Abstract
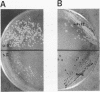
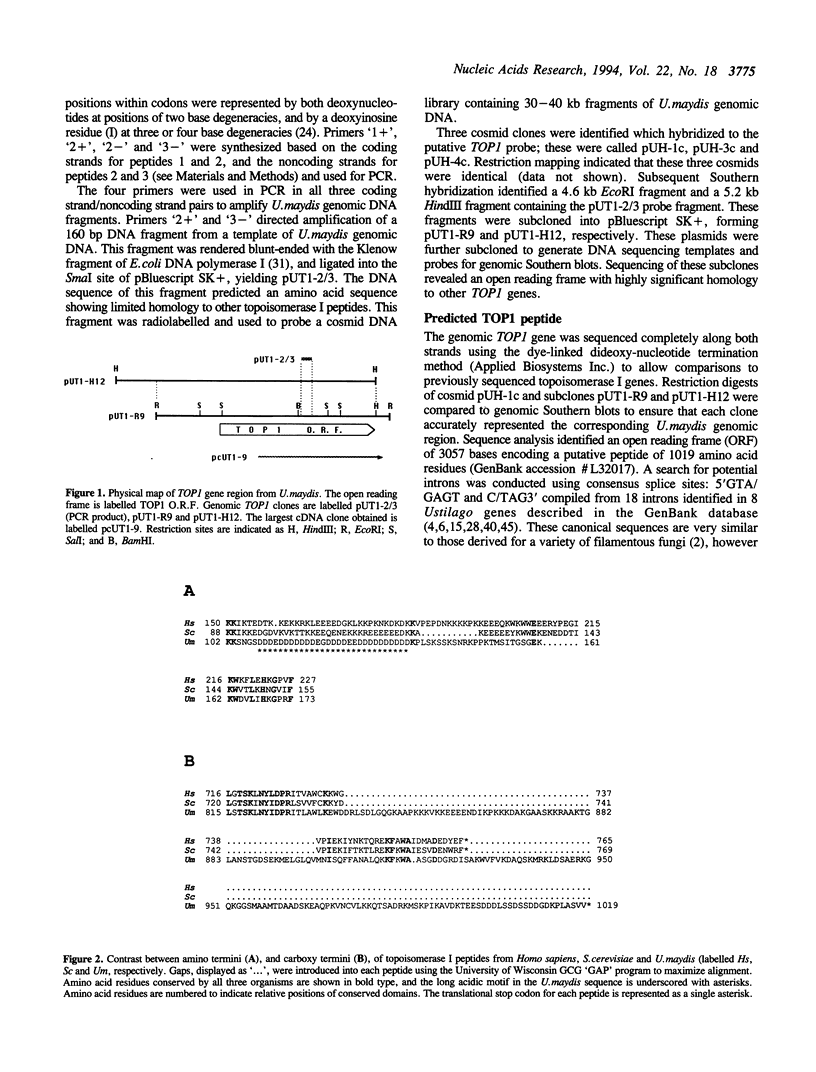
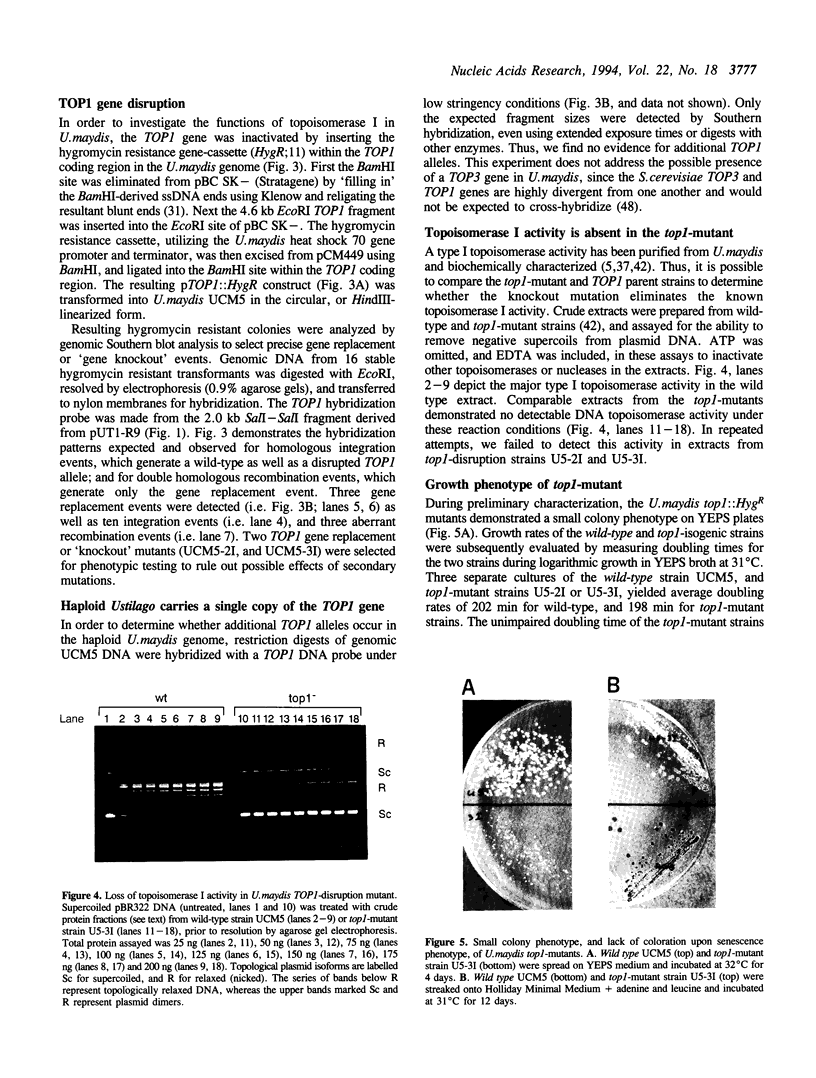
The Ustilago maydis genomic TOP1 gene encoding DNA topoisomerase I was cloned by amplifying a gene fragment using the polymerase chain reaction, and using this fragment to search a genomic DNA library by hybridization. The predicted peptide sequence exhibited 30-40% identity to other eukaryotic TOP1 genes, yet differed in several features. First, an unusually long acidic region was identified near the amino terminus (28/29 residues are acidic), which resembles other nucleolar peptide motifs. Second, an atypical carboxy-terminal 'tail', absent in other TOP1 genes, followed the active site tyrosine residue. A top1 gene disruption mutant was constructed by replacing the genomic TOP1 gene, with a top1::HygR null allele. This mutant lost the abundant topoisomerase I activity evident in wild-type U.maydis, and displayed a subtle coloration phenotype evident during cell senescence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailis A. M., Arthur L., Rothstein R. Genome rearrangement in top3 mutants of Saccharomyces cerevisiae requires a functional RAD1 excision repair gene. Mol Cell Biol. 1992 Nov;12(11):4988–4993. doi: 10.1128/mcb.12.11.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballance D. J. Sequences important for gene expression in filamentous fungi. Yeast. 1986 Dec;2(4):229–236. doi: 10.1002/yea.320020404. [DOI] [PubMed] [Google Scholar]
- Banks G. R., Shelton P. A., Kanuga N., Holden D. W., Spanos A. The Ustilago maydis nar1 gene encoding nitrate reductase activity: sequence and transcriptional regulation. Gene. 1993 Sep 6;131(1):69–78. doi: 10.1016/0378-1119(93)90670-x. [DOI] [PubMed] [Google Scholar]
- Brougham M. J., Rowe T. C., Holloman W. K. Topoisomerase from Ustilago maydis forms a covalent complex with single-stranded DNA through a phosphodiester bond to tyrosine. Biochemistry. 1986 Nov 18;25(23):7362–7368. doi: 10.1021/bi00371a018. [DOI] [PubMed] [Google Scholar]
- Bölker M., Urban M., Kahmann R. The a mating type locus of U. maydis specifies cell signaling components. Cell. 1992 Feb 7;68(3):441–450. doi: 10.1016/0092-8674(92)90182-c. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. DNA is linked to the rat liver DNA nicking-closing enzyme by a phosphodiester bond to tyrosine. J Biol Chem. 1981 May 25;256(10):4805–4809. [PubMed] [Google Scholar]
- Chang X. B., Wilson J. H. Modification of DNA ends can decrease end joining relative to homologous recombination in mammalian cells. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4959–4963. doi: 10.1073/pnas.84.14.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choder M. A general topoisomerase I-dependent transcriptional repression in the stationary phase in yeast. Genes Dev. 1991 Dec;5(12A):2315–2326. doi: 10.1101/gad.5.12a.2315. [DOI] [PubMed] [Google Scholar]
- Christman M. F., Dietrich F. S., Fink G. R. Mitotic recombination in the rDNA of S. cerevisiae is suppressed by the combined action of DNA topoisomerases I and II. Cell. 1988 Nov 4;55(3):413–425. doi: 10.1016/0092-8674(88)90027-x. [DOI] [PubMed] [Google Scholar]
- Eng W. K., Pandit S. D., Sternglanz R. Mapping of the active site tyrosine of eukaryotic DNA topoisomerase I. J Biol Chem. 1989 Aug 15;264(23):13373–13376. [PubMed] [Google Scholar]
- Fotheringham S., Holloman W. K. Pathways of transformation in Ustilago maydis determined by DNA conformation. Genetics. 1990 Apr;124(4):833–843. doi: 10.1093/genetics/124.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G. N., Wang J. C. Supercoiling of intracellular DNA can occur in eukaryotic cells. Cell. 1988 Dec 2;55(5):849–856. doi: 10.1016/0092-8674(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Gillissen B., Bergemann J., Sandmann C., Schroeer B., Bölker M., Kahmann R. A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell. 1992 Feb 21;68(4):647–657. doi: 10.1016/0092-8674(92)90141-x. [DOI] [PubMed] [Google Scholar]
- Goto T., Wang J. C. Yeast DNA topoisomerase II is encoded by a single-copy, essential gene. Cell. 1984 Apr;36(4):1073–1080. doi: 10.1016/0092-8674(84)90057-6. [DOI] [PubMed] [Google Scholar]
- Halligan B. D., Davis J. L., Edwards K. A., Liu L. F. Intra- and intermolecular strand transfer by HeLa DNA topoisomerase I. J Biol Chem. 1982 Apr 10;257(7):3995–4000. [PubMed] [Google Scholar]
- Holliday R. Radiation sensitive mutants of Ustilago maydis. Mutat Res. 1965 Dec;2(6):557–559. doi: 10.1016/0027-5107(65)90022-9. [DOI] [PubMed] [Google Scholar]
- Holm C., Stearns T., Botstein D. DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage. Mol Cell Biol. 1989 Jan;9(1):159–168. doi: 10.1128/mcb.9.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T. S., Brown S. D., Huang P., Fostel J. Isolation and characterization of a gene encoding DNA topoisomerase I in Drosophila melanogaster. Nucleic Acids Res. 1992 Dec 11;20(23):6177–6182. doi: 10.1093/nar/20.23.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan C. C., Hwang J. L., Liu A. A., Whang-Peng J., Knutsen T., Huebner K., Croce C. M., Zhang H., Wang J. C., Liu L. F. Human DNA topoisomerase I is encoded by a single-copy gene that maps to chromosome region 20q12-13.2. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8910–8913. doi: 10.1073/pnas.85.23.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R. A., Wang J. C. Function of DNA topoisomerases as replication swivels in Saccharomyces cerevisiae. J Mol Biol. 1989 Jul 20;208(2):257–267. doi: 10.1016/0022-2836(89)90387-2. [DOI] [PubMed] [Google Scholar]
- Lee M. P., Brown S. D., Chen A., Hsieh T. S. DNA topoisomerase I is essential in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6656–6660. doi: 10.1073/pnas.90.14.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Lowndes N. F., Johnson A. L., Johnston L. H. Coordination of expression of DNA synthesis genes in budding yeast by a cell-cycle regulated trans factor. Nature. 1991 Mar 21;350(6315):247–250. doi: 10.1038/350247a0. [DOI] [PubMed] [Google Scholar]
- Lynn R. M., Bjornsti M. A., Caron P. R., Wang J. C. Peptide sequencing and site-directed mutagenesis identify tyrosine-727 as the active site tyrosine of Saccharomyces cerevisiae DNA topoisomerase I. Proc Natl Acad Sci U S A. 1989 May;86(10):3559–3563. doi: 10.1073/pnas.86.10.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maridor G., Nigg E. A. cDNA sequences of chicken nucleolin/C23 and NO38/B23, two major nucleolar proteins. Nucleic Acids Res. 1990 Mar 11;18(5):1286–1286. doi: 10.1093/nar/18.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei B., Budde A. D., Leong S. A. sid1, a gene initiating siderophore biosynthesis in Ustilago maydis: molecular characterization, regulation by iron, and role in phytopathogenicity. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):903–907. doi: 10.1073/pnas.90.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T., Pfund W. P., Mehta V. B., Trask D. K. Eukaryotic type I topoisomerase is enriched in the nucleolus and catalytically active on ribosomal DNA. EMBO J. 1985 May;4(5):1237–1243. doi: 10.1002/j.1460-2075.1985.tb03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peculis B. A., Gall J. G. Localization of the nucleolar protein NO38 in amphibian oocytes. J Cell Biol. 1992 Jan;116(1):1–14. doi: 10.1083/jcb.116.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. Nuclear location signal-mediated protein transport. Biochim Biophys Acta. 1989 Aug 14;1008(3):263–280. doi: 10.1016/0167-4781(89)90016-x. [DOI] [PubMed] [Google Scholar]
- Rowe T. C., Rusche J. R., Brougham M. J., Holloman W. K. Purification and properties of a topoisomerase from Ustilago maydis. J Biol Chem. 1981 Oct 25;256(20):10354–10361. [PubMed] [Google Scholar]
- Schiestl R. H., Dominska M., Petes T. D. Transformation of Saccharomyces cerevisiae with nonhomologous DNA: illegitimate integration of transforming DNA into yeast chromosomes and in vivo ligation of transforming DNA to mitochondrial DNA sequences. Mol Cell Biol. 1993 May;13(5):2697–2705. doi: 10.1128/mcb.13.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S. Recombination mediated by vaccinia virus DNA topoisomerase I in Escherichia coli is sequence specific. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10104–10108. doi: 10.1073/pnas.88.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. L., Leong S. A. Isolation and characterization of a Ustilago maydis glyceraldehyde-3-phosphate dehydrogenase-encoding gene. Gene. 1990 Sep 1;93(1):111–117. doi: 10.1016/0378-1119(90)90143-f. [DOI] [PubMed] [Google Scholar]
- Spanos A., Kanuga N., Holden D. W., Banks G. R. The Ustilago maydis pyr3 gene: sequence and transcriptional analysis. Gene. 1992 Aug 1;117(1):73–79. doi: 10.1016/0378-1119(92)90492-8. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan M. M., Kotani H., Holloman W. K., Kmiec E. B. DNA relaxation mediated by Ustilago maydis type I topoisomerase; modulation by chromatin associated proteins. Biochim Biophys Acta. 1993 May 28;1173(2):155–164. doi: 10.1016/0167-4781(93)90176-e. [DOI] [PubMed] [Google Scholar]
- Thrash C., Bankier A. T., Barrell B. G., Sternglanz R. Cloning, characterization, and sequence of the yeast DNA topoisomerase I gene. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4374–4378. doi: 10.1073/pnas.82.13.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang T., Copeland V., Bowden G. T. A set of cassette cloning vectors for rapid and versatile adaptation of restriction fragments. Biotechniques. 1991 Mar;10(3):330–330. [PubMed] [Google Scholar]
- Tsukuda T., Bauchwitz R., Holloman W. K. Isolation of the REC1 gene controlling recombination in Ustilago maydis. Gene. 1989 Dec 28;85(2):335–341. doi: 10.1016/0378-1119(89)90426-5. [DOI] [PubMed] [Google Scholar]
- Uemura T., Morino K., Uzawa S., Shiozaki K., Yanagida M. Cloning and sequencing of Schizosaccharomyces pombe DNA topoisomerase I gene, and effect of gene disruption. Nucleic Acids Res. 1987 Dec 10;15(23):9727–9739. doi: 10.1093/nar/15.23.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Ohkura H., Adachi Y., Morino K., Shiozaki K., Yanagida M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell. 1987 Sep 11;50(6):917–925. doi: 10.1016/0092-8674(87)90518-6. [DOI] [PubMed] [Google Scholar]
- Wallis J. W., Chrebet G., Brodsky G., Rolfe M., Rothstein R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989 Jul 28;58(2):409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Caron P. R., Kim R. A. The role of DNA topoisomerases in recombination and genome stability: a double-edged sword? Cell. 1990 Aug 10;62(3):403–406. doi: 10.1016/0092-8674(90)90002-v. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]