Abstract
Selective attention filters information to limit what is encoded and maintained in working memory. Although the prefrontal cortex (PFC) is central to both selective attention and working memory, the underlying neural processes that link these cognitive abilities remain elusive. Using functional magnetic resonance imaging to guide repetitive transcranial magnetic stimulation with electroencephalographic recordings in humans, we perturbed PFC function at the inferior frontal junction prior to participants performing a selective-attention, delayed-recognition task. This resulted in diminished top-down modulation of activity in posterior cortex during early encoding stages, which predicted a subsequent decrement in working memory accuracy. Participants with stronger fronto-posterior functional connectivity displayed greater disruptive effects. Data further suggested that broad alpha band (7–14 Hz) phase coherence subserved this long distance top-down modulation. The results establish top-down modulation mediated by the prefrontal cortex as a causal link between early attentional processes and subsequent memory performance.
Selective attention describes goal-directed behavior achieved by orienting the focus of conscious awareness towards relevant stimuli and away from irrelevant or competing stimuli1. Working memory is the cognitive operation that underlies our ability to temporarily maintain and manipulate attended information in mind when it is no longer accessible in the environment to guide behavior2. The functional overlap between selective attention and working memory is intuitive, such that attended items are more likely to be remembered than ignored items. The reverse dependency has also been documented, as selective attention seems to utilize an internal template3 or attentional trace4 based on working memory that is used to resolve competition among multiple elements in the environment. However, only recently has empirical research begun to identify overlapping neural mechanisms engaged during both selective attention and working memory tasks5, 6. Despite evidence suggesting a shared neural substrate between these two cognitive operations, a direct causal link via a common control region and underlying neural process has yet to be established.
The neural process of top-down modulation serves as a fundamental physiological mechanism for selective attention. It underlies our critical ability to selectively focus our attentional resources on relevant stimuli and ignore distractions. It is a bi-directional process, accomplished by differentially enhancing and suppressing neural activity in sensory cortical regions based on the relevance of information to our goals7, 8. By generating neural contrast via the enhancement and suppression of activity, top-down signals are also thought to bias the likelihood of successfully representing and maintaining relevant information in a competitive system9–11. Recent neuroimaging research has provided indirect evidence that successful visual working memory performance in the setting of distracting information (i.e., requiring selective attention) is associated with top-down modulation of activity in visual cortices during early visual processing stages12, 13. However, direct evidence that selective attention and working memory are bound by this common neural mechanism has not yet been documented.
Neural network communication has been proposed to be the basis by which top-down modulation is achieved. Evidence in support of this comes from tract-tracing studies in monkeys that identified an anatomical framework of reciprocal cortico-cortical projections between areas in the parietal, frontal and visual association cortices14, 15. In addition, functional neuroimaging studies in humans have revealed that top-down modulation during visual processing engages this frontal-parietal-visual network7, 16, 17. Functional connectivity analysis offers further evidence that the prefrontal cortex (PFC) is a source of this activity modulation18. Lesion studies in humans19, and more recently transcranial magnetic stimulation (TMS) used to perturb function in frontal and parietal regions, provide causal evidence that these areas are a source of top-down activity modulation in visual cortex20–23. However, the mechanism underlying this long-distance communication and its influence on working memory performance has not been evaluated from a causal perspective.
Recent research has identified a region within the PFC, the inferior frontal junction (IFJ), which based on fMRI functional connectivity analysis may serve as a source of top-down modulation underlying attention to visual features (i.e. color and motion)24. Furthermore, electroencephalographic (EEG) results suggest that the IFJ may exert an influence on visual processing as early as 100 ms post-stimulus onset (during the P1 component of the event related potential (ERP)). This time point has previously been shown to be modulated by attention to color and motion stimuli25, 26, as well as related to subsequent working memory performance12. Moreover, we have recently determined that top-down modulation may be subserved by long-distance alpha band phase coherence24, consistent with the suggestion that alpha phase synchronization coordinates top-down control and access to memory traces27. However, this hypothesis has not been directly addressed with a methodology that permits causal inferences.
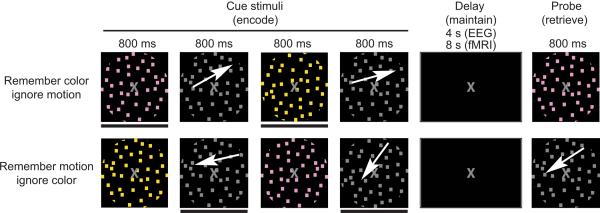
The current experiment directly addressed both the causal role and mechanisms of PFC-mediated top-down modulation, as driven by selective attention, on subsequent working memory performance. This was accomplished by perturbing function within the IFJ via repetitive TMS (rTMS) prior to participants performing a selective-attention, delayed-recognition task and recording the consequences with EEG. The paradigm utilized in this two-session experiment required participants to selectively attend to relevant visual features of sequentially presented stimuli (motion or color), ignore the irrelevant stimuli and maintain the attended features until the information was probed (Fig.1). The first session utilized functional magnetic resonance imaging (fMRI) to identify neural networks associated with top-down modulation. Based on each participant's functional connectivity data, during the second session, the right IFJ was targeted for 1 Hz rTMS to disrupt the network subserving top-down modulation. Immediately following rTMS, participants performed the same experimental task as the first session while EEG and working memory performance data were acquired. We assessed the rTMS influence on posterior P1 amplitude modulation and fronto-posterior alpha phase coherence modulation during stimulus presentation, as well as the impact on working memory accuracy. Our goals were to determine the extent to which early top-down activity modulation in visual cortices was driven by the IFJ, how this impacted working memory performance, and identify neural mechanisms underlying the long-distance modulatory influences.
Figure 1.
Experimental Paradigm. White arrows indicate motion and were not present during the experiment. A black bar below a cue stimulus (also not present during the experiment) indicates it is an item to be remembered. The pictures approximate stimuli appearance (see methods for details).
Results
Neural networks subserving top-down modulation
Areas V4 and V5/hMT+ are selectively responsive to color and motion features, respectively28, and activity in each region is modulated by top-down attentional processes29. Thus, color and motion features were used as the stimuli during a selective-attention, delayed-recognition task12, 24 to induce top-down modulation in the visual association cortex (Fig. 1). During the first session, areas V4 and V5 were identified as regions of interest (ROIs) for each participant, and were shown to be modulated by attention during the task (Supplementary Fig. 1; see Supplementary Results for more details).
In order to identify neural networks involved in top-down modulation, functional connectivity analysis using the beta series correlation approach30, 31 was conducted on the fMRI data using V4 and V5 ROIs as seed regions. Four group-level network connectivity maps were generated for the encoding period regressor by correlating each seed region with voxels from the rest of the brain during either the congruent or incongruent condition: V4 attend network (V4 seed and remember color condition), V4 ignore network (V4 seed and Remember Motion condition), V5 attend network (V5 seed and Remember Motion condition) and V5 ignore network (V5 seed and Remember Color condition). Connectivity maps were submitted to a repeated measures analysis of variance (ANOVA) with ROI (V4 / V5) and relevance (attend / ignore feature) as factors and corrected for multiple comparisons by thresholding p-values with a cluster extent determined by a Monte-Carlo simulation resulting in a corrected P-value of 0.01.
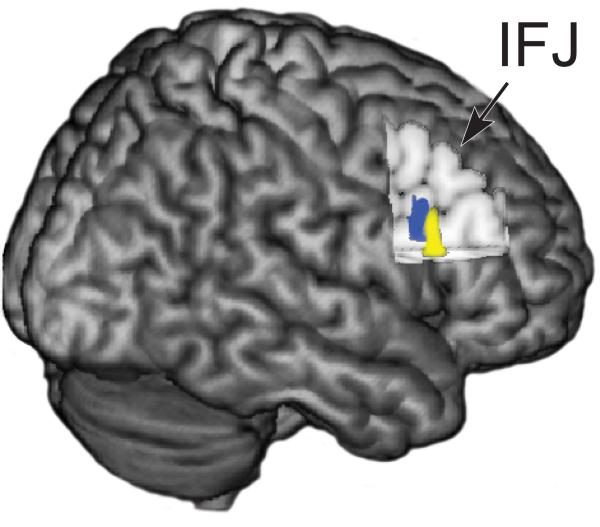
Main effects for ROI and relevance, as well as an ROI X relevance interaction were observed in widespread fronto-parietal regions. To further explore the neural networks underlying top-down modulation, paired t-tests contrasted attend and ignore network maps for each seed region. This functional connectivity analysis revealed frontoparietal neural networks were engaged during top-down modulation for both color (Supplementary Table 1) and motion features (Supplementary Table 2). Although the color and motion top-down network maps were largely distinct from each other, the right inferior frontal junction (IFJ) within the PFC was shown to be utilized by both color and motion neural networks (Fig. 2). This finding replicates recent results from a separate cohort of participants engaged in the same task, which suggested the right IFJ as a putative control region for long-distance top-down modulation of both color and motion feature processing24. Thus, the right IFJ region served as the target site for rTMS in the second experimental session. Of note, data from the current and a previous study24 both revealed bilateral IFJ engagement only in the motion network. We hypothesized that rTMS to the right IFJ would affect neural measures and working memory performance more for the Remember Color condition than for the Remember Motion condition, since color processing utilized only the right IFJ, and motion processing recruited bilateral IFJ. Therefore, this prediction served as an internal control of the specificity of rTMS effects.
Figure 2.
Functional connectivity analysis revealed a fronto-parietal region associated with both motion and color top-down modulation, the right inferior frontal junction (IFJ). However, distinct sub-regions within the IFJ distinguish motion (blue area) from color (yellow area) networks.
Effects of rTMS on top-down modulation and working memory
During the second session, 1 Hz rTMS was applied for 10 minutes to the right IFJ in order to perturb function in this region32, potentially impacting the neural network underlying top-down modulation of activity in visual regions and subsequent working memory performance. Each participant received two blocks of rTMS (one preceding each condition), as well as two blocks of sham rTMS (coil angled 90 degrees away from head as a control) followed by 64-channel EEG recordings while they were engaged in the experimental paradigm. rTMS effects decrease over time33, therefore, data from each experimental block was divided in half for analysis. It was hypothesized that rTMS-related changes in neural activity and working memory performance would be most prominent during the first half of a block. This prediction served as another important internal control.
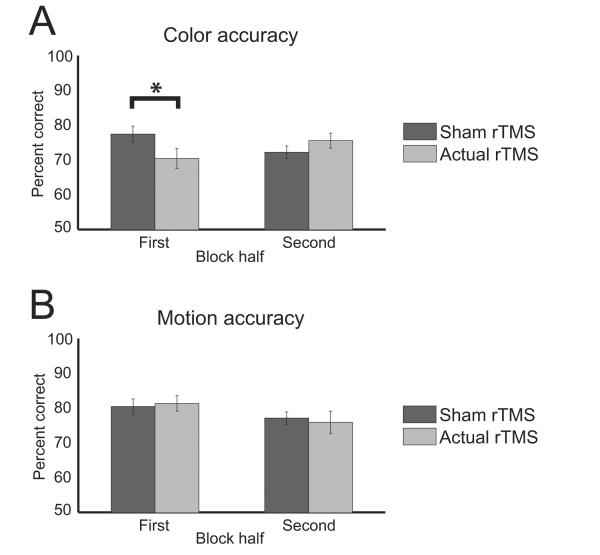
Measures of working memory accuracy were submitted to a repeated measures ANOVA with condition (Remember Color / Motion), rTMS (sham / actual), and block half (1st / 2nd) as factors. Results indicated a main effect for condition (F1,18 = 7.21, P < 0.05), a two-way interaction between rTMS and block half (F1,18 = 6.31, P < 0.05), and a three-way interaction (F1,18 = 5.62, P < 0.05). Post-hoc paired t-tests evaluating the three-way interaction indicated that working memory accuracy for the Remember Color condition decreased following actual rTMS relative to sham rTMS levels during the first half of the experimental block (t18 = 2.29, P < 0.05; Fig. 3a). However, this effect was not observed during the second half of the Remember Color condition (P > 0.1). Moreover, rTMS did not affect working memory performance during either half of the Remember Motion condition (P > 0.5, each comparison; Fig. 3b), revealing that the right IFJ has varying importance based on stimulus feature type. The findings confirmed our predictions that rTMS (and not sham rTMS) would impact working memory performance more during the Remember Color than the Remember Motion condition with a greater influence during the first half of the block.
Figure 3.
Working memory accuracy for the, (a) Remember Color and (b) Remember Motion conditions. Accuracy declined due to rTMS only for color working memory during the first half of the experimental block. Error bars represent s.e.m. Asterisk indicates p < 0.05.
We have previously identified the P1 amplitude of the ERP (at posterior electrodes) in response to stimulus presentation during the encoding period as a neural marker of top-down modulation for visual features12, 25. To confirm the utility of the P1 as a marker of color and motion feature processing, source localization was conducted on the P1 from each attended feature following sham rTMS. Analysis indicated the P1 source as the striate and extrastriate visual cortex as well as portions of the inferior temporal cortex. Of interest, these sources included V4 when attending to color and V5 when attending to motion (Supplementary Fig. 2). Therefore, initial analysis of the EEG data focused on P1 amplitude modulation and assessed whether it was altered by rTMS.
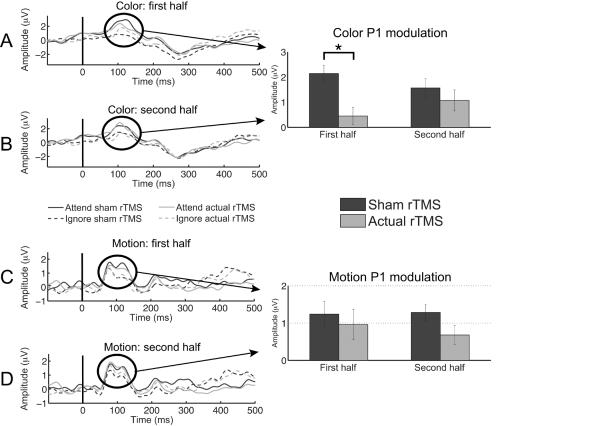
Measures of top-down modulation using P1 amplitude differences (attend – ignore) were submitted to a repeated measures ANOVA with stimulus (color / motion), rTMS (sham / actual), and block half (1st / 2nd) as factors. Results indicated a main effect for rTMS (F1,18 = 4.58, P < 0.05), as well as a three-way interaction (F1,18 = 4.51, P < 0.05). Post-hoc paired t-tests evaluating the three-way interaction showed that during the first half of the Remember Color condition, the neural signature of top-down modulation at the P1 decreased following actual rTMS relative to sham stimulation (t18 = 3.00, P < 0.01; Fig. 4a). The magnitude of P1 modulation during the second half of the Remember Color condition showed no difference between actual and sham rTMS (t18 = 0.86, P > 0.4; Fig. 4b). These results show that the magnitude of top-down modulation for color processing was decreased due to rTMS only during the first half of the experimental block, comparable to the working memory accuracy findings. Post-hoc paired t-tests of P1 modulation during the Remember Motion condition showed no differences between sham and actual rTMS during either the first (t18 = 0.43, P > 0.6; Fig. 4c) or second half (t18 = 1.93, P > 0.05; Fig. 4d) of the experimental block. These results were comparable to the lack of rTMS effects on working memory accuracy for motion, and further confirm our prediction that neural measures of color processing are more affected by rTMS than motion processing with a greater influence during the first half of the block.
Figure 4.
Attentional modulation of the P1 during color processing (a,b) and motion processing (c,d). (a) P1 modulation declined to non-significant levels during the first half of the experimental block following actual rTMS compared to sham. (b) Attentional modulation was observed during the second half of each block following both sham and actual rTMS. Bar graphs indicate the magnitude of attentional modulation (attend – ignore). rTMS altered the magnitude of P1 attentional modulation only during the first half of the actual rTMS block. Asterisk indicates p < 0.05. (c and d) Attentional modulation of the P1 during motion processing was observed during both the first and the second half of each experimental block. rTMS did not change the magnitude of attentional modulation to motion stimuli. Error bars represent s.e.m.
To explore whether the observed changes in P1 modulation during the first half were due to changes in enhancement of relevant stimuli or suppression of irrelevant stimuli, data from sham and actual rTMS were directly compared. Results indicated that rTMS reduced the degree that ignored color stimuli were suppressed (t18 = 2.43, P < 0.05). Additionally, an rTMS related decrease in the amount relevant color stimuli was enhanced strongly trended towards significance (t18 = 1.95, P = 0.067). Thus, the rTMS-induced decrease in top-down modulation was due to combined effects of a decline in the participant's ability to enhance P1 activity to relevant color stimuli and suppress P1 activity to irrelevant color stimuli. This implies that a single region in the PFC drives increases and decreases in cortical activity of a distant sensory region based solely on task goals.
To assess whether the absence of a right IFJ rTMS effect for the Remember Motion condition was due to bilateral IFJ control (as suggested by the fMRI connectivity data), participants were split into two groups based on the magnitude of left IFJ-V5 functional connectivity in the attend network. Measures of P1 modulation from the posterior left hemisphere during the first half of the Remember Motion condition were submitted to an ANOVA with subgroup (higher left IFJ-V5 connectivity, lower left IFJ-V5 connectivity) and rTMS (sham rTMS, actual rTMS) as factors. A significant subgroup X rTMS interaction was observed (F1,16 = 4.21, P = 0.05). Post-hoc t-tests revealed no significant P1 modulation difference between sham and actual rTMS for the higher left IFJ-V5 connectivity group (t8 = 0.61 P > 0.5), while the lower left IFJ-V5 connectivity group displayed reduced P1 modulation following actual rTMS (t8 = 3.89 P < 0.005). Direct comparisons between the higher and lower left IFJ-V5 connectivity groups indicate no difference following sham rTMS (t16 = 1.32, P > 0.2), but for actual rTMS, lower left IFJ-V5 functional connectivity was associated with diminished P1 modulation compared to higher left IFJ-V5 connectivity (t16 = 2.14, P < 0.05). This supports our hypothesis that bilateral IFJ control compensates for the disruptive effects of rTMS to the right IFJ for motion processing.
Previous research has also identified attentional modulation in posterior electrodes to stimulus features at later processing stages, reflected as the selection negativity (200–400 ms post-stimulus onset)25, 34. Mean amplitudes of the selection negativity were submitted to a repeated measures ANOVA with stimulus (color / motion), rTMS (sham / actual), and block half (1st / 2nd) as factors. Results indicated no main effects or interactions. Therefore, the right IFJ rTMS effects on activity modulation appear limited to early visual processing, highlighting how rapidly frontally-dependent biasing of posterior areas may occur19.
Correlations between EEG, fMRI, and working memory performance data
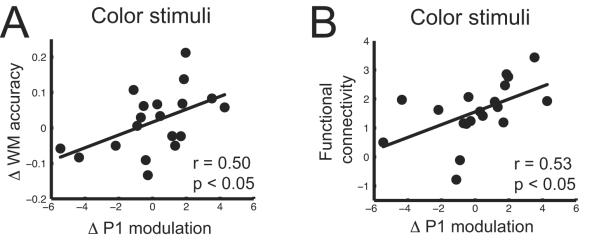
To further elucidate the relationship between IFJ control of top-down modulation and working memory performance, an across-participant regression analysis was conducted on indices of rTMS effects on P1 modulation and working memory accuracy for the Remember Color condition. P1 modulation was measured as the difference between attended and ignored stimuli. Indices of the rTMS-induced change in P1 modulation and working memory accuracy were calculated as the difference between sham and actual rTMS (sham – actual rTMS). The analysis for color stimuli showed that participants who experienced greater P1 modulation decreases following right IFJ rTMS (larger index values) exhibited greater working memory accuracy decline (larger index values) (r = 0.50, P < 0.05; Fig. 5a. The same analysis for motion stimuli displayed no such relationship (r = 0.12, P > 0.6). This is yet stronger evidence that disrupting neural networks subserving top-down modulation results in decreased working memory performance.
Figure 5.
Regression between the rTMS-related change in P1 modulation and, (a) working memory accuracy and (b) V4 / IFJ functional connectivity when attending to color. Δ = sham − actual rTMS.
The right IFJ was targeted for rTMS because the fMRI functional connectivity analysis identified it as being involved in top-down modulation during both color and motion processing. rTMS to the right IFJ induced a decline in P1 modulation in posterior brain regions for color stimuli during the first half of the experimental block, which in turn directly related to decrements in working memory performance. We therefore explored the relationship between rTMS-induced changes in P1 modulation for color stimuli and the magnitude of IFJ-V4 fMRI functional connectivity when attending to color stimuli. Results indicated that participants who displayed stronger measures of functional connectivity exhibited greater rTMS-induced declines in P1 modulation (r = 0.53, P < 0.05; Fig. 5b), suggesting that those who engaged the right IFJ more for top-down modulatory control of color processing were more affected by rTMS to that region.
Phase coherence subserving top-down modulation
Thus far, we have shown that the right IFJ is functionally connected to visual cortical regions during a selective working memory task and that perturbing right IFJ function with rTMS disrupts activity modulation in posterior cortical areas, as well as subsequent working memory performance. Although this data suggests that rTMS disrupts the neural network subserving top-down modulation, it does not provide a direct measure of the disruption of functional connectivity between frontal and posterior cortices. We have recently identified anticipatory signatures of neural modulation for color stimuli as alpha-band phase coherence between electrodes located around the right IFJ and posterior regions24. Therefore, anticipatory phase coherence (−200 to 0 ms post stimulus onset) in the alpha (7–14 Hz), beta (14–30 Hz) and gamma (30–50 Hz) bands were explored as markers of functional connectivity underlying top-down modulation.
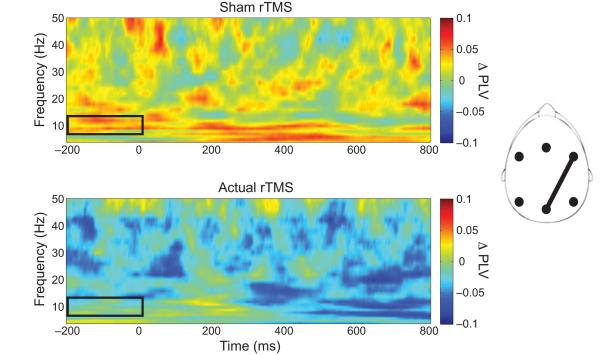
Alpha band phase coherence was assessed between right-frontal and central-posterior regions, whereas beta and gamma bands were assessed between right-frontal and right-posterior regions, as these regions exhibited the greatest attentional modulation when collapsed across conditions and rTMS treatment. The data was split in half by experimental block to evaluate the decay of rTMS effects, and the analysis focused on the color condition. The magnitudes of the phase locking value (PLV) modulation (attend – ignore) were submitted to repeated measures ANOVA with rTMS (sham / actual) and block half (1st / 2nd) as factors. In the alpha band, a main effect of rTMS (F1,18 = 4.57, P < 0.05) indicated that the magnitude of PLV modulation decreased following actual rTMS (Fig. 6 and Supplementary Fig. 3). No other main effect or interaction was observed in the alpha band. Additionally, no main effects or interactions were observed in the beta and gamma bands. Although an interaction between rTMS and block half was not observed, the alpha band results are similar to the P1 modulation findings and indicate that anticipatory alpha-band phase coherence may serve as a mechanism for top-down modulation to operate over long cortical distances.
Figure 6.
Alpha-band phase coherence between right-frontal and central-posterior regions immediately preceding the onset of color stimuli. Time-frequency maps depict the modulation (attend – ignore) of the phase locking values (PLVs) following sham (top row) and actual rTMS (bottom row). Black boxes highlight the time-frequency region of interest.
Discussion
In the current study, we disrupted function in a PFC control region, the right IFJ, with rTMS and assessed the consequences on top-down modulation in posterior cortical regions and working memory performance. Results indicated that top-down modulation during very early visual processing stages of the memoranda and subsequent working memory performance were causally related. There are four results supporting this conclusion. First, color processing showed an rTMS-related decline in both P1 modulation and working memory accuracy. Second, as the P1 modulation recovered with time (i.e., in the second half of the block), so did working memory performance. Third, on an individual participant basis, the rTMS-induced effect on the P1 modulation during color processing predicted changes in working memory accuracy. Finally, motion processing, which exhibited bilateral connectivity, did not show rTMS-related effects on P1 modulation or working memory accuracy. Thus, we conclude that early top-down activity modulation during stimulus processing driven by attentional demands is causally related to subsequent working memory performance (see Supplementary Discussion for further justification). Notably, the data revealed the IFJ to be a PFC control region that mediates this causal connection between top-down modulation in the service of attentional goals and subsequent working memory performance. Moreover, top-down modulation may be subserved by alpha phase coherence for long-distance communication.
Although functional connectivity analysis is informative in characterizing neural networks that may be involved in top-down modulation, it is a correlational measure and cannot be used to make strong statements of causality. In recent years, rTMS has been coupled with physiological recordings to provide causal evidence in humans that fronto-parietal regions are a true source of top-down modulation of activity in sensory cortical areas. However, only a few of the fronto-parietal regions proposed to be cognitive control regions underlying top-down modulation have been evaluated with this approach. TMS coupled with neuroimaging has been used to show that visual ERPs are under top-down influences from frontal eye fields (FEF)20, 35 and posterior parietal cortex (PPC)36. Top-down influences operate on areas V1 through V5 and have differential roles based on whether frontal (FEF) or parietal (interparietal sulcus) cortex is the source of modulation22. Research within the last two years has indicated that neural modulation occurs early during visual attention to features (~100 ms post stimulus onset)12, 26. Here, we contribute to this literature by showing that the right IFJ modulates early visual cortical processing. Specifically, both enhancement and suppression of the P1 amplitude for relevant and irrelevant color stimuli, respectively, were diminished post-rTMS. This suggests that suppression is not simply the lack of enhancement. Indeed, recent research has shown a selective decline in older adults' ability to suppress irrelevant information, suggesting that enhancement and suppression rely on distinct mechanisms37. An interesting question remains as to how one frontal region may be involved in both enhancing and suppressing posterior neural activity based solely on the goals of the task. One possibility is that functional segregation of the IFJ may exist at a scale smaller than the area impacted by rTMS, consistent with the notion of a topographical organization and functional specialization of this region38.
Despite the largely distinct fronto-parietal networks associated with attention to color and motion features, the right IFJ was common to both networks (albeit neighboring subregions within the IFJ). Recent research has shown the IFJ to be involved in many different tasks, including task switching, interference control and working memory39. As such, it has been hypothesized that the IFJ plays a role in updating relevant task representations40. The current data supports this hypothesis and extends it by suggesting that updating relevant task representations may occur via goal-directed biasing of neural activity in distal cortical regions. Furthermore, we show here that this neural biasing optimizes working memory performance. Interestingly, the IFJ and other frontal cortical regions display topographic organization and functional specialization across multiple spatial tasks, including memory-guided saccades, spatial working memory, and finger pointing38. Thus, spatial encoding cannot be discounted as an alternative hypothesis for the proposed role of the IFJ. However, given the many different tasks that have now been shown to utilize the IFJ that do not rely on spatial information39, 40, it seems more plausible that the IFJ has a generalized role in updating task representations. This is not to imply a lack of topographical organization within the IFJ. Color and motion processing engage ventral and dorsal visual processing streams, respectively, and this domain specificity is thought to be maintained in frontal regions41. The current data replicated our previous report that IFJ activity during color processing is localized ventrally within the IFJ relative to regions engaged for motion processing24, thereby supporting selective topographical organization and functional specialization of this region.
While long-distance cortical communication seems likely to occur via phase information42, evidence that top-down modulation utilizes such a mechanism has only recently been appreciated. Alpha band activity appears to be a prime candidate for such processes, as it is modulated by attention43 and linked to fronto-parietal networks subserving attention21 and working memory44. Here, we show pre-stimulus alpha-band phase coherence between frontal and posterior regions is modulated by attention and that perturbing function in the IFJ disrupts this modulation. These results support recent findings that top-down modulation mediated by PFC networks bias activity in sensory cortical regions prior to stimulus onset24, 45 and extends it to phase coherence being an operational mechanism. Given that all stimuli within each trial were presented randomly, anticipatory top-down modulation must have occurred as a cognitive set, as opposed to “switching” between enhancement and suppressive modes. A recent review has suggested alpha phase synchronization may be interpreted in terms of processes that coordinate top-down control and access to memory traces27. Here, we provide direct evidence to support this hypothesis by showing that alpha phase coherence is modulated by attention and that IFJ rTMS disrupts alpha phase coherence modulation.
Based on the current results and previous research, we hypothesize that when participants are instructed to remember color, the right IFJ biases visual cortical regions via alpha phase coherence prior to stimulus onset. Anticipatory biasing allows visual information (i.e. color features) to be enhanced or suppressed early in the visual processing stream, depending on their relationship to task goals. This contrast between enhanced and suppressed activity yields increased fidelity of the representation of the memoranda, which leads to improved working memory performance. The current study identified P1 modulation as a bridge between selective attention and working memory performance, and lends support to the supposition that selective attention and working memory encoding may not be dissociable at the neural level46. However, this may not be true in all cases. Attention operates during both perceptual and post-perceptual stages of stimulus processing, and its influence on working memory may depend on the timing of activity modulation, as well as the type of attention and working memory task47. Although the current results were identified during the encoding period, this does not preclude that top-down modulation during other stages of the task (e.g., the delay period) influence working memory performance. Furthermore, it should be noted that the IFJ is only one region identified in widespread fronto-parietal neural networks utilized by selective color and motion processing. Future research will assess the necessity of other putative control regions within the frontal and parietal cortex.
Methods
Participants
Twenty healthy individuals (mean age 24.25 years; range 18–31 years; 7 males) participated in the experiment. All participants gave informed consent to engage in the study according to procedures approved by the University of California. Each participant had normal or corrected to normal vision. One participant was excluded from all data analysis due to excessive artifacts.
Stimuli
The stimuli consisted of a circular aperture of 290 dots (0.08° × 0.08° each) that subtended 8° of visual angle centered at the fovea. Two types of dots were used during the experiment: 1) gray and moving coherently at 10° per second or 2) stationary and colored along the tritan axis. Stimuli were presented with a gray fixation cross in the center of the circular aperture on a black background. All colored and gray dots were equated for brightness by minimizing heterochromatic flicker in tests carried out prior to the experiment for each subject. After all stimuli were equated for brightness, participants engaged in two thresholding procedures (one for motion, one for color) in order to minimize perceptual discriminability differences between participants13, 25. A staircase procedure required participants to determine whether two stimuli (directions of motion or colors) were different from each other. The two stimuli were presented for 800 ms each and separated by 2000 ms. The procedure continued until a “just 100%” level of performance was reached, meaning if the stimuli were any more similar, performance would drop below 100%. Thresholding determined the twelve possible directions of motion (three per quadrant, cardinal axes excluded) and the six possible colors each participant receive during the experiment.
Several steps were taken to minimize the role of verbalization during feature encoding. Colors were selected from the tritan axis, which are not easily nameable, and the directions of motion never fell along a cardinal axis to prevent them being verbalized as `left', `right', `up' or `down'. Moreover, all stimuli were selected using a thresholding procedure based on the participant's ability to discriminate color and motion differences. Thus, participants who may have tried using verbalization as a strategy would find it difficult to attribute specific labels to subtle differences between the stimuli. Additionally, a post-experimental questionnaire specifically asked what type of strategy the participant employed during each task. Only a few participants reported attempting a verbal strategy, while the majority reported using a mental imagery technique. Lastly, analysis focused on early measures of perceptual processing (e.g. P1) which precedes typical neural signatures of semantic processing (e.g. N400).
Experimental procedure
The experimental paradigm is depicted in Figure 1, as previously reported12. Participants were required to either 1) remember the two directions of motion (ignore the two colors) or 2) remember the two colors (ignore motion). Each condition was presented in two blocks, and the four blocks were randomized across the experiment and participants. Prior to beginning each block, participants were given task instructions. Additionally, a brief (1 s) task reminder was provided at the start of each trial during the EEG session. Both conditions required viewing four sequentially presented images: two differently colored stimuli and two different directions of motion. Every image was presented for 800 ms with a 1200 ms inter-stimulus-interval (ISI; an 800 ms ISI was used in the EEG experiment). After the four images were presented, there was an eight second delay (4 s in EEG) followed by a probe stimulus (800 ms duration). Participants responded with a button press whether the probe matched any of the items held in memory. Participants responded by pressing one of two buttons. One half of the probe stimuli matched a previously attended object. Participants were instructed to respond as quickly as possible and yet retain accuracy during all conditions. Prior to beginning the experiment, participants were given 12 practice trials for each of the two conditions, split into two blocks (6 trials each). During the experiment, participants received 30 trials per condition (60 trials per EEG condition). The stimuli for each trial were randomly selected from pre-determined sets of stimuli, constraining the directions of motion to one quadrant.
MR imaging
All fMRI data was collected on a Siemens 3T MAGNETOM Trio with stimuli presented on an LCD monitor positioned behind the head of the participants and viewed using a mirror rigidly attached to a 12-channel head-coil. Echo planar imaging data was acquired (FA=90°, TE = 25 ms, TR = 2 sec) with 33 interleaved axial slices (0.5 mm gap) and a 1.8 × 1.8 × 3 mm voxel size (FOV = 23 cm; 128 × 128 matrix). All pre-preprocessing of the data was conducted in SPM5 (Wellcome Department of Imaging Neuroscience, London, England). Raw blood oxygen level dependent (BOLD) data was corrected offline for slice-timing acquisition and motion-artifacts. A 5 mm isotropic Gaussian smoothing kernel was applied prior to modeling the data. To aid in anatomical localizations of BOLD activity, high-resolution T1-MPRAGE images were acquired (1 × 1 × 1 mm voxel size; FOV = 160 × 240 × 256 mm, TR = 2300 ms, TE = 3 ms, FA = 9°).
fMRI region of interest localization
A functional localizer task was run prior to beginning the fMRI experiment in order to identify feature selective regions of interest (ROIs) in the extrastriate visual cortex (i.e. V4 for color, V5 for motion) that are known to be modulated by attention29, 48. Participants were instructed to perform a 1-back task where circular apertures of color and motion stimuli (as described above) were presented in separate blocks. Each stimulus type (color and motion) was presented in ten, 16 sec blocks interleaved with 16 sec of rest when participants passively viewed stationary gray dots. Within each block, stimuli were presented for 300 ms with a 500 ms ISI. Upon identifying a 1-back matched stimulus, participants were instructed to press the right-sided button. There were two random matches within each color and motion block. BOLD data from the color and motion localizers were analyzed using a general linear model (GLM) and contrasted against each other. ROIs were selected in native space as the most significant cluster of activation (p < 0.01) within the respective anatomical region of the right hemisphere, fusiform gyrus (specifically, V4) for color and middle temporal gyrus (specifically, V5/hMT+) for motion. For all GLM analyses, epochs spanning the duration of stimulus presentation were convolved with the SPM canonical hemodynamic response function.
fMRI functional connectivity analysis
Functional connectivity network maps were created for each subject as described previously using a beta series connectivity analysis approach30, 31. For each condition, the encoding, maintenance and retrieval stages from every trial were modeled with their own separate regressor within the GLM and a mean beta value was extracted for each ROI (per trial). Although three stages were modeled during each trial, only the encoding period was subject to analysis. Thus, the ROI beta values from the encoding period were correlated across trials with every voxel in the brain to find regions with covariant activity. This procedure produced a whole brain Pearson's r-value map for each participant and a Fisher's r-to-z transformation was applied. The z-values were subsequently normalized to the Montreal Neurological Institute (MNI; 2 × 2 × 2 mm voxel size) template and Gaussian smoothed (5 mm FWHM) for group level analysis.
Transcranial magnetic stimulation
A Magstim Standard Rapid TMS Unit (Jali Medical Inc) was used to generate pulses with a 70 mm figure-of-eight induction coil. The magnetic stimulus had a biphasic waveform with a pulse width of about 300 μs. The Brainsight frameless stereotaxic software (Rogue Research, Montreal, Canada) is used to co-register the subject's head, coil and high- resolution T1-weighted MRI images into a common digital workspace. The IFJ target for rTMS was identified by each individual participant's functional connectivity data (V5 seed and Remember Motion condition) that was subsequently overlaid onto their T1-weighted MRI image. The IFJ was targeted based on fMRI connectivity data from the Remember Motion condition since this condition served as a control in the rTMS experiment, and therefore, the control condition results would not be biased towards showing no effect. Repetitive 1 Hz TMS was applied to the right IFJ for 10 minutes while participants remain seated upright with the EEG cap on. Electrodes beneath the rTMS coil were removed prior to stimulation in order to minimize the distance between the coil and the head, which were replaced prior to EEG recording. The coil was held such that the handle protruded toward the back of the head and was approximately perpendicular to the precentral sulcus. During sham rTMS, the coil handle was held at a similar angle, but the coil face was angle 90 degrees away from the participant's head. rTMS pulse intensity was held at 65% maximum stimulator output for each participant. This intensity was chosen based on pilot data that found it to be on average 120% the active motor threshold. Following rTMS application (sham or actual), electrodes that were previously removed were replaced and participants began the task described above. Approximately one minute elapsed between the offset of rTMS and onset of the task with EEG data collection. All participants wore earplugs during both sessions of the experiment as protection from the fMRI noise (session 1) and rTMS clicking (session 2). The order of presentation for all conditions and application of sham / actual rTMS were counterbalanced across participants.
EEG recordings
Electrophysiological signals were recorded at 1,024 Hz through a 24-bit BioSemi ActiveTwo 64-channel Ag-AgCl active electrode EEG acquisition system (Cortech Solutions, LLC). Electrode offsets were maintained between +/− 20 mV. Raw EEG data were referenced to the average off-line. Eye artifacts were removed through an independent component analysis by excluding components consistent with the electrooculogram time-series and topographies for blinks and eye movements. One-second epochs were extracted from the data beginning 200 ms pre-stimulus onset and ending 800 ms post-stimulus onset. This preprocessing was conducted in Brain Vision Analyzer (Cortech Solutions, LLC) and exported to Matlab (The Mathworks, Inc.) for all subsequent analyses. Epochs that contained an eye-related artifact were discarded from subsequent analysis. Each trial contained four stimulus epochs, two attended and two ignored, resulting in 120 epochs per block per stimulus of interest (60 per half block). Trials where the participant responded incorrectly were discarded from analysis to ensure neural measures reflected task-related activity. Epochs were band-pass filtered from 1–30 Hz and those that exceeded a voltage threshold of +/− 50 μV were rejected. A 200 ms pre-stimulus baseline was subtracted from each epoch prior to calculating the ERP. Peak P1 values were chosen as the largest amplitude between 50–150 ms and mean ERP amplitudes (+/− 5 ms around peak) were used for subsequent analysis. An electrode of interest was selected13 from the posterior right hemisphere (P4, P6, P8, P10, PO4, PO8, O2) for each subject and stimulus type (color / motion) based on the largest magnitude of modulation (attend – ignore) after averaging over the rTMS conditions (sham / actual). A repeated measures ANOVA was implemented for ERP analysis with a Greenhouse-Geisser correction when appropriate. Post-hoc analysis consisted of paired t-tests. All regression analyses utilized Pearson's linear correlation coefficient.
EEG source localization
Sources of the P1 from the ERP were estimated using a distributed linear inverse solution. The inverse matrices were based on a local auto-regressive average (LAURA) model of the unknown current density in the brain49. A realistic head model was used with a solution space within the grey matter of the MNI template brain. P1 activity was averaged across subjects and over time (between 75–125 ms) and submitted to analysis through the CARTOOL software (http://brainmapping.unige.ch/Cartool.php). Current source density estimates were transformed to z-scores and retained if greater than 3.
EEG phase coherence analysis
Six regions of interest (ROIs) were identified (three frontal and three posterior). Each ROI was calculated as the average of 5 electrodes: F4, FC2, FC4, FC6, C4 front-right; above the right IFJ previously identified by source localization in24; F3, FC1, FC3, FC5, C3 (front-left); AFZ, FZ, F1, F2, FCZ (front-central); P4, P6, P8, PO4, PO8 (right-posterior); P3, P5, P7, PO3, PO7 (left-posterior); and POZ, OZ, O1, O2, IZ (central-posterior). Unfiltered raw epoched data from each ROI whose voltage exceeded a threshold of +/− 100 μV were rejected. Artifact free trials were then convolved with complex Morlet wavelets (family ratio: fo/σf = 7) to resolve frequencies from 4 to 70 Hz. Phase was computed from the wavelet coefficients for each ROI at every time-frequency point. Phase locking values (PLV) between two ROIs were computed by measuring the intertrial variability of the phase difference at each time-frequency point50. This procedure yields a PLV measure bound from 0 to 1 such that 0 represents random phase differences across trials while 1 indicates a consistent phase difference. PLV frequencies were averaged between 7–14 Hz (alpha band), 14–30 Hz (beta band), and 30–50 Hz (gamma band) and from −200 to 0 ms post stimulus onset in order to calculate pre-stimulus phase coherence. One ROI pair was selected for each frequency band for statistical analysis. Each ROI pair was selected based on the greatest attentional modulation when averaged across all conditions (alpha: front-right to central-posterior; beta: front-right to right-posterior; gamma: front-right to right-posterior).
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Health Grants 1F32AG030249-01A2 (TZ) and NIH Grant 5R01AG030395 (AG). We would like to thank Victor Barres, Celya Gruson-Daniel, and Chips McSteeley Jr. III for their assistance.
References
- 1.Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 2.Baddeley A. Working Memory. Oxford University Press; Oxford: 1986. [Google Scholar]
- 3.Duncan J, Humphreys GW. Visual search and stimulus similarity. Psychol. Rev. 1989;96:433–458. doi: 10.1037/0033-295x.96.3.433. [DOI] [PubMed] [Google Scholar]
- 4.Naatanen R. Processing negativity - An evoked potential reflection of selective attention. Psychol. Bull. 1982;92:605–640. doi: 10.1037/0033-2909.92.3.605. [DOI] [PubMed] [Google Scholar]
- 5.LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- 6.Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- 7.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 8.Gazzaley A, Cooney JW, McEvoy K, Knight RT, D'Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- 9.Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- 10.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 11.Fuster JM, Bauer RH, Jervey JP. Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res. 1985;330:299–307. doi: 10.1016/0006-8993(85)90689-4. [DOI] [PubMed] [Google Scholar]
- 12.Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. Journal of Neuroscience. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutman AM, Clapp WC, Chadick JZ, Gazzaley A. Early top-down control of visual processing predicts working memory performance. Journal of Cognitive Neuroscience. 2010;22:1224–1234. doi: 10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp Brain Res. 1989;76:473–484. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- 15.Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- 16.Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Exp. Brain Res. 2009;192:489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 18.Gazzaley A, et al. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb. Cortex. 2007;17:I125–I135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- 20.Taylor PCJ, Nobre AC, Rushworth MFS. FEF TMS affects visual cortical activity. Cereb. Cortex. 2007;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- 21.Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal Cortex Controls Spatial Attention through Modulation of Anticipatory Alpha Rhythms. Journal of Neuroscience. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruff CC, et al. Distinct causal influences of parietal versus frontal areas on human visual cortex: Evidence from concurrent TMS-fMRI. Cereb. Cortex. 2008;18:817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller BT, Vytlacil J, Fegen D, Pradhan S, D'Esposito M. The Prefrontal Cortex Modulates Category Selectivity in Human Extrastriate Cortex. Journal of Cognitive Neuroscience. 2011;23:1–10. doi: 10.1162/jocn.2010.21516. [DOI] [PubMed] [Google Scholar]
- 24.Zanto TP, Rubens MT, Bollinger J, Gazzaley A. Top-down modulation of visual feature processing: The role of the inferior frontal junction. Neuroimage. 2010;53:736–745. doi: 10.1016/j.neuroimage.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanto TP, Toy B, Gazzaley A. Delays in neural processing during working memory encoding in normal aging. Neuropsychologia. 2010;48:13–25. doi: 10.1016/j.neuropsychologia.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang WW, Luck SJ. Feature-based attention modulates feedforward visual processing. Nat. Neurosci. 2009;12:24–25. doi: 10.1038/nn.2223. [DOI] [PubMed] [Google Scholar]
- 27.Klimesch W, Freunberger R, Sauseng P. Oscillatory mechanisms of process binding in memory. Neurosci. Biobehav. Rev. 2010;34:1002–1014. doi: 10.1016/j.neubiorev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Zeki S, et al. A direct demonstration of functional specialization in human visual cortex. Journal of Neuroscience. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat. Neurosci. 1999;2:671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- 30.Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 31.Gazzaley A, Rissman J, Desposito M. Functional connectivity during working memory maintenance. Cognitive Affective and Behavioral Neuroscience. 2004;4:580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- 32.Pascual-Leone A, et al. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J. Clin. Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Thut G, Pascual-Leone A. A Review of Combined TMS-EEG Studies to Characterize Lasting Effects of Repetitive TMS and Assess Their Usefulness in Cognitive and Clinical Neuroscience. Brain Topography. 2010;22:219–232. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AnlloVento L, Hillyard SA. Selective attention to the color and direction of moving stimuli: Electrophysiological correlates of hierarchical feature selection. Percept. Psychophys. 1996;58:191–206. doi: 10.3758/bf03211875. [DOI] [PubMed] [Google Scholar]
- 35.Morishima Y, et al. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat. Neurosci. 2009;12:85–91. doi: 10.1038/nn.2237. [DOI] [PubMed] [Google Scholar]
- 36.Fuggetta G, Pavone EF, Walsh V, Kiss M, Eimer M. Cortico-cortical interactions in spatial attention: A combined ERP/TMS study. J. Neurophysiol. 2006;95:3277–3280. doi: 10.1152/jn.01273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gazzaley A, et al. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proceedings of the National Academy of Science USA. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn. Sci. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derrfuss J, Brass M, von Cramon DY. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. Neuroimage. 2004;23:604–612. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. Journal of Cognitive Neuroscience. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- 41.Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Natl Acad Sci U S A. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sauseng P, Klimesch W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev. 2008;32:1001–1013. doi: 10.1016/j.neubiorev.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Thut G, Nietzel A, Brandt SA, Pascual-Leone A. alpha-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. Journal of Neuroscience. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sauseng P, Klimesch W, Schabus M, Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. International Journal of Psychophysiology. 2005;57:97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Bollinger J, Rubens MT, Zanto TP, Gazzaley A. Expectation-driven changes in cortical functional connectivity influence working-memory and longterm memory performance. Journal of Neuroscience. 2010;30:14399–14410. doi: 10.1523/JNEUROSCI.1547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desimone R, Miller EK, Chelazzi L. Interaction of neural systems for attention and memory. In: Koch C, Davis J, editors. Large-scale theories of neuronal function. MIT Press; Cambridge, MA: 1994. pp. 75–91. [Google Scholar]
- 47.Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience. 2006;139:201–208. doi: 10.1016/j.neuroscience.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 48.Schoenfeld MA, et al. Spatio-temporal analysis of feature-based attention. Cereb. Cortex. 2007;17:2468–2477. doi: 10.1093/cercor/bhl154. [DOI] [PubMed] [Google Scholar]
- 49.Menendez RGD, Murray MM, Michel CM, Martuzzi R, Andino SLG. Electrical neuroimaging based on biophysical constraints. Neuroimage. 2004;21:527–539. doi: 10.1016/j.neuroimage.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 50.Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.