Abstract
Considerable variability across individuals has been reported in both the behavioral and fMRI blood oxygen level-dependent (BOLD) response to nicotine. We aimed to investigate (1) whether there is a heterogeneous effect of nicotine on behavioral and BOLD responses across participants and (2) if heterogeneous BOLD responses are associated with behavioral performance measures. In this double-blind, placebo-controlled, cross-over study, 41 healthy participants (19 smokers)—drawn from a larger population-based sample—performed a visual oddball task after acute challenge with 1 mg nasal nicotine. fMRI data and reaction time were recorded during performance of the task. Across the entire group of subjects, we found increased activation in the anterior cingulate cortex, middle frontal gyrus, superior temporal gyrus, post-central gyrus, planum temporal and frontal pole in the nicotine condition compared with the placebo condition. However, follow-up analyses of this difference in activation between the placebo and nicotine conditions revealed that some participants showed an increase in activation while others showed a decrease in BOLD activation from the placebo to the nicotine condition. A reduction of BOLD activation from placebo to nicotine was associated with a decrease in reaction time and reaction time variability and vice versa, suggesting that it is the direction of BOLD response to nicotine which is related to task performance. We conclude that the BOLD response to nicotine is heterogeneous and that the direction of response to nicotine should be taken into account in future pharmaco-fMRI research on the central action of nicotine.
Electronic supplementary material
The online version of this article (doi:10.1007/s00213-010-2145-8) contains supplementary material, which is available to authorized users.
Keywords: Nicotine, Functional magnetic resonance imaging (fMRI), Visual oddball, Reaction time
Introduction
Nicotine is known to enhance cognitive functions in animals and humans (Levin et al. 2006). Given that nicotinic agonists are being investigated as treatments for cognitive deficits associated with a range of neuropsychiatric conditions including Alzheimer dementia, schizophrenia, and attention deficit disorder (Newhouse et al. 2004; Taly et al. 2009), a thorough understanding of the neural mechanisms by which nicotine improves cognitive performance is necessary. Considerable variability across studies has been reported in both the behavioral and functional magnetic resonance imaging (fMRI) blood oxygen level-dependent (BOLD) response to nicotine. For example, while numerous animal and human experiments illustrate the cognitive enhancing properties of nicotine (Kumari et al. 2003; Lawrence et al. 2002; Levin et al. 2002; Levin and Chen 2004; Thiel et al. 2005), significant improvements in performance are not always observed on all behavioral measures (Ettinger et al. 2009; Giessing et al. 2006; Jacobsen et al. 2004). Equivocal findings regarding the effects of nicotine on task-related BOLD responses have also been reported (Ettinger et al. 2009). Some studies find a decrease in task-related BOLD activation in response to nicotine (Giessing et al. 2006; Thiel et al. 2005; Thiel and Fink 2008) others find an increased BOLD activation in response to nicotine (Jacobsen et al. 2004; Kumari et al. 2003; Lawrence et al. 2002) while some further studies report that nicotine enhances task-induced BOLD deactivations (Hahn et al. 2007, 2009). This heterogeneity in responses could be due to many factors, such as type of task/cognitive function under investigation, dose of nicotine, method of nicotine administration, and sample characteristics. However, it could also be related to individual responses to nicotine and the associated task performance.
At present, the precise relationship between the changes in BOLD activation in response to nicotine and performance measures has not been sufficiently clarified. Some studies suggest that a reduction in BOLD response under nicotine compared with placebo represents more “efficient” processing and is therefore representative of improved performance (Giessing et al. 2006; Thiel et al. 2005). Other studies, however, suggest that an increase in BOLD activation in response to nicotine compared with placebo is indicative of improved performance (Kumari et al. 2003; Lawrence et al. 2002). In addition, not all studies find a relationship between BOLD responses to nicotine and the effects of nicotine on behavioral measures. For example, in a study by Ettinger et al. (2009), both the behavioral and BOLD responses to nicotine were found to be heterogeneous, but the nicotine effects on behavioral measures and BOLD were found to be unrelated. Given the apparent inter-subject heterogeneity of both the behavioral and BOLD response to nicotine, investigation of the relationships between these response modalities could be key to understanding the effects of nicotine on cognition—and by extension the nicotinic system's properties in the brain.
The study presented here focuses on responses to a visual two-choice reaction time task with infrequent target stimuli similar to that of an oddball task. The oddball task is used in event-related potential (ERP) studies to elicit the P3 component of the ERP that represents target detection/event categorization (Halgren et al. 1998; Picton 1992) or more broadly, is considered to reflect selective attention and—to some extent—working memory processes (Gur et al. 2007; Javitt et al. 2008). The fMRI response to tasks that also evoke the ERP P3 in electrophysiological experiments involves a large distributed network including the supramarginal gyrus, frontal, insula, thalamus, cerebellum, occipital–temporal, superior temporal, and cingulate regions (Bledowski et al. 2004; Thiel et al. 2005; Gur et al. 2007; Musso et al. 2006; Winterer et al. 2007; Strobel et al. 2008). Given that a nicotine challenge has been shown to influence behavioral and electrophysiological responses to the oddball task (Froelinger et al. 2009; Polich and Criado 2006) and that the task elicits a robust BOLD response, it provides a suitable framework for investigating the effects of nicotine on behavioral and fMRI measures of attention and working memory. Thus, the aims of the present study were twofold: (1) To investigate whether there is a heterogeneous effect of nicotine on behavioral and BOLD responses to target stimuli across participants and (2) if heterogeneous BOLD responses are present to investigate whether an improvement in performance is related to the heterogeneous changes in the BOLD response.
Methods
Participants
Forty-five healthy smoking and non-smoking participants were recruited from a large population-based database in Germany (Mobascher et al. 2010) with no history of medical, neurological, or psychiatric illness (DSM-IV axis 1) or alcohol and drug abuse within past 6 months as assessed by a full medical interview and examination, routine laboratory tests, a drug screening test, an electrocardiogram, and a standardized psychiatric interview (First et al. 1995). Smokers were only included in the study if their Fagerström test for nicotine dependence (FTND) score was ≥4 (Heatherton et al. 1991). Non-smokers were included if they had smoked less than 20 cigarettes/lifetime. All subjects were right-handed, as assessed by Edinburgh Handedness Inventory (Oldfield 1971).
Data were discarded of two participants due to motion artifacts during imaging measurements, of one participant because of poor task performance (defined as fewer than 90% correct responses), one participant due to technical difficulties and two participants due to being left handed (handedness was not an exclusion criterion for the broader clinical trial from which this sample was drawn). This resulted in data for 39 healthy participants [18 smokers, six male; mean age 33.6 years (SD = 10.9), and 21 non-smokers, 11 male, mean age 32.6 years (SD = 10.5)] being included in the analysis (see Table 1).
Table 1.
Demographic and clinical information
| Variable | Smokers | Non-smokers |
|---|---|---|
| Subject number | 18 | 21 |
| Age, mean (SD),years | 33.6 (10.9) | 32.6 (10.5) |
| Male (N) | 6 | 11 |
| Female (N) | 13 | 10 |
| IQa | 101.8 (10.2) | 102.6 (9.8) |
| FTND score, mean (SD) | 5.1 (2.0) | – |
| CO, mean (SD), ppm | 17.3 (11.18) | – |
| QSU, mean (SD) | 104.47 (30.23) | – |
| Plasma cotinine, mean (SD) (ng/ml)b | 127.9 (116.2) | – |
FTND Fagerström test for nicotine dependence score was ≥ 4 (Heatherton et al. 1991)
QSU Questionnaire on Smoking Urges (Tiffany and Drobes 1991) to assess craving
CO levels of carbon monoxide in expired air
IQ Wechsler Memory Scale (Wechsler, 1987).
aSmokers N = 18, non-smokers, N = 18, data unavailable for three subjects
bSmokers N = 16, plasma cotinine data unavailable for two subjects
Study procedure
The study (ClinicalTrial.gov Identifier: NCT00618280) employed a double-blind, placebo-controlled, within-subject, randomized, cross-over (counterbalanced) design and was conducted in compliance with the declaration of Helsinki in its latest version and according to ICH-GCP (good clinical practice) guidelines following a strict standard operating procedure with regular external monitoring. Written informed consent was obtained from all participants. The study was approved by the ethics committee of the Heinrich-Heine University, Düsseldorf and the federal drug agency in Germany, i.e., the Bundesinstitut für Arzneimittel and Medizinprodukte (BfArM).
Participants (current smokers and non-smokers) were investigated in the context of a multi-session pharmacological fMRI study before and after overnight nicotine withdrawal. The interim analyses presented here focus on the experimental sessions from the first day, i.e., before overnight nicotine withdrawal. Participants were admitted to the clinical research unit of the Research Center Jülich for the entire duration of the study. Before admission, smokers were asked to smoke ad libitum with most participants taking the possibility to have their last cigarette right before admission. After admission, smokers remained abstinent throughout the course of the study. Within 1 h after arrival at the research center, participants completed the Questionnaire on Smoking Urges (QSU; Tiffany and Drobes 1991) which is a state-sensitive measure to assess nicotine craving, levels of exhaled carbon monoxide (CO) were measured using a Micro 4 Smokerlyzer® (Bedfont Scientific Ltd.) and plasma was collected for cotinine immunoassay measurements (DRI® Cotinine Assay, Microgenics, Passau, Germany). Participants also completed the Wechsler IQ Scale (Wechsler 1987) as a measure of intelligence.
All participants completed two 1-h experimental sessions in the MRI scanner 4 h apart with the first session being approximately 2 h after admission. The experimental sessions were conducted after acute challenge with 1 mg nasal nicotine spray® (0.5 mg each nostril) or placebo (pepper) spray. One milligram nicotine delivered by nasal spray is largely bioequivalent with nicotine consumption by smoking one cigarette (Benowitz and Jacob 1984). A between-session interval of 4 h was chosen for the following reasons. Half-life time of nicotine is 2 h (Benowitz et al. 1982), i.e., 75% of nicotine (administered by nasal spray) is metabolized after 4 h (including the duration of the first MR scan of 1 h). At the same time, this relatively short time interval is considered a reasonable compromise to avoid that smokers develop serious withdrawal symptoms which may themselves have an effect on brain function (Polich and Ochoa 2004). In order to account for the unavoidable remaining effects of lingering nicotine and beginning withdrawal, the order of the placebo and nicotine challenge was randomized and counterbalanced across participants.
Behavioral task
Participants performed a visual choice reaction oddball task consisting of 64 infrequent “target” stimuli and 256 frequent stimuli (one additional cognitive task was also performed but is not reported here). Stimuli were black and white checkerboards, the infrequent target stimuli were identified as a reversal of the pattern of the frequent stimuli. A black screen was presented between stimuli. Stimuli were presented using Presentation version 11.3 (Neurobehavioural systems®, Albany, CA, USA) via a screen situated behind the scanner. Participants were able to view the screen via a mirror mounted on the head coil. Participants' responses were recorded using Lumitouch key pads® (Photon Control Inc, Burnaby, BC, Canada). For infrequent stimuli, participants responded with their right index finger, and for frequent stimuli, they responded with their left index finger; they were asked to respond quickly and accurately to each stimulus and reaction time was recorded for each response. Stimuli were presented with duration of 1,000 ms and a pseudorandomized interstimulus interval (ISI) of 4,000 (±500) ms. Only responses to target stimuli were of interest (see fMRI analysis section below); the target-to-target ISI was 22 s (SD 23 s).
fMRI data acquisition
Functional MR-images were acquired using a 3T scanner (TIM-Trio®, Siemens, Erlangen, Germany). Using echo planar imaging (EPI), 630 volumes were obtained applying the following EPI parameters of 33 slices; slice thickness, 3 mm; interslice gap, 0.3 mm; field of view (FOV), 200 × 200 mm; 64 × 64 matrix; repetition time, 2,000 ms; echo time, 30 ms; and flip angle, 90°. To facilitate localization and co-registration of functional data, structural scans were acquired using T1-weighted MRI sequences [magnetization prepared rapid gradient echo: TR/TE = 2250/3.03 ms, flip angle = 9°, 176 sagittal slices, FOV 200 × 200mm, 64 × 64 matrix, voxel size 1 × 1 × 1 mm.
Simultaneous EEG recordings were also conducted during fMRI scanning. These data will not be reported in the current study.
fMRI analysis
Single-subject imaging analyses were conducted blind for drug condition (placebo vs. nicotine). fMRI analysis was performed with FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl); employing different modules of the FSL-software package, motion correction was performed using MCFLIRT (FMRIBs Linear Registration Tool, Jenkinson et al. 2002), non-brain removal using BET (Smith 2002), spatial smoothing using a Gaussian kernel of FWHM = 8 mm, mean based intensity normalization of all volumes by the same factor, and high-pass temporal filtering (sigma = 125 seconds). General linear model (GLM) time-series statistical analysis of individual data sets was carried out using FILM (FMRIB's Improved Linear Model) with local autocorrelation correction (Woolrich et al. 2001). Registration of functional images to high-resolution structural images was done with FLIRT (FMRIB's Linear Image Registration Tool, Forman et al. 1995; Jenkinson et al. 2002). Responses to target stimuli were modeled with an explanatory variable constructed using onset times of target stimuli only, convolved with a gamma hemodynamic response function. An explanatory variable containing the onsets of the frequent (non-target) stimuli was also included as a variable of no interest. The resulting activation maps represent BOLD responses to target stimuli compared with baseline (target stimuli > baseline). Group-level mixed-effect analyses were conducted using FLAME (FMRIB's Local Analysis of Mixed Effects; Behrens et al. 2003) with spatial normalization to MNI (Montreal Neurological Institute) space and applying a cluster significance threshold of Z > 2.3 (Forman et al. 1995; Friston et al. 1994; Worsley et al. 1992). The following group-level analyses were conducted: Group means were created for the placebo and nicotine sessions separately to determine the overall activation pattern. Differences between groups (smokers and never-smokers) were investigated using an independent sample t test; differences between the placebo and nicotine sessions were investigated with a paired sample t test. To investigate the relationship between the nicotine effect on BOLD response and the nicotine effect on reaction time, additional analyses were conducted with change in reaction time and change in reaction time standard deviation included as covariates. A second-level fixed-effects analysis (placebo vs nicotine) was performed for each subject to give a statistic representing the difference between the placebo and nicotine conditions. These data were then taken through to group-level mixed-effects analyses where the reaction difference values were included as covariates. Functional data were imported to MRIcron (Rorden et al. 2007) for visual display purposes.
Region-of-interest analysis
The nicotine>placebo group-level contrast (for target stimuli > baseline) revealed a pattern of increased activation in the nicotine condition compared with placebo (see Results section). To investigate whether all participants showed an increase in activation from placebo to nicotine a region-of-interest (ROI) mask was created based on overall activation in this contrast. This mask was 3,406 voxels in size and encompassed clusters in the following regions: anterior cingulate cortex (ACC), middle frontal gyrus, frontal orbital cortex, superior frontal gyrus, and frontal pole. (see Results section for details). Mean percent signal change (parameter estimate) in the region-of-interest was exported for each participant for each session. A difference value for nicotine-placebo was then calculated to assess whether each participant showed a decrease or an increase in BOLD activation from placebo to nicotine. This difference in activation between the placebo and nicotine conditions is not to be confused with deactivation which is considered to be a reduction in BOLD signal compared with baseline in response to a task and has been associated with the nicotine response (Hahn et al. 2007, 2009). What we are looking at here is the difference in the BOLD response between the placebo and nicotine condition, whether a particular subject has more or less activation (target>baseline) in the nicotine condition compared with the placebo condition.
Statistical analysis
A 2 × 2 (drug × smoking status) analysis of variance (ANOVA) was conducted to test for nicotine and smoking status effects on the following dependent variables: mean BOLD percent signal change, mean reaction time, and reaction time standard deviation.
Relationships between the following variables were tested with Pearson correlation coefficient r: difference in mean percent signal change between the placebo and nicotine conditions and the difference in reaction time (RT) measures between placebo and nicotine conditions; and between smoking-related variables (QSU, FTND, CO, cotinine) and mean percent signal change in the ROI and RT variables.
Results
Behavioral data
All participants performed the task with an average of 98.9% (SD 2.43%) and 99.2% (SD 2.2%) correct responses to target stimuli for the placebo and nicotine session, respectively. No false responses were recorded, but an average of 1.0% (SD 2.4 %) and 0.8% (SD 2.2%) target stimuli were missed for the placebo and nicotine sessions, respectively. Mean RT to target stimuli for the placebo session was 630.3 ms (SD = 75.5) and for the nicotine session was 626.3 ms (SD 79.9). A 2 × 2 (drug × smoking status) ANOVA revealed no differences in mean reaction time or reaction time standard deviation between the placebo and nicotine conditions (F(1,37) = 0.22, P = 0.64, F(1,37) = 0.38, P = 0.54, respectively) or between smokers and non-smokers [F(1,37) = 0.93, P = 0.34, F(1,37) = 0.1.21, P = 0.28, respectively). Furthermore, the drug × smoking status interactions failed to reach significance [F(1,37) = 0.001, P = 0.98, F(1,37) = 0.18, P = 0.67, respectively).
fMRI data—overall nicotine effects
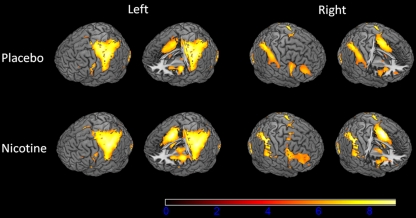
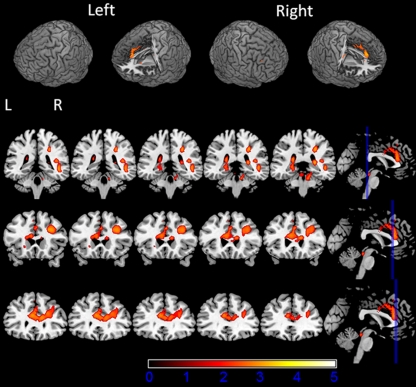
The BOLD analysis (N = 39) revealed activation in response to infrequent target stimuli in the post-central gyrus, pre-central gyrus, cerebellum, supramarginal gyrus, insula, frontal operculum, inferior frontal gyrus, middle frontal gyrus, anterior cingulate cortex, and lateral occipital cortex (Fig. 1.; see Table 2 for MNI coordinates and Z values). Group-level analyses revealed no significant differences in whole-brain voxelwise BOLD activation between smokers and non-smokers for both the placebo and nicotine conditions. Within the group of smokers, smoking behavior-related variables, FTND, QSU, expired CO, and plasma cotinine, were not related to any of the behavioral or fMRI measures (Supplemental Table 1). Since no differences were found between the smokers and non-smokers on any measure and no relationships were found between the smoking-related variables and BOLD or reaction time measures, the smokers and non-smokers were considered as one group in all further analyses. Across all participants, there was a significant difference in BOLD activation between the placebo and nicotine condition in the anterior cingulate cortex, middle frontal gyrus, superior frontal gyrus, pre-central gyrus, planum temporal, lateral occipital cortex, supramarginal gyrus, and frontal pole (see Fig. 2; Table 3) with there being more activation in the nicotine condition than the placebo condition (nicotine > placebo contrast).
Fig. 1.
BOLD activation for the group-level analysis (second-level mixed-effects FLAME; N = 39, cluster-corrected threshold Z = 2.3, p = 0.05)
Table 2.
Brain regions and local maxima of responses to target stimuli
| Region (Harvard-Oxford, maximum probability) | MNI coordinates of local maxima (X, Y, Z)a | Max. Z score | Region (Harvard-Oxford, maximum probability) | MNI coordinates of local maxima (X, Y, Z) | Max. Z score |
|---|---|---|---|---|---|
| Left hemisphere | Right hemisphere | ||||
| Post-central gyrus | −44, −24, 50 −56, −26, 48 | 6.84 6.43 | |||
| Pre-central gyrus | −40, −16, 56 −36, −18, 56 | 6.44 6.38 | Pre-central gyrus | 46, 8, 26 | 5.16 |
| Parietal opercular cortex | −50, −26, 20 | 6.65 | |||
| Supramarginal gyrus | −54, −28, 42 | 6.35 | Supramarginal gyrus | 44, −36, 42 | 4.72 |
| Cerebelluma (left) | −32, −54, −26 | 3.78 | Cerebelluma (right) | 16, −52, −26 | 5.87 |
| Inferior lat occ cortex, superior lat occ cortex | −44, −78, −12 | 4.86 | Inferior lat occ cortex, superior lat occ cortex | 34, −90, −4 | 5.82 |
| −32, −88, 14 | 3.96 | 20, −66, 50 | 4.88 | ||
| Superior parietal lobule | 28, −54, 52 | 5.05 | |||
| Insular cortex | −44, 2, −2 | 5.52 | Insular cortex | 34, 24, −2 | 5.25 |
| −40, 6, −2 | 5.07 | ||||
| Supplementary motor area | −6, 2, 46 | 5.41 | Supplementary motor area | 8, 6, 50 | 3.63 |
| Cingulate gyrus (anterior) | −6, 2, 44 | 5.26 | Cingulate gyrus (anterior) | 6, 20, 32 | 4.81 |
| Cingulate gyrus (posterior) | −2, −30, 26 | 3.72 | |||
| Occipital pole | −30, −98, 0 | 5.22 | Occipital pole | 34, −90, 2 | 5.57 |
| −34, −92, −6 | 5.02 | 26, −100, 4 | 5.36 | ||
| Temporal occipital fusiform cortex | 30, −52, −20 | 5.37 | |||
| Frontal orbital cortex | −30, 26, −2 | 3.61 | Frontal orbital cortex | 34, 26, −2 | 5.2 |
| Inferior frontal gyrus | 48, 10, 28 | 5.12 | |||
| Thalamus | −12, −20, 4 | 5.09 | |||
| Brain-stem | −4, −28, −12 | 4.89 | |||
| Occipital fusiform gyrus | −40, −66, −12 | 4.89 | Occipital fusiform gyrus | 42, −64, −14 | 3.75 |
| Superior frontal gyrus | −22, −8, s72 | 4.86 | Superior frontal gyrus | 12, 2, 72 | 3.55 |
| Frontal pole | 44, 44, 18 | 3.87 |
Whole-brain voxelwise analysis (N = 39, smokers and non-smokers, cluster-corrected at Z = 2.3, p = 0.05)
aMontreal Neurological Institute (MNI) label
lat occ lateral occipital
Fig. 2.
BOLD activation for the placebo versus nicotine contrast (paired t test) (second-level mixed-effects FLAME. N = 39, cluster-corrected threshold Z = 2.3, p = 0.05)
Table 3.
Regions activated in the nicotine vs placebo contrast
| Region (Harvard-Oxford, maximum probability) | MNI coordinates of local maxima (X, Y, Z) | Maximum Z value | ||
|---|---|---|---|---|
| Middle frontal gyrus (R) | 30 | 16 | 32 | 3.83 |
| Middle frontal gyrus (L) | −54 | 16 | 38 | 2.94 |
| ACC (R) | 2 | 24 | 34 | 3.09 |
| Frontal orbital cortex (R) | 32 | 20 | −24 | 3.86 |
| Frontal orbital cortex (L) | −42 | 30 | −4 | 2.34 |
| Pre-central gyrus (R) | 50 | −6 | 34 | 2.78 |
| Pre-central gyrus (L) | −60 | 8 | 34 | 3.8 |
| Lateral occipital cortex (L) | −24 | −64 | 50 | 3.64 |
| Frontal pole (R) | 30 | 50 | −16 | 2.82 |
Whole-brain voxelwise analysis (N = 39, smokers and non-smokers, cluster-corrected at Z = 2.3, p = 0.05)
ROI analyses
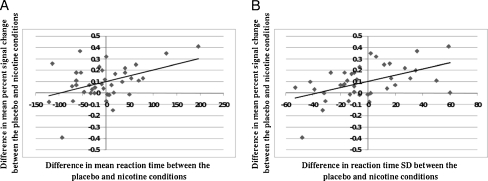
A ROI mask was created from the overall (nicotine > placebo) activation map. The mean percent signal change in this ROI was then extracted for each participant for each session. The difference in mean percent signal changes between the placebo and nicotine sessions were calculated for each participant. Some participants showed an increase in activation from placebo to nicotine, and others showed a decrease. Again, this difference in activation between the placebo and nicotine conditions is not to be confused with deactivation; we are looking at whether a subject shows more or less activation in the nicotine condition compared with the placebo condition. In addition to individual subjects showing differential BOLD responses to nicotine (i.e., some show an increase while others show a decrease), significant relationships between this difference value and performance measures were observed. As depicted in Fig. 3, shortening of reaction time from placebo to nicotine condition was accompanied by a decrease in BOLD activation [r(37) = 0.43, P = 0.007]. Likewise, a decrease in reaction time variability from placebo to nicotine condition was related to a reduction of BOLD activation [r(37) = 0.51, P = 0.001]. For clarification of the absence of differences between smokers and non-smokers, a 2 × 2 (drug × smoking status) ANOVA was performed on mean percent signal change in the ROI. There was a significant main effects for drug [F(1,37) = 14.69, P < 0.001], which was to be expected due to the ROI being based on the nicotine > placebo contrast. However, no difference was found between smokers and non-smokers [F(1,37) = 1.09, P = 30], the drug by smoking status also failed to reach significance [F(1,37) = 0.11, P = 0.74).
Fig. 3.
Scatter plots showing the relationship between fMRI BOLD and behavioral responses to nicotine. a The difference in mean reaction time (RT) and the difference in mean percent signal (BOLD in the placebo vs nicotine ROI between the placebo and nicotine conditions. Difference values were calculated by subtracting the value for the placebo condition from the value for the nicotine condition. For mean percent signal change, a negative difference value represents a decrease in activation from placebo to nicotine. A positive value represents an increase from placebo to nicotine. For mean RT, a negative value indicates a reduction in reaction time from placebo to nicotine and a positive value represents an increase in reaction time from placebo to nicotine. A decrease in reaction time from placebo to nicotine is related to a decrease in BOLD activation from placebo to nicotine (b). The difference in reaction time standard deviation (RT_SD) and the difference in mean percent signal change in the placebo vs nicotine ROI between the placebo and nicotine conditions. For RT_SD, a negative value indicates a reduction in reaction time variability from placebo to nicotine and a positive value represents an increase in reaction time variability from placebo to nicotine. A decrease in reaction time variability from placebo to nicotine is related to a decrease in BOLD activation from placebo to nicotine
fMRI data—relationship to behavioral response
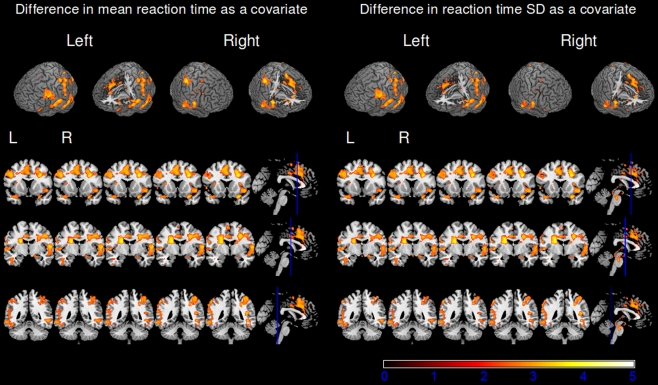
To further investigate the relationship between nicotine effects on the BOLD response and nicotine effects on behavioral measures, the difference in reaction time and reaction time standard deviation were included as covariates in the GLM (see Methods for details). The change in mean reaction time was positively related to the activation in the nicotine > placebo contrast (Fig. 4). In other words, an increase in BOLD activation from placebo to nicotine was related to an increase in reaction time from placebo to nicotine and vice versa. The regions in which BOLD activation correlated with mean reaction time were as follows: middle frontal gyrus, planum temporale, frontal orbital cortex, superior parietal lobule, lateral occipital cortex, post-central gyrus, pre-central gyrus, and anterior cingulate cortex. A positive relationship to BOLD activation was also found for reaction time standard deviation in the following regions: middle frontal gyrus, frontal orbital cortex, inferior temporal gyrus, lateral occipital cortex, planum temporale, pre-central gyrus, and post-central gyrus. Both mean reaction time and reaction time standard deviation were related to the nicotine effect on the BOLD response in similar regions, and these regions largely overlap with the overall nicotine effect on BOLD response, suggesting that the behavioral response and BOLD response are indeed closely linked (Table 4).
Fig. 4.
BOLD activation for the placebo versus nicotine contrast with reaction time data included as a covariate (second-level mixed-effects FLAME, N = 39, cluster-corrected threshold Z = 2.3, p = 0.05). The left panel shows activation associated with the nicotine effects on mean reaction time. The right panel shows activation associated with the nicotine effects on reaction time standard deviation
Table 4.
Brain regions and local maxima where BOLD activation is related to behavioral performance
| Region (Harvard-Oxford, maximum probability) | MNI *coordinates of local maxima (X, Y, Z) | Max. Z score | Region (Harvard-Oxford, maximum probability) | MNI coordinates of local maxima (X, Y, Z) | Max. Z value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean reaction time | Reaction Time SD | ||||||||
| Middle frontal gyrus (R) | 30 | 16 | 32 | 3.92 | Middle frontal gyrus (R) | 30 | 16 | 32 | 3.86 |
| Planum temporale (L) | 40 | −38 | 14 | 3.90 | Frontal orbital cortex (L) | 32 | 20 | −24 | 3.87 |
| Frontal orbital cortex (R) | 32 | 20 | −24 | 3.85 | Inferior temporal gyrus | −44 | −52 | −16 | 3.83 |
| Superior parietal lobule (R) | 44 | −38 | 58 | 3.80 | Lateral occipital cortex (L) | −24 | −62 | 64 | 3.83 |
| Lateral occipital cortex (L) | −24 | −62 | 50 | 3.76 | Planum temporale (R) | 40 | −38 | 14 | 3.79 |
| Post-central gyrus (R) | −50 | −36 | 60 | 3.71 | Pre-central gyrus (L) | −62 | 10 | 32 | 3.73 |
| Pre-central gyrus (R) | −60 | 10 | 32 | 3.70 | Post-central gyrus (R) | −48 | −40 | 58 | 3.68 |
| Anterior cingulate cortex (L) | −6 | −6 | 38 | 3.10 | Anterior cingulate cortex (L) | −6 | −6 | 40 | 2.91 |
Whole-brain voxelwise analysis (N = 39, smokers and non-smokers, cluster-corrected at Z = 2.3, p = 0.05)
Discussion
We investigated the effects of acute nasal spray nicotine challenge on BOLD fMRI and behavioral responses to a visual oddball task. Group-level analysis revealed BOLD activation in response to infrequent target stimuli in brain regions consistent with previous research (Halgren et al. 1995a, b; Gur et al. 2007; Strobel et al. 2008; Winterer et al. 2007). Smokers and non-smokers did not differ on any behavioral or BOLD measures. Applying standard group contrast analyses between the nicotine and placebo conditions, increased activation was found in the post-central gyrus, pre-central gyrus, cerebellum, supramarginal gyrus, insula, frontal operculum, inferior frontal gyrus, middle frontal gyrus, anterior cingulate cortex, and lateral occipital cortex while no significant behavioral group differences (mean reaction time and reaction time standard deviation) were found. However, when individual response patterns were examined more closely, it was found that some subjects showed an increase in activation in the nicotine condition compared with the placebo condition while others showed a decrease. This difference in BOLD activation between the two conditions was also related to nicotine effects on behavioral performance.
Contrasting the nicotine with the placebo condition on the group level, we found increased activation in the post-central gyrus, pre-central gyrus, cerebellum, supramarginal gyrus, insula, frontal operculum, inferior frontal gyrus, middle frontal gyrus, anterior cingulate cortex, and lateral occipital cortex in the nicotine condition compared with the placebo condition (nicotine > placebo). By and large, this result is consistent with several earlier reports of an increased BOLD response for varying task conditions (Jacobsen et al. 2004; Kumari et al. 2003; Lawrence et al. 2002). Furthermore, the direction and magnitude of the nicotine effects on the BOLD response in these regions was related to the nicotine effects on mean reaction time and reaction time standard deviation. Specifically, faster reaction times and reduced reaction time variability were associated with reduced BOLD activation under nicotine compared with placebo.
The diversity of regions that are apparently influenced by nicotine across studies and paradigms suggests that nicotine acts on several sub-processes of attention and working memory whereby different task conditions emphasize different cortical regions. The rather uniform nicotine effect across regions is in accordance with neuroimaging and neuroanatomical studies showing a rather homogeneous density of nicotinic receptors throughout human cortex (Hellström-Lindahl et al. 1999; Gallezot et al. 2005) although one post-mortem study reported a relative increase of nicotinic binding sites in parietal cortex (Scheperjans et al. 2005). The finding from our study also fits with what is known about nicotinic/cholinergic neurotransmission and cognition. For instance, binding of nicotine to nicotinic receptors enhances visuospatial and sustained attention (Hahn et al. 2009; Newhouse et al. 2004). Furthermore, dopaminergic neurotransmission plays a central role in working memory, and nicotine-stimulated dopamine release has been shown to impact on working memory task performance (Jacobsen et al. 2006).
While our overall group-level response to nicotine is consistent with previous research, the degree and direction of the difference in BOLD activation between the placebo and nicotine condition showed substantial inter-individual variation. This variation was also related to reaction time performance. In response to nicotine compared with placebo, some participants showed faster reaction times together with reduced reaction time variability, and this was associated with reduced BOLD activation. The opposite observation was made for those participants with a nicotine response-related slow-down of reaction time and increase of reaction time variability. Thus, despite an overall increase of BOLD activation in response to nicotine, inter-individual variability in responses was observed. Overall, the observation of heterogeneous nicotine effects on BOLD response with regard to the direction of change is in line with other recent studies (Ettinger et al. 2009; Giessing et al. 2007). However, we show here for the first time that this heterogeneous nicotine response is functionally significant as reflected by its relationship to performance.
The reaction time-related nicotine effect in the ACC, superior parietal, inferior temporal, and lateral occipital regions is particularly interesting, since activation in these regions is consistent with previous research and expected modulation in relation to our task. The ACC is associated with executive and evaluative functions, and its involvement in our task is consistent with other fMRI studies of target detection and decision making (Gur et al. 2007; Philiastides and Sajda 2007; Sridharan et al. 2008). Specifically, we found activation in the left and right dorsal ACC. The dorsal ACC is connected to the prefrontal cortex and parietal cortex as well as the motor system and the frontal eye fields, i.e., it plays an important role in processing top-down and bottom-up stimuli and assigning appropriate control to other areas in the brain (Paus 2001). The ACC is also thought to be part of the so-called Salience Network (Sridharan et al. 2008) that is involved in processing the degree of subjective salience and typically shows an increase in activation in response to a cognitive task. Since ACC activation also depends on cholinergic neurotransmission (Sarter et al. 2006), it is therefore not unexpected that the ACC is particularly strongly engaged in the target detection task used in the present nicotine challenge study. If an increase in ACC activation represents increased processing or effort, then a reduction in task-related ACC activation in response to nicotine compared with placebo could be interpreted as more “efficient” processing under nicotine. This might provide a phenomenology-based explanation of the relationship found between reduced ACC activation and improved reaction time performance between the placebo and nicotine conditions.
We also found that activation in the parietal cortex (specifically, the superior parietal lobule and post-central gyrus) differed between the placebo and nicotine conditions, consistent with other studies (Thiel et al. 2005; Thiel and Fink. 2008; Giessing et al. 2007; Vossel et al. 2008). The studies by Thiel and colleagues showed a decrease in parietal BOLD activation under nicotine compared with placebo, and this was accompanied by a tendency for a reduction in reaction time under nicotine compared with placebo. These findings thus support the idea that a reduction in BOLD activation in response to nicotine compared with placebo is related to improvements in performance. Similar performance efficiency-related reductions in BOLD signal have also been observed for other compounds. For example, Dodds et al. (2008) observed that methylphenidate reduced BOLD signal in the ventral putamen during a switching task after negative feedback and speculated that, in absence of any behavioral effect of the drug, the reduction in BOLD signal may reflect an increase in the efficiency of executive control (Dodds et al. 2008). In addition, the COMT (catecholamine-O-methyltransferase) inhibitor tolcopone was shown to reduce BOLD activation in the dorsolateral prefrontal cortex in tasks involving memory and executive function; this was associated with an improvement in performance (Apud et al. 2007). This matches our own findings in that a reduction in BOLD activation from placebo to nicotine was related to a reduction in reaction time variability from placebo to nicotine. Given that, for some participants activation decreased from placebo to nicotine and for others activation increased from placebo to nicotine, this activation in the parietal cortex could reflect a more efficient processing in some participants. So, those that improve their performance also show reduced parietal activation under nicotine. The same principle can be applied to the activation observed in the inferior temporal gyrus and lateral occipital cortex. The inferior temporal gyrus is part of the higher level of the ventral stream of visual processing and is involved in object recognition. The lateral occipital cortex is not only indicative of processing visual stimuli but has also been implicated in attention allocation (Weissman et al. 2006; Goldman et al. 2009). These regions also showed a reaction time-related decrease in activation in response to nicotine, suggesting that they are also involved in this improved efficiency due to enhanced cholinergic signaling under nicotine. It is interesting to note that this more efficient processing is also reflected in less variable performance as well as an improvement in mean reaction time. Vossel et al. (2008) also found that nicotine reduced the variability of reaction times in a cue validity task, but only when validity was high. The reduction in activation in the parietal regions could therefore be the neural mechanism underlying the reduction in response variability observed in our data.
Finally, the question arises whether the group-level difference in response to nicotine between our investigation and, for instance, the study of Thiel et al. (2005) or Vossel et al. (2008) is simply dependent on different task conditions. We found an overall, group-level increase in BOLD activation in response to nicotine, which, given the relationship between BOLD and reaction time revealed in our data, suggests that our group performed less well under nicotine. However, our task was a simple target detection task, and the tasks employed in other studies have different cognitive demands. An alternative explanation, also to be taken into account, is that the heterogeneous nicotine response reflects—at least, in part—sample heterogeneity. Thus, as opposed to previous imaging studies on nicotine effects, our study was population-based rather than recruiting, for instance, mainly students for the experiment. In future investigations of nicotine effects, these questions should be addressed more systematically.
In summary, we confirm the observation of considerable variability in the response direction of BOLD signaling to nicotine across participants. For the first time, it is shown that this response direction is behaviorally meaningful. We suggest that this is likely to contribute to the equivocal findings observed across studies reported in literature. There is a wealth of research suggesting that nicotine does have an effect on cognition, in terms of performance on attention and working memory tasks, but many factors may influence whether nicotine has a beneficial, detrimental, or no effect on the individual. For example, factors to investigate in the future include genetic factors, receptor availability, and withdrawal effects. Furthermore, aspects of experimental design, such as task, mode of nicotine administration, and sample characteristics can also influence the measured responses to nicotine. Consideration of all factors is beyond the scope of this paper; however, it would be interesting to find out whether the response direction to nicotine has any implications for psychiatric disorders or patients who are thought to potentially benefit from nicotine agonists.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 52 kb)
Acknowledgments
This study was conducted within the framework of the Priority Program SPP1226 of the German Research Foundation (DFG, Deutsche Forschungsgemeinschaft): “Nicotine: Molecular and Physiological Effects in CNS” [http://ww.nicotine-research.com], grant number: Wi1316/7-1. We thank Pfizer/McNeil AB (Sweden) and Johnson&Johnson/Janssen Pharmaceutica (Belgium) for providing us with nicotine/pepper spray, Christian Ohmann (Coordination Center for Clinical Studies, University of Düsseldorf) for data management support, and Petra Engels, Barbara Elghahwagi, Dorothe Krug, Cordula Kemper, and Veronika Ermer for assistance with fMRI scanning.
Disclosure/conflict of interest
TW, AM, FM, JB, SV, TS, and NJS have no disclosures/conflicts of interest to declare. GW has received sponsorship to attend scientific meetings, speaker honorariums, and consultancy fees from pharmaceutical companies (Johnson & Johnson/Janssen Pharmaceutica, Janssen Cilag, Pfizer Inc., and AstraZeneca). Beyond this, the authors declare that, except for income received from the primary employer and from a grant of the Deutsche Forschungsgemeinschaft to GW, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, Alce G, Iudicello JE, Akbar N, Egan MF, Goldberg TE, Weinberger DR. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32(5):1011–20. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- Behrens T, Woolrich MW, Smith S. Multi-testing using a fully subject null hypothesis Bayesian framework: theory. New York City: Human Brain Mapping Meeting; 2003. [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Jones RT, Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982;221(2):368–72. [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, Linden LEJ. Localizing P300 generators in visual target and distractor processing: a combined event-related potential and functional magnetic resonance imaging study. J Neurosci. 2004;24(42):9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds CM, Müller U, Clark L, van Loon A, Cools R, Robbins TW. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 2008;28(23):5976–5982. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Williams SCR, Patel D, Michel TM, Nwaigwe A, Caceres A, Mehta MA, Anilkumar AP, Kumari V. Effects of acute nicotine on brain function in healthy smokers and non-smokers: estimation of inter-individual response heterogeneity. Neuroimage. 2009;45:549–561. doi: 10.1016/j.neuroimage.2008.12.029. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Giddon M, Williams JB. The structured clinical interview for DCM-IV axis I disorders research version (SCID-1) New York: American Psychiatric Press; 1995. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:214–220. doi: 10.1002/hbm.460010207. [DOI] [PubMed] [Google Scholar]
- Froelinger B, Gilbert DG, McClernon FJ. Effects of nicotine on novelty detection and memory recognition performance: double-blind, placebo-controlled studies of smokers and non-smokers. Psychopharmacology. 2009;205:625–633. doi: 10.1007/s00213-009-1571-y. [DOI] [PubMed] [Google Scholar]
- Gallezot J-D, Bottlaender M, Grégoire M-C, Roumenov D, Deverre J-R, Coulon C, Ottaviani M, Dollé F, Syrota A, Valette H. In vivo imaging of human cerebral nicotinic acetylcholine receptors with 2–18 F-fluoro-A-85380 and PET. J Nucl Med. 2005;46:240–247. [PubMed] [Google Scholar]
- Giessing C, Thiel CM, Rösler F, Fink GR. The modulatory effects of nicotine on parietal cortex depend on cue reliability. Neuroscience. 2006;137:853–864. doi: 10.1016/j.neuroscience.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Giessing C, Fink GR, Roesler F, Thiel CM. fMRI data predict individual differences of behavioural effects of nicotine: a partial least square analysis. J Cogn Neurosci. 2007;9(4):658–670. doi: 10.1162/jocn.2007.19.4.658. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Wei C-Y, Philiastides MG, Gerson AD, Friedman D, Brown TR, Sajda P. Single-trial discrimination for integrating simultaneous EEG and fMRI: identifying cortical areas contributing to trial-to-trial variability in the auditory oddball task. Neuroimage. 2009;47:136–147. doi: 10.1016/j.neuroimage.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Loughead J, Waxman J, Snyder W, Ragland JD, Elliott MA, Bilker WB, Arnold SE, Gur RE. Hemodynamic responses in neural circuitries for detection of visual target and novelty: an event-related fMRI study. Hum Brain Mapp. 2007;28:263–274. doi: 10.1002/hbm.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27(13):3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Wolkenberg FA, Shakleya M, Huestis MA, Stein EA. Performance effects of nicotine during selective attention, divided attention, and simple stimulus detection: an fMRI study. Cereb Cortex. 2009;19:1990–2000. doi: 10.1093/cercor/bhn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K, Chauvel K. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol. 1998;106:156–164. doi: 10.1016/S0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Liégeois C, Chauvel P, Musolino A. Intracerebral potentials to rare target and distractor auditory and visual stimuli I. Superior temporal plane and parietal lobe. Electroencephalogr Clin Neurophysiol. 1995;94:191–220. doi: 10.1016/0013-4694(94)00259-N. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal J-P, Biraben A. Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroencephalogr Clin Neurophysiol. 1995;94:229–250. doi: 10.1016/0013-4694(95)98475-N. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hellström-Lindahl E, Mousavi M, Zhang X, Ravid R, Nordberg A. Regional distribution of nicotinic receptor subunit mRNAs in human brain: comparison between Alzheimer and normal brain. Mol brain res. 1999;66:94–103. doi: 10.1016/S0169-328X(99)00030-3. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, D’Souza DC, Mencl EW, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Mencl WE, Gelernter J. C957T polymorphism of the dopamine D2 receptor gene modulates the effect of nicotine on working memory performance and cortical processing efficiency. Psychopharmacology. 2006;188:530–540. doi: 10.1007/s00213-006-0469-1. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajós M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady J, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, Vythelingum GN, Williams SCR, Simmons A, Sharma T. Cognitive effects of nicotine in humans: an fMRI study. Neuroimage. 2003;19:1002–1013. doi: 10.1016/S1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Rose TJ, Stein EA. Cognitive mechanism of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/S0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioural characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal alpha 7 and alpha 4 beta 2 nicotine receptors and working memory. Neuroscience. 2002;109:757–765. doi: 10.1016/S0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicol Teratol. 2004;26:731–735. doi: 10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Mobascher A, Brinkmeyer J, Warbrick T, Wels C, Wagner M, Gründer G, Spreckelmeyer KN, Wienker T, Diaz Lacava A, Dahmen N, Böttcher M, Thuerauf N, Clepce M, Kiefer F, de Millas W, Gallinat J, Winterer G. The P300 event-related potential and smoking—a population-based case-control study. Int J Psychophysiol. 2010;77(2):166–75. doi: 10.1016/j.ijpsycho.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Musso F, Konrad A, Vucurevic G, Schäffner C, Friedrich B, Frech P, Stoeter P, Winterer G. Distributed BOLD-response in association cortex vector state space predicts reaction time during selective attention. Neuroimage. 2006;29:1311–1318. doi: 10.1016/j.neuroimage.2005.07.059. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Philiastides MG, Sajda P. EEG-informed fMRI reveals spatiotemporal characteristics of perceptual decision making. Cereb Cortex. 2007;16:509–518. doi: 10.1093/cercor/bhi130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event related potential. J Clin Neurophysiol. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Polich J, Ochoa CJ. Alcoholism risk, tobacco smoking, and P300 event-related potential. Clin Neurophysiol. 2004;115(6):1374–83. doi: 10.1016/j.clinph.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Palomero-Gallagher N, Grefkes C, Schleicher A, Zilles K. Transmitter receptors reveal segregation of cortical areas in the human superior parietal cortex: relations to visual and somatosensory regions. Neuroimage. 2005;28(2):362–79. doi: 10.1016/j.neuroimage.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel A, Debener S, Sorger B, Peters JC, Kranczioch C, Hoechstetter K, Engel A, Brocke B, Goebel R. Novelty and target processing during an auditory novelty oddball: a simultaneous event-related potential and functional magnetic resonance imaging study. Neuroimage. 2008;40:869–883. doi: 10.1016/j.neuroimage.2007.10.065. [DOI] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8(9):733–50. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Fink GR. Effects of the cholinergic agonist nicotine on reorienting of visual spatial attention and top-down attentional control. Neuroscience. 2008;152:381–390. doi: 10.1016/j.neuroscience.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology. 2005;30:810–820. doi: 10.1038/sj.npp.1300633. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Vossel S, Thiel CM, Fink GR. Behavioral and neural effects of nicotine on visuospatial attentional reorienting in non-smoking subjects. Neuropsychopharmacology. 2008;33:731–738. doi: 10.1038/sj.npp.1301469. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale—revised manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff G. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Winterer G, Musso F, Konrad A, Vucurevic G, Stoeter P, Sander T, Gallinat J. Association of attentional network function with exon 5 variations of the CHRNA4 gene. Hum Mol Genet. 2007;16(18):2165–2174. doi: 10.1093/hmg/ddm168. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modelling of fMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 52 kb)