Abstract
We report six patients with array deletions encompassing 12q14. Out of a total of 2538 array investigations carried out on children with developmental delay and dysmorphism in three diagnostic testing centres, six positive cases yielded a frequency of 1 in 423 for this deletion syndrome. The deleted region in each of the six cases overlaps significantly with previously reported cases with microdeletions of this region. The chromosomal range of the deletions extends from 12q13.3q15. In the current study, we report overlapping deletions of variable extent and size but primarily comprising chromosomal bands 12q13.3q14.1. Four of the six deletions were confirmed as de novo events. Two cases had deletions that included HMGA2, and both children had significant short stature. Neither case had osteopoikilosis despite both being deleted for LEMD3. Four cases had deletions that ended proximal to HMGA2 and all of these had much better growth. Five cases had congenital heart defects, including two with atrial septal defects, one each with pulmonary stenosis, sub-aortic stenosis and a patent ductus. Four cases had moderate delay, two had severe developmental delay and a further two had a diagnosis of autism. All six cases had significant speech delay with subtle facial dysmorphism.
Keywords: 12q14 microdeletion, array-CGH, HMGA2, short stature
Introduction
Chromosomal microdeletions and duplications are known to be a significant cause of developmental delay and congenital malformation in humans. In recent times, the impact of array-CGH technologies has allowed the rapid identification and delineation of many new syndromic conditions in patients with idiopathic learning disability and congenital dysmorphism. Microdeletions of chromosomal region 12q14 have been associated with characteristic features that include low birth weight, failure to thrive, short stature, developmental delay and facial dysmorphism.1, 2, 3, 4 To date, six cases with deletions spanning this region and with similar phenotype have been reported as 12q14 microdeletion syndrome. Similar deletions have also been described in children with features of Silver–Russell syndrome and/or primordial dwarfism.4 The severe growth retardation in these cases is thought to be due to loss of HMGA2 which lies within this region. Osteopoikilosis, due to deletion of the LEMD3 gene, has been described in some but not all cases reported to date.
We present a further six children with developmental delay and array-CGH-detected deletions within 12q14. Two of the children have deletions that included HMGA2 and LEMD3. The other four have deletions that ended proximal to these two genes. The two children with the more distal deletions, extending to and including HMGA2 and LEMD3, were severely growth retarded, with growth of four SDS below the mean. Neither child had osteopoikilosis. The other four children with more proximal deletions have much milder evidence of growth retardation. Our cases confirm the association between severe growth retardation and loss of HMGA2, and highlight the possibility that cases of primordial dwarfism and/or Silver–Russell syndrome with developmental delay should be investigated for deletions within this region.
Clinical cases
Case 1
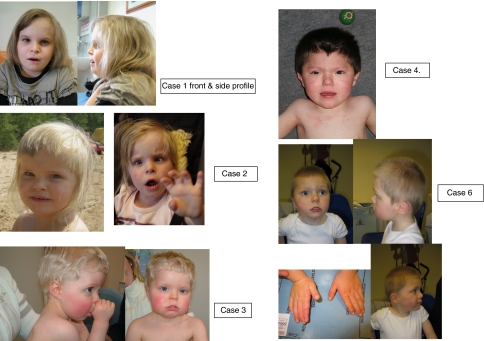
The child, a girl, was born by caesarean section at 36 weeks weighing 2.05 kg at birth (Figure 1a). Her head circumference was 32 cm and length was 45 cm. Her failure to thrive resulted in a provisional diagnosis of Silver—Russell syndrome being made, although there was no asymmetry, no café au lait patches and no history of excess sweating. She sat independently at 18 months and ‘bottom shuffled' until the age of 4 years, when she eventually walked. She has speech delay, although this is predominantly expressive. At the age of 11 years she is moderately developmentally delayed and is quite hyperactive, although she can concentrate. She can speak full sentences in both English and Polish; however, she had significant expressive speech delay as a young child. She cannot read or write. She can recognise colours, is good at jigsaws and likes singing and music. She can dress herself and manage basic hygiene. She is described as a naughty but popular child. She suffers from gastrointestinal reflux and previously suffered from constipation. On examination at the age of 7 years and 11 months, her Ht was 101.5 cm (−4.21 SDS), Wt was 14.2 kg (−4.42 SDS) and OFHC was 53.2 cm (+1.13 SDS). Her mother and father are 161 cm and 176 cm tall, respectively. She has relative macrocephaly. Her height velocity has been 3–4 cm per year. Her leg length (−2.4 SDS) is greater than her sitting height (4.9 SDS). She has a convergent squint, a high nasal bridge, long eyelashes and a cupid's bow. She has clinodactyly and her terminal phalanges are broad. She had fifth finger clinodactyly bilaterally. She has truncal obesity since the age of 10 years, with her weight velocity being greater than her height velocity.
Figure 1.
Clinical photographs of five affected individuals with 12q14 deletions. Informed consent was obtained for publication of photographs of cases 1–4 and 6.
Clinical investigations
The following investigations were normal: U and E, TFTs, routine chromosome analysis, uniparental disomy for chromosome 7, MLPA telemore testing, IGF 1 18.7 nmol/l (12–50) and IGFBP3 2.6 mg/l (1.3–4.66). Cortisol levels were measured on several occasions and were always within normal limits and she produced a good cortisol response to insulin-induced hypoglycaemia. A small anterior pituitary gland was seen on brain MRI scan and no other abnormality was detected. A skeletal survey noted a mild scoliosis but there was no evidence of osteopoikilosis. Bone age was 8 years and 10 months at a chronological age of 10.5 years. An insulin tolerance test revealed growth hormone deficiency.
Overnight growth hormone levels were subnormal for growth hormone secretion with only two peaks >3.3 within 12 h (4.5 and 4.66 mg/l). A normal result is established if at least three peaks per night are identified.5 As this is less than the normal range, she has recently been started on growth hormone treatment. It is noteworthy that this child suffered a fracture to her left frontal bone as a young child and this may have been a contributory factor to the growth hormone deficiency.
Case 2
The second child, a girl was born weighing 1.93 kg at 37+4 weeks (Figure 1b). She failed to thrive from birth. She was found to have an atrial septal defect. She is severely growth retarded with growth parameters as follows: at 3 months: Ht 50.2 cm (>−4 SDS), Wt 4.0 kg (−3 SDS), OFHC 37.5 cm (−2 SDS); at 17 months: Ht 67.5 cm (>−4 SDS), Wt 7.5 kg (−4 SDS), head circumference 45.5 cm (−1.5 SDS); at 3 years: Ht 81 cm (>−3.1 SDS), Wt 9.9 kg (−3.4 SDS). She weighed only 10 kg at 3 years and 8 months of age. The mother's height is 160 cm and her father's height is 175 cm. She fails to thrive and is currently being fed by gastrostomy. She has relative macrocephaly and is hyperactive. She has a bilateral convergent squint. She has an aversion to food and very rarely consumes a drink. She sat independently at 1 year and started walking at the age of 2 years. She is incapable of speech but can express herself by showing what she wants. She has had several ear infections and pneumonia. She has the following dysmorphic signs: a short-broad neck, frontal bossing, hypertelorism, upslanting palpebral fissures, a high nasal bridge and a short nose. The philtrum is flat, the upper lip is thin and her mouth is rather big. She has a micrognathia and low set ears. She has a single palmar crease on the left hand and a sacral dimple. X-ray at the age of 3 years showed no signs of skeletal/bone abnormalities. She has been diagnosed with moderate mental retardation with autism and significant behavioural difficulties. The 12q14 microdeletion was not present in the mother, but DNA from a phenotypically normal father was not available for testing.
Case 3
The third case, a male, was born at 39 weeks weighing 3.6 kg (Figure 1c). He had feeding difficulties as a neonate. At 4½ years of age, his Ht was 101 cm (−0.6 SDS), Wt was 15 kg (−1.12 SDS) and head circumference was 51.5 cm (+0.79 SDS). He has moderate global developmental delay and no speech at the age of 6 years. He walked at 2½ years. He has a mild degree of pulmonary stenosis and a progressive pectus deformity. Dysmorphic features include long eyelashes, low post hairline, frontal hair whorl and mild brachydactyly. He has small joint hypermobility and poor oromotor control, and constantly dribbled until the age of 4 years. The 12q14 microdeletion occurred as a de novo event.
Case 4
The fourth case, a male, was born at 36 weeks weighing 1.9 kg (Figure 1d). He was diagnosed with pyloric stenosis for which he underwent surgery. He is severely developmentally delayed, hyperactive and is thought to be on the autistic spectrum. At the age of 2 years and 3 months, his growth parameters were as follows: Ht 83.5 cm (−0.66 SDS), Wt 12.7 kg (−0.05 SDS) and OFC 48.5 cm. At the age of 2 years and 9 months he spoke no words. His growth parameters at the of age 2 years and 11 months were as follows: OFC 48.5 cm, 25th centile; Ht 87.5 cm 50th centile; and Wt IUGR 4 lbs 11 oz, 50th centile at 36 weeks. At the age of 5 years and 8 months, his height was 105.5 cm and weight was 18.5 kg. It was not possible to measure his OFHC. He has a history of several upper respiratory tract infections. Dysmorphic features include a marked widow's peak, upslanting palpebral fissures and a pouty lower lip with protruding tongue. He has strikingly short, broad fingers and toes, particularly his thumbs. His genitalia are normal and other examinations were unremarkable. At 6 years and 5 months he has much more interaction with people around him. He has developed the use of single words and is using sounds in addition to defining by action very well. However, he is still extremely active and still in nappies. He is not interested in food and seems to need only a little sleep. He has started to bite his arms a bit and get frustrated with communication at times. Skeletal survey showed broad spatulate tips to distal phalanges. His parents declined genetic testing but were phenotypically normal.
Case 5
The pregnancy was complicated by a car accident at 28 weeks. The baby, a boy was born weighing 2.89 kg at 36 weeks (Figure 1e). He was admitted to SCBU overnight and fed rather poorly from the beginning. At the age of 8 months he was diagnosed with global developmental delay. He sat at 8 months and walked at 18 months. He has marked speech delay. He is now 20 years old and attends a special school. Dysmorphic features include upslanting palpebral fissures, pinched nose, high palate, flat face, broad hands, flat feet and 2/3 syndactyly. A provisional earlier diagnosis of Lujan Fryns was made. Growth at 17.7 years was as follows: Ht 161.9 cm (−0.04 SDS), Wt 51.1 kg (−0.80 SDS) and OFC 53.2 cm (−0.99 SDS). He had an atrial septal defect that required closure. The 12q14 microdeletion occurred as a de novo event.
Case 6
The sixth case (Figure 1f) was the second child of non-consanguineous parents, born at term by emergency caesarean section for fetal distress, weighing 2.85 kg. This followed an uncomplicated pregnancy. There were no feeding problems. There were concerns from the beginning as the parents noticed that he showed a delay in achieving his milestones. At 2 years and 6 months, he was diagnosed to have a moderated sub-aortic stenosis. At 4 years and 4 months, he was assessed to have moderate global developmental delay and was still incapable of putting two words together. He has hypermetropia. His growth parameters were as follows: Ht 95 cm (−2.3 SDS), Wt 15.1 kg (−1.1 SDS) and OFHC 49 cm (−2.3 SDS). Dysmorphic features include brachycephaly, deep set eyes, bilateral low set posteriorly rotated ears, short nasal bridge with an upturned nose and a long simple philtrum with a thin upper lip. He was seen to have focal cortical dysplasia in the insular cortex on brain MRI scan. The 12q14 microdeletion occurred as a de novo event.
Methods
Microarray analysis
Following initial diagnostic investigations by karyotyping and multiplex ligation-dependent probe amplification (MLPA (subtelomere kits P036D and P070) MRC Holland) in which no imbalances were detected, whole genome screening was carried out using whole-genome 0.5 Mb ‘CytoChip' microarrays (BlueGnome, Cambridge, UK) for cases 1 and 5, SNP6.0 analysis for case 2 (Affymetrix, Santa Clara, CA, USA), 44K oligo (Agilent, Santa Clara, CA, USA) microarray for cases 3–4 and 135K oligo (Roche-Nimblegen, Madison, WI, USA) for case 6. Microarray procedures were carried out on the respective microarray platforms using standard procedures. Higher resolution screening using a 135K oligonucleotide arrays (Roche-Nimblegen) was subsequently used to more precisely define deleted breakpoints for case 1 and 3–6. To refine breakpoints in case 2, an Affymetrix Genome-Wide SNP Array 6.0 (Affymetrix) was carried out in accordance with the manufacturer's instructions. All CytoChip BAC microarrays were scanned at 10-μm resolution using a GenePix Pro 5.0 array scanner Axon (Molecular Devices, Sunnyvale, CA, USA) and analysed using BlueFuse for Microarrays analysis software version 3.4 (BlueGnome). All oligo and SNP arrays were carried out according to the manufacturer's protocols, scanned at 2–5 μm, and analysed using SignalMap v1.9 (135K), DNA Analytics v4.0.76 (44K), or Genotyping Console v4.0 (SNP6.0).
Confirmation of array-CGH results
FISH techniques were carried out on metaphase spreads prepared from peripheral blood for patients 1, 3 and 5 and for both parents wherever possible, using standard techniques.6 Microdeletions of 12q14 were confirmed in each case using BAC probes within each respective deleted region. In case 2, three specifically designed MLPA probes were designed to cover the region of interest.7 Data analysis was carried out with GeneMarker software 1.85 (Softgenetics, State College, PE, USA). The parents of case 4 declined genetic testing but were identified as being phenotypically normal. Quantitative (real-rime) PCR (qPCR) was carried out in quadruplicate (case 5 only) and data were analysed using the relative quantification method (ddCT).8 The control used was pooled DNA from 12 normal individuals from the blood bank, and the reference/endogenous control was the single copy RNase P. Microarray-CGH investigations using a Roche-Nimblegen 135K array were carried outfor confirmatory studies in case 6.
Results
Detection of deletions encompassing 12q14
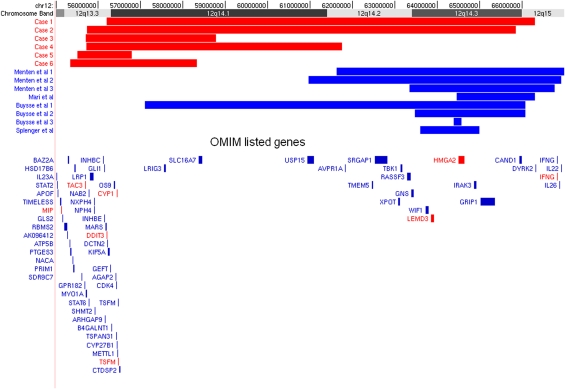
The deletions in all six affected individuals were initially detected as part of an array-CGH screen for cryptic rearrangements in patients with moderate–severe learning disability and/or congenital anomalies following normal karyotype and subtelomere MLPA analysis. In total, we had six positive cases out of 2538 cases referred with developmental delay for array analysis, giving a frequency of 1 in 423 cases. It is not possible to estimate an overall frequency of this deletion syndrome as the cases tested by array are a biased cohort, selected by clinical geneticists and would not represent the developmental delay population in general. High-resolution oligo/SNP arrays were used to estimate the minimum size of the deletion breakpoints (median probe spacing ∼12 524 bp (135K), and ∼40 000 bp (44K), ∼680 bp (SNP6.0)): case 1, 56 192 860–66 309 484 bp; case 2, 55 728 406–65 850 933 bp; case 3, 55 704 299–58 779 521 bp; case 4, 55 723 270–61 759 877 bp; case 5, 55 508 340–56 797 233 bp; and case 6, 55 344 266–58 329 514 (NCBI Human Genome Build 36). The deleted region in each of the six cases (Figure 2) overlaps significantly with previously reported cases with microdeletions of this region.1, 2, 3, 4 In the current study, the chromosomal range of deletions extends from 12q13.3q15. With the exception of case 5 in the current study, cases 1–4 and 6 carry overlapping deletions of variable size but comprise chromosomal bands 12q13.3q14.1. No other copy number variants were identified elsewhere in the genome in the six cases that have not been previously identified as high-frequency CNVs in normal population studies according to the Database of Genomic Variants (http://projects.tcag.ca/variation/). FISH verification experiments were carried out on metaphase spreads from cases 1, 3 and 5 (MLPA was carried out in case 2, parents were unavailable in case 4 and array-CGH was used for confirmation in case 6), to validate each copy number imbalance as detected by array-CGH. All FISH probes used to confirm the array-CGH findings for each of the cases were within each deleted 12q region. Normal FISH signals for each probe were seen for parental cases 1, 3 and 5 (data not shown) confirming that these three cases were de novo deletions of the 12q13.3q15 region. For case 2, the microdeletion was not identified in the mother, and the father was not available for testing. The parents of case 4 declined genetic testing. qPCR analysis using the TaqMan/Universal Probe Library sequence-specific probes to RDH16, together with FISH was used to confirm the de novo deletion in case 5. Microarray analysis using a 135k oligo array (Roche-Nimblegen) was carried out to confirm a de novo deletion in case 6.
Figure 2.
Schematic illustration of the six 12q14 deletions detected by array-CGH using the Ensembl browser. Deleted regions for the respective cases in this communication are shown as red bars. The colour reproduction of this figure is available on the html full text version of the manuscript.
Discussion
In the current communication, we report further six cases with deletions encompassing 12q14 which brings the total number to 12 reported cases. At a time when phenotypic variability is often associated with newly identified microdeletion syndromes, 12q14 deletions have been reported as a relatively uniform and constant phenotype of low birth weight, poor feeding in infancy, short stature and learning disability.1, 2, 3, 4 There have been reports of some cases presenting with osteopoikilosis and skin lesions as well.3 A further case reported by Jurenka and van Allen,9 presenting with short stature, learning disability and a mixed sclerosing bone dysplasia, is also suspected of having a 12q14 microdeletion by Mari et al.2 The 12q14 deletions in our cases varied in size from 1.28 to 10.12 Mb. The apparent lack of low copy repeats near the breakpoints in each of our cases indicates that the underlying genetic mechanism of non-homologous end joining may be responsible for recurrent deletions within this region. The number of HGNC genes within the deleted regions of all six cases ranged from 35 to 58 with 15–39 OMIM-listed genes. Although there is substantial genomic overlap with the previously reported 12q14 microdeletion cases, it should be reported that the shortest region of overlap (SRO) between all six cases reported in this communication differs substantially from the SRO of the reported cases in literature. This will inevitably explain phenotypic variation between cases. The SRO of the previously reported 12q14 microdeletion cases includes HMGA2. Genotype–phenotype correlations are difficult to ascertain, as only cases 1 and 2 are severely growth retarded; however, but common characteristics seen include moderate–severe learning disability, speech delay and congenital heart defects (not known for case 4); therefore, so it is possible that deletion of one or more of these genes could be responsible for any of these aspects of the phenotype (see Table 1). DCTN2 and KIF5A have been associated with disruptions in axonal transport;10, 11 therefore, in theory, haploinsufficiency could account for the neurodevelopmental anomalies seen in these cases. Congenital heart defects have not been reported in any of the six reported 12q14 microdeletions in literature, which suggests that the candidate cardiac gene lies upstream of the 5′ ends of previously reported cases. The largest region of overlap seen in our cases was between case 1 and 2 comprising 9.65 Mb, and includes both HMGA2 and LEMD3.
Table 1. Summary of the clinical features presented in six patients with deletion of 12q14.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Sex | F | F | M | M | M | M |
| Birth weight (kg) | 2 kg at 36 weeks | 1.9 at 37+4 weeks | 3.6 kg at 39 weeks | 1.9 at 36 weeks | 2.9 at 36 weeks | 2.86 at 40 weeks |
| Development | Moderate delay | Severe delay | Moderate delay | Severe delay | Moderate delay | Moderate delay |
| Behaviour | Pleasant and friendly personality | Autism | Autism | |||
| Feeding | Poor feeder gastrointestinal reflux | Poor feeder gastrostomy fed | Poor feeder | Pyloric stenosis | Poor feeder | Normal feeding |
| Malformations | ?patent ductus | Atrial septal defect | Pulmonary stenosis and pectus excavatum | Atrial septal defect | Sub-aortic stenosis | |
| Eyes | Convergent squint | Convergent squint | Hypermetropia | |||
| Speech | Expressive speech delay | No speech | No speech age 6 years | Single words aged 6 years | Marked speech delay | Speech delay |
The first report of the 12q14 microdeletion syndrome by Menten in 20071 described three patients with a deletion of 12q14 who presented with growth retardation and osteopoikilosis. There was a 3.44-Mb common deleted region that contained LEMD3, the causal gene for osteopoikilosis.12 However, growth retardation, failure to thrive and learning disability have not been reported in patients with osteopoikilosis or melorheostosis. Mari et al2 described a case with primordial dwarfism and a smaller deletion of the 12q14.4 region. The child, a boy, had severe prenatal and postnatal growth failure and mild developmental delay. There was a history of poor feeding and failure to thrive, similar to the phenotype of primordial dwarfism or severe Silver–Russell syndrome (SRS). There was no evidence of osteopoikilosis but this child's deletion started distally to that of the cases in Menten et al1 and did not include LEMD3. Buysse et al3 described two children, one of whom had an intragenic deletion of HMGA2 and short stature, providing evidence that HMGA2 is the likely growth gene. HMGA2 lies at 12q14.3, which would explain the normal growth in our cases 3–6 whose deletion ended proximally. Spengler et al4 identified one further case with a 12q14 deletion in a series of 20 children with a diagnosis of SRS. This child's deletion included HMGA2 and LEMD3 although he had no evidence of osteopoikilosis. Cases in which HMGA2 remains intact had growth within the normal centiles. This supports the proposal that HMGA2 is the most significant gene associated with growth in this region. Loss of function of HMGA2 in the mouse results in the pygmy phenotype that combines prenatal and postnatal growth failure, with resistance to the adipogenic effect of overfeeding. As such it has been suggested that haploinsufficiency of HMGA2 could be associated with both reduced growth and poor feeding, failure to thrive and lack of adipose tissue, which has been reported in some cases.2 This would not explain the poor feeding and failure to thrive that was seen in cases 3–6 in this report in which HMGA2 was not shown to be deleted.
Interestingly, case 1 had documented low overnight growth hormone levels. As this child had a significant head injury resulting in a frontal fracture as a baby, it is difficult to determine whether the low growth hormone levels are related to the deletion or are a result of the head injury, or perhaps a combination of both events. This child has just recently commenced growth hormone therapy and we wait to see how this affects growth. The case reported by Mari et al2 had been treated with growth hormone without any obvious benefit. Growth hormone levels in the other cases have not been documented. There were some dysmorphic features common to cases 1–4 including, a cupid's bow upper lip. Two of the children (cases 1 and 3) had similar facial dysmorphism with long eyelashes and a cupid's bow. A prognathic appearance is also present in cases 1–3. There was no evidence of osteopoikilosis in any of our cases despite a deletion of LEMD3 in cases 1 and 2. Five of the six cases had congenital heart anomalies. Two cases had an atrial septal defect (case 2 and 5), one had pulmonary stenosis (case 3), one had sub-aortic stenosis and case 1 presented with a patent ductus. One child had pyloric stenosis, but all except case 6 had feeding difficulties. Interestingly, severe speech delay was noted in cases 2–6. Case 1, having expressive speech delay early on, can currently speak full sentences in both Polish and English at the age of 11 years. Two of the cases (2 and 4) were also identified as having ‘autistic features'. The dysmorphism in these children is subtle. They all had a cupid's bow. Cases 1 and 2 had deep set eyes and a squint and these two children have a very similar facial appearance. The dysmorphism in the other four cases was subtle. All cases except case 6 had a tall forehead. Cases 1, 3, 4 and 6 had arched eyebrows.
We conclude that the 12q14 microdeletion syndrome is a recurrent deletion syndrome with a dysmorphic phenotype, which may be recognisable in some cases. One needs to consider the diagnosis in children with severe growth retardation and developmental delay. Our cases, particularly cases 3–6, whose growth was within normal centiles and whose deletion ended proximal to HMGA2, provide further evidence that HMGA2 is a good candidate gene for growth retardation. We would recommend growth hormone level measurements in other cases with deletions of HMGA2 to determine whether growth hormone deficiency is a common finding. Further testing for loss of function mutations in HMGA2 is also required to test for idiopathic short stature.
Acknowledgments
We thank all of the families for agreeing to participate in this study.
The authors declare no conflict of interest.
References
- Menten B, Buysse K, Zahir F, et al. Osteopoikilosis, short stature & mental retardation as key features of a new microdeletion syndrome on 12q14. J Med Genet. 2007;44:264–268. doi: 10.1136/jmg.2006.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari F, Hermanns P, Giovannucci-Uzielli ML, et al. Refinement of the 12q14 microdeletion syndrome: primordial dwarfism and developmental delay with or without osteopoikilosis. Eur J Hum Genet. 2009;17:1141–1147. doi: 10.1038/ejhg.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse K, Reardon W, Mehta L, et al. The 12q14 microdeletion syndrome: additional patients and further evidence that HMGA2 is an important genetic determinant for human height. Eur J Med Genet. 2009;52:101–117. doi: 10.1016/j.ejmg.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Spengler S, Schönherr N, Binder G, et al. Sub microscopic chromosomal imbalances in idiopathic Silver–Russell syndrome (SRS): the SRS phenotype overlaps with the 12q14 microdeletion syndrome. J Med Genet. 2010;47:356–360. doi: 10.1136/jmg.2009.070052. [DOI] [PubMed] [Google Scholar]
- Brook CGD, Hindmarsh PC.Clinical Pediatric Endocrinology Blackwell Science4th edition2001
- Chong SS, Pack SD, Roschke AV, et al. A revision of the lissencephaly and Miller-Dieker syndrome critical regions in chromosome 17p13.3. Hum Mol Genet. 1997;6:147–155. doi: 10.1093/hmg/6.2.147. [DOI] [PubMed] [Google Scholar]
- Stern RF, Roberts RG, Mann K, Yau SC, Berg J, Ogilvie CM. Multiplex ligation-dependent probe amplification using a completely synthetic probe set. Biotechniques. 2004;37:399–405. doi: 10.2144/04373ST04. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Jurenka SB, Van Allen MI. Mixed sclerosing bone dysplasia, small stature, seizure disorder and mental retardation: a syndrome. Am J Med Genet. 1995;57:6–9. doi: 10.1002/ajmg.1320570103. [DOI] [PubMed] [Google Scholar]
- LaMonte BH, Wallace KE, Holloway BA, et al. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- Fichera M, Lo Giudice M, Falco M, et al. Evidence of kinesin heavy chain (KIF5A) involvement in pure hereditary spastic paraplegia. Neurology. 2004;63:1108–1110. doi: 10.1212/01.wnl.0000138731.60693.d2. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Preobrazhenska O, Willaert A, et al. Loss of function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Nat Genet. 2004;36:1213–1218. doi: 10.1038/ng1453. [DOI] [PubMed] [Google Scholar]