures due to hyperacute rejection and technical failure, processes felt unlikely to be APOL1-related. Excluding 2/8 graft losses from APOL1 risk donors and 3/17 graft losses from APOL1 non-risk donors, two APOL1 G1/G2 risk variants in donors (HR 2.32, p=0.028) and number of HLA mismatches (HR 1.68, p=0.025) remained significantly associated with graft survival, with weaker effect from cold ischemia time (HR 1.06, p=0.10).
Discussion
In this single center study, significantly shorter renal allograft survival was observed in patients receiving deceased donor kidneys from African Americans with two APOL1 nephropathy risk variants compared to patients receiving kidneys from African American donors with fewer than two risk variants. Differences in graft survival were seen after approximately 20 months. Accounting for risk variation in APOL1, overall African ancestry did not significantly impact renal allograft survival after transplantation. HLA mismatch also significantly contributed to graft survival; however, the hazard ratio was weaker than that for APOL1 risk variants. In addition, we have shown that long-term renal graft survival rates in African American deceased donors lacking two APOL1 risk variants are similar to the rates seen in both non-African American deceased donor recipients listed in the U.S. Renal Data Systems between 1994–1998 6 and in standard criteria deceased donor recipients listed between 1997–2007 in the U.S. Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients.22 Therefore, our preliminary findings in a small sample suggest that the shorter renal allograft survival rates seen in kidneys donated by African Americans relative to Caucasians could be substantially related to variation in APOL1.
The mechanism(s) whereby APOL1 gene polymorphisms or ApoL1 risk variant proteins contribute to the development of glomerulosclerosis in native kidneys is unknown. The Kaplan-Meier renal allograft survival curves based on APOL1 genotypes of African American deceased kidney donors separate substantially by 20 months after renal transplantation in this report. Since 75% of failed grafts from donors with two APOL1 risk variants had FSGS or severe donor-acquired microvascular disease on biopsies shortly after transplantation, this suggests that the presence of two APOL1 risk variants with the stress of cold ischemia and exposure to calcineurin inhibitors led to accelerated loss of kidney function. We postulate that subclinical nephropathy was likely present in some kidneys donated by APOL1 risk homozygotes prior to procurement; especially since patients with FGGS often lack significant proteinuria.2 Nephrotoxicity relating to the presence of abnormal circulating ApoL1 proteins in transplant recipients is unlikely. ApoL1 is synthesized in the liver and would not be impacted by the genotype of the donor kidney. This does not preclude a role for nephrotoxicity initiated by circulating ApoL1 proteins (either free or HDL bound) in the kidneys of risk donors prior to donation. The initiating mechanisms for nephropathy could also differ in patients based upon their histologic subtype of APOL1-associated disease (e.g., FSGS, collapsing FSGS as in HIV associated nephropathy, and FGGS). This remains an important area of research.
Messenger RNA encoding APOL1 is expressed in liver, lung, placenta, endothelial cells, weakly in heart and pancreas, and possibly macrophages.12 ApoL1 is a secretory protein that associates with plasma high density lipoprotein (HDL) cholesterol.13 We hypothesize three potential pathways whereby APOL1 variants may lead to nephropathy in native (non-transplanted) kidneys. First, ApoL1 variant proteins may circulate in individuals harboring two APOL1 risk alleles; these proteins may bind less well to HDL particles, remain free to be filtered and reabsorbed by the proximal nephron and cause kidney disease. Circulating ApoL1 proteins have been implicated in rapidly recurring FSGS after kidney transplantation; a syndrome that can be improved with plasmapheresis.14–17 Second, altered HDL concentrations may contribute to the development of renal microvascular disease, a common accompaniment of FSGS and HA-ESRD. This effect may be accelerated in the presence of calcineurin inhibition. Finally, messenger RNA encoding ApoL1 protein is expressed in cultured human podocytes and other renal cell types (unpublished observation; Barry Freedman and Jeffrey Kopp). As such, podocyte expression could lead to injury or failure to support a critical cellular function. ApoL1 shares structural and functional similarities with the Bcl2 family of proteins involved in apoptosis.18 Apoptosis in podocytes may lead to glomerulosclerosis. These pathways, singly or in concert, could contribute to the development of subclinical APOL1-associated kidney disease in native kidneys, with subsequent loss of graft function after donation in the presence of cold ischemia and nephrotoxic medications including calcineurin inhibitors.
Additional risk factors for post-kidney transplant allograft failure include deceased donor category, preservation and recipient issues, and exposure to BK polyomavirus infection. These factors could accelerate graft loss in donor kidneys harboring two APOL1 risk variants. Importantly, significant differences in terminal serum creatinine concentration, donor age, percentage of African American recipients, type of immunosuppression (based on year of transplantation), and percentage of recipients with diabetic ESRD were not observed between study groups. Differential antigen presentation and immune system cell-cell interactions in APOL1 risk carriers may exist. Known immune-modulatory effects of apolipoprotein E (ApoE), another lipoprotein constituent of HDL, include inhibition of antigen-stimulated T cell proliferation, down regulation of intracellular signaling molecules downstream of the interleukin 2 receptor, suppression of pro-inflammatory cytokine formation by macrophages, regulation of major histocompatibility complex class II and co-stimulatory molecules (CD80, CD86) expression on dendritic cells, and inhibited expression of adhesion molecules on endothelial cells.19, 20
Weaknesses of this report include the small sample size, lack of APOL1 genotype determinations in recipients, few post-implantation biopsies, and results limited to a single transplant center. Recipient APOL1 genotypes would have permitted analysis of donor-recipient genotype interactions on graft survival 21; an effect relevant only for African American recipients as APOL1 nephropathy risk variants are rare in Caucasians. In this study, African Americans received 50.0% of donor kidneys harboring two APOL1 risk variants and 50.9% of kidneys harboring zero or one risk variants (p=0.94), nonetheless recipient genotypes remain of interest. Finally, although this was a single center study, all African American deceased donor kidneys with DNA for APOL1 genotyping transplanted at our center during the study period were included. This analysis clearly requires replication in other centers, as well as in recipients of live donor renal transplants. We did not adjust for the presence of donor specific antibodies, body mass index, rejection, compliance to medical therapy and follow-up. While we do not believe that differences in these factors contributed to our results, these are additional limitations.
At present, we do not suggest changes in kidney donor selection based on APOL1 genotypes, as our finding needs to be replicated at other transplant centers. However, our findings suggest that donor kidney APOL1 genotypes have the potential to improve the donor selection process, potentially resulting in optimization of long-term renal allograft function. If replicated, studies delineating the molecular basis of this finding and the testing of novel strategies to prevent early failure of African-ancestry donor kidneys related to APOL1 nephropathy risk variants are warranted, in this era of global shortages of suitable kidneys for transplantation.
Acknowledgments
This work was supported by grants RO1 DK070941 (BIF) and DK 084149 (BIF). The authors wish to thank all individuals associated with the WFUBMC Transplant Program, as well as our patients, organ donors and their families.
Alphabetical list of abbreviations
- AA
African American
- ApoE
apolipoprotein E
- APOL1
apolipoprotein L1 (gene)
- ApoL1
apolipoprotein L1 (protein)
- DGF
delayed graft function
- DNA
deoxyribonucleic acid
- ESRD
end-stage renal disease
- ECD
expanded criteria donor
- FGGS
focal global glomerulosclerosis
- FSGS
focal segmental glomerulosclerosis
- HA-ESRD
hypertension-attributed end-stage renal disease
- HDL
high density lipoprotein
- HIV
human immunodeficiency virus
- HLA
human leukocyte antigen
- HR
hazard ratio
- MMF
mycophenolate mofetil
- PRA
panel reactive antibody
- rATG
rabbit anti-thymocyte globulin
- RNA
ribonucleic acid
- SNPs
single nucleotide polymorphisms
- WFUBMC
Wake Forest University Baptist Medical Center
Footnotes
Disclosures
The authors have nothing to disclose.
Reference List
- 1.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman BI, Kopp JB, Langefeld CD, et al. The Apolipoprotein L1 (APOL1) Gene and Nondiabetic Nephropathy in African Americans. J Am Soc Nephrol. 2010;21(9):1422–1426. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128(3):345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooke JN, Bostrom MA, Hicks PJ, et al. Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. J Am Soc Nephol (Abstract) 2010 doi: 10.1093/ndt/gfr522. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int. 2000;58(3):1311–1317. doi: 10.1046/j.1523-1755.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 6.Swanson SJ, Hypolite IO, Agodoa LY, et al. Effect of donor factors on early graft survival in adult cadaveric renal transplantation. Am J Transplant. 2002;2(1):68–75. doi: 10.1034/j.1600-6143.2002.020112.x. [DOI] [PubMed] [Google Scholar]
- 7.Chakkera HA, O’Hare AM, Johansen KL, et al. Influence of race on kidney transplant outcomes within and outside the Department of Veterans Affairs. J Am Soc Nephrol. 2005;16(1):269–277. doi: 10.1681/ASN.2004040333. [DOI] [PubMed] [Google Scholar]
- 8.Callender CO, Cherikh WS, Traverso P, Hernandez A, Oyetunji T, Chang D. Effect of donor ethnicity on kidney survival in different recipient pairs: an analysis of the OPTN/UNOS database. Transplant Proc. 2009;41(10):4125–4130. doi: 10.1016/j.transproceed.2009.06.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sale MM, Smith SG, Mychaleckyj JC, et al. Variants of the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in an African-American population enriched for nephropathy. Diabetes. 2007;56(10):2638–2642. doi: 10.2337/db07-0012. [DOI] [PubMed] [Google Scholar]
- 10.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distribution. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 11.Liang K-Y, Zeger SL. Longitudinal data anlysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 12.Monajemi H, Fontijin RD, Pannekoek H, Horrevoets AJ. The apolipoprotein L gene cluster has emerged recently in evolution and is expressed in human vascular tissue. Genomics. 2002;79:539–546. doi: 10.1006/geno.2002.6729. [DOI] [PubMed] [Google Scholar]
- 13.Duchateau PN, Pullinger CR, Orellana RE, et al. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J Biol Chem. 1997;272(41):25576–25582. doi: 10.1074/jbc.272.41.25576. [DOI] [PubMed] [Google Scholar]
- 14.Sharma M, Sharma R, McCarthy ET, Savin VJ. “The FSGS factor:” enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma. J Am Soc Nephrol. 1999;10(3):552–561. doi: 10.1681/ASN.V103552. [DOI] [PubMed] [Google Scholar]
- 15.Gohh RY, Yango AF, Morrissey PE, et al. Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipients. Am J Transplant. 2005;5(12):2907–2912. doi: 10.1111/j.1600-6143.2005.01112.x. [DOI] [PubMed] [Google Scholar]
- 16.Candiano G, Musante L, Zennaro C, et al. Inhibition of renal permeability towards albumin: a new function of apolipoproteins with possible pathogenetic relevance in focal glomerulosclerosis. Electrophoresis. 2001;22(9):1819–1825. doi: 10.1002/1522-2683(200105)22:9<1819::AID-ELPS1819>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Candiano G, Musante L, Carraro M, et al. Apolipoproteins prevent glomerular albumin permeability induced in vitro by serum from patients with focal segmental glomerulosclerosis. J Am Soc Nephrol. 2001;12(1):143–150. doi: 10.1681/ASN.V121143. [DOI] [PubMed] [Google Scholar]
- 18.Zhaorigetu S, Wan G, Kaini R, Jiang Z, Hu CA. ApoL1, a BH3-only lipid-binding protein, induces autophagic cell death. Autophagy. 2008;4(8):1079–1082. doi: 10.4161/auto.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang HL, Wu J, Zhu J. The immune-modulatory role of apolipoprotein E with emphasis on multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Dev Immunol. 2010;2010:1–10. doi: 10.1155/2010/186813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stannard AK, Riddell DR, Sacre SM, et al. Cell-derived apolipoprotein E (ApoE) particles inhibit vascular cell adhesion molecule-1 (VCAM-1) expression in human endothelial cells. J Biol Chem. 2001;276(49):46011–46016. doi: 10.1074/jbc.M104812200. [DOI] [PubMed] [Google Scholar]
- 21.Freedman BI, Nagaraj SK, Lin JJ, et al. Potential donor-recipient MYH9 genotype interactions in post-transplant nephrotic syndrome after pediatric kidney transplantation. Am J Transplant. 2009;9(10):2435–2440. doi: 10.1111/j.1600-6143.2009.02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Organ and Procurement Transplantation Network (OPTN) Based on OPTN data. 2008 October 17; http://www.ustransplant.org.annual_reports/current.