Abstract
The archaeological site of Sagalassos is located in Southwest Turkey, in the western part of the Taurus mountain range. Human occupation of its territory is attested from the late 12th millennium BP up to the 13th century AD. By analysing the mtDNA variation in 85 skeletons from Sagalassos dated to the 11th–13th century AD, this study attempts to reconstruct the genetic signature potentially left in this region of Anatolia by the many civilizations, which succeeded one another over the centuries until the mid-Byzantine period (13th century BC). Authentic ancient DNA data were determined from the control region and some SNPs in the coding region of the mtDNA in 53 individuals. Comparative analyses with up to 157 modern populations allowed us to reconstruct the origin of the mid-Byzantine people still dwelling in dispersed hamlets in Sagalassos, and to detect the maternal contribution of their potential ancestors. By integrating the genetic data with historical and archaeological information, we were able to attest in Sagalassos a significant maternal genetic signature of Balkan/Greek populations, as well as ancient Persians and populations from the Italian peninsula. Some contribution from the Levant has been also detected, whereas no contribution from Central Asian population could be ascertained.
Keywords: ancient DNA, mtDNA, Anatolia, Byzantine
Introduction
Anatolia, the Asian part of Turkey (Asia Minor) is at the crossroad between the Balkans, the Near East and the Caucasus, and has acted during major population expansions as a bridge between Europe, Africa and Asia. Presence of modern humans in Anatolia is attested since the upper Palaeolithic.1 This region was also suggested to be a refuge during the Last Glacial Maximum,2 at the end of which an improving climate allowed the coastal hunter-gatherers to venture inland, where they established temporary camps in search of game and flint.3 Anatolia is also considered as an area through which the ‘Neolithic revolution' spread into Europe.4, 5, 6, 7, 8 However, the major cultural changes in Anatolia occurred from the Bronze Age, when various civilization and empires succeeded one another over time and increasing interactions in the eastern Mediterranean resulted in the development of trading networks, military campaigns and human colonisations, particularly during the Mycenaean, the Greco-Hellenistic, Roman Republican and Imperial and later the Islamic periods.9, 10, 11, 12, 13
From the early Middle Bronze Age onwards city-states and federations of the Hittites and the Luwians, two originally ethnically and linguistically related groups from Transcaucasia, started to infiltrate Central to Eastern and Western to Southern Anatolia, respectively.14, 15 Shortly afterward, from the 16th millennium BC, Minoan and Mycenaean colonization took place on the Anatolian west and southwest coast.
The Early Iron Age in Central and West Anatolia is characterized by the kingdom of the Phrygians (10th to early 7th century BC), followed after its collapse by the Lydians. From 546 until 334/333 BC, Anatolia was incorporated into the Achaemenid Persian Empire and later into that of Alexander the Great. Then, from the early 2nd century BC onwards, Pisidia was part of the realm of the Romans.16, 17 It has been estimated that in the Late Roman Period, the size of the Anatolian population reached 12 million individuals.18
In the mid of the 6th century AD the late Roman civilisation and Empire, to which Anatolia belonged, became part of the Byzantine culture and Empire, Greek in language and ideology, but Christian in religion (6th–15th century AD). Under Byzantine rule the intermittent Arabic invasions took place, before the Seljuk Turks conquered Anatolia in the late 11th century AD. After their crushing by the Mongols, followed by the Beylik period (Turkish principalities), the Anatolian peninsula was unified again into the Ottoman Empire, from the 14th to the early 20th century AD, when it became the major part of the current Republic of Turkey.9, 10, 19
Evidently, the Anatolian population history is the result of complex processes involving different population groups, the impact and contribution of which to the genetic pool of the Anatolian population is still relatively unknown. Molecular genetic analyses of modern human populations provided important clues to reconstruct the past migratory trajectories in the eastern Mediterranean and Anatolia.2, 20, 21, 22, 23, 24 Nevertheless, these results are limited by the fact that various demographic events could have obscured over time the evolutionary history inferred from modern populations. In this context, genetic analysis of ancient populations appears to be a promising way to investigate the origin of human populations and to validate hypotheses based on modern genetic data and other fields of study (eg historical and archaeological data).
In this study, we describe the mitochondrial DNA (mtDNA) variation of a Byzantine population (11th–13th century) from the archaeological site of Sagalassos, in Southwestern Anatolia. The importance of this population is because of both (i) a continuous human occupation of its territory from the later 6th millennium BP up to the 13th century AD25 and (ii) its very well-known historical and archaeological background resulting from 25 years of research (http://www.sagalassos.be), which revealed the different influences that Sagalassos had undergone since the Bronze Age through its affiliation to successive empires/civilizations in this region (see Ricaut and Waelkens26 for a more detailed review of the historical context of Sagalassos).
The purpose of this study is to estimate the maternal genetic signature potentially left in the Byzantine population from Sagalassos by the historic events impacting the Sagalassos region and South-western Anatolia since the Bronze Age, and whether these events may have been associated to large population movements, to more restricted elite dominance or only to cultural diffusion. More particularly, on the basis of the available historical and archaeological records, our objective is to test whether differential contributions to Sagalassos population were made by populations from the Mediterranean area (Balkan and Italian peninsula), the Caucasus, the Near/Middle East and Central Asia.
Samples and methods
Samples
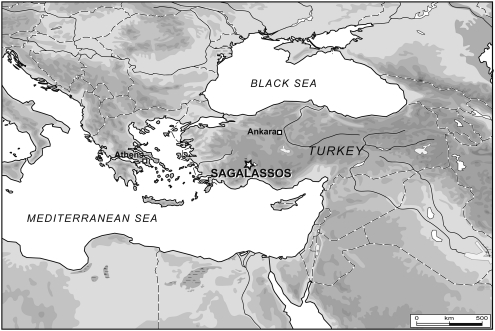
The Classical to early Byzantine archaeological site of Sagalassos is located in the Taurus Mountains of Southwestern Turkey at an altitude of around 1500 m (Figure 1). The excavations of this site revealed a city with monumental architecture and several necropoles, which were given up during the 13th century AD.11
Figure 1.
Geographic location of Sagalassos.
This work analysed 85 individuals excavated from two different locations: 57 skeletons were recovered from pits in the context of a slope composed of earthquake debris caused by a seismic event in the late 6th century AD, flanking the area of the ‘Lower Agora', whereas 28 individuals were found in a neighbouring churchyard, within the adjoining ‘Apollo Klarios Shrine'.11 The archaeological context pointed to low social status burials that followed the Christian tradition (see Supplementary Text).
All graves have been dated to the 11th–13th century cal AD by AMS carbon-14 dating of human bones (Analytic Inc. http://www.radiocarbon.com) and/or from the stratigraphical sequencing coupled with the general historical reconstruction of the site. DNA extractions were made from more than 169 bone and tooth samples from the 85 skeletons.
Contamination avoidance
Genetic analyses were performed in the ancient DNA (aDNA) facilities of the Laboratory of Forensic Genetics and Molecular Archaeology in Leuven. Standard contamination precautions as previously described in literature were employed for the aDNA analysis,27, 28, 29 amongst which: pre- and post-PCR procedures carried out in physically separated laboratories, restricted and controlled access to the pre-PCR laboratory, mock-extraction controls in the DNA extractions and amplification reactions, independent replication of data, analysis of associated fauna remains. More details about contamination avoidance procedures are reported as Supplementary Text.
DNA extraction and PCR amplifications
After bone and tooth samples decontamination and pulverization, aliquots of 0.5–1 g powder were digested in a Proteinase-K lysis buffer and DNA was extracted through silica-based spin columns.30 Analysis of the first and the second hypervariable segments (HVS-I and HVS-II) of the mtDNA control region was accomplished by amplifying and direct sequencing, respectively, five and two overlapping fragments (ranging from 109–166 bp in size), in order to read, respectively, 357 bp, from nucleotide position (np) 16009–16365, and 217 bp, from np 49–265. Furthermore, a selection of samples was used for cloning of the PCR products (Supplementary Table 11). This allowed us to confirm the results obtained by direct sequencing and to have an indication about post-mortem DNA damage in the samples (Supplementary Text). Eight diagnostic markers in the mtDNA coding region were selected and analysed by means of multiplex amplification and minisequencing reactions in order to confirm and in some instances to resolve the haplogroup classification. List of primers used and details about the amplification, sequencing and minisequencing reactions are reported in Supplementary Table 1 and Supplementary Text.
Data analysis
On the basis of the HVS-I/HVS-II haplotype and the SNPs in the mtDNA coding region, each individual was assigned to a haplogroup according to the latest mtDNA phylogeny31 (http://www.phylotree.org/). Comparative surveys with mtDNA data of up to 157 African and Eurasian populations reported in literature (Supplementary Tables 2,3,4, Supplementary Text) were done in order to detect the genetic signature left by the populations considered descendant of those which, from historical documents and archaeological evidence, may have contributed culturally and/or demographically to the ethnogenesis of the Sagalassos population since the Bronze Age.11, 16, 32 Search of haplotypes shared and analysis of Fst genetic distances based on the HVS-I and HVS-II variation were carried out in order to infer relationship between Sagalassos and the comparative populations (ARLEQUIN 3.11 software, http://cmpg.unibe.ch/software/arlequin/). The statistical significance of Fst values was estimated by permutation analysis, using 10 000 permutations. The pattern of genetic differentiation was visualised by MDS (XLstat v.7.5.2, http://www.xlstat.com/en/home/) and geographical interpolation method (Surfer v.8.0, Golden Software Inc., 2002, Golden, CO, USA). Furthermore, a Principal Component Analysis (PCA) was carried out on the basis of haplogroup frequencies from 80 Eurasian populations present in literature (Statistica v.8, StatSoft Inc., Tulsa, OK, USA). See Supplementary Text for detailed method.
Results
Even when applying strict precautions, contamination from modern human DNA cannot yet be totally ruled out,29 and a strong logical chain of evidence is required to authenticate aDNA results.33 In this study, on the basis of the procedures followed and the criteria used to assess the reliability of results, we could exclude contamination with a high level of confidence and attest to the authenticity of our aDNA data (Supplementary Text).
Genetic diversity and kinship
HVS-I and HVS-II sequences were determined in 53 out of the total 85 initial individuals (62%). Analysis of SNPs in the coding region of the mtDNA was done in a selection of samples, and haplogroup affiliation could be ascertained in all the individuals (Supplementary Table 5). A total of 10 haplotypes were shared between two or more individuals. On the basis of the burial location in the cemetery, we assumed a putative maternal relationship in two couples of individuals buried in relatively close graves (SA2005 AK41a, SA2005 AK41b2 and SA2005 AK41b, SA2005 AK41c; see Supplementary Text).
After excluding the related individuals, the final Sagalassos dataset encompassed 51 individuals, revealing 39 different haplotypes. This accounts for the fairly high haplotype diversity observed in Sagalassos (H=0.989±0.006).
All Sagalassos sequences fall into a set of haplogroups within macrohaplogroups N (X, W and N1b) and R (R0a, H, V, HV, U, K, J and T) that are characteristic of West Eurasians. None of the sequences from Sagalassos belong to the East/South Asian macrohaplogroup M and subSaharan haplogroups (L1, L2 and L3A).
Population comparisons
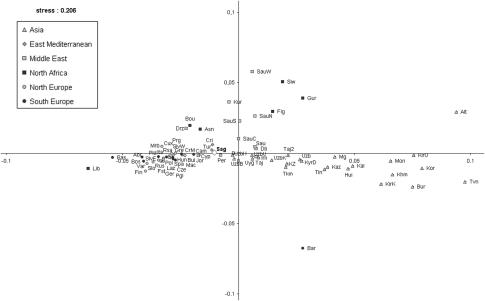
To infer phylogenetic relationships between the Sagalassos population and 76 Eurasian and African populations, we performed a non-metric multidimensional scaling (MDS) analysis based on the Fst distance matrix calculated from the HVS-I/HVS-II haplotypes (Supplementary Table 6). The results of the MDS (Figure 2) suggest that geography can explain the grouping of populations on the two-dimensional plot. Sagalassos is located very close to the samples from Turkey, Crimea and Iran,34, 35, 36 which present a nonsignificant differentiation with Sagalassos (Fst<0.01) (Supplementary Table 8) and their position in the middle of the plot reflects a geographic bridge between Asia and Europe. Regarding Asian populations, a genetic proximity in the MDS plot of some Uzbek samples (Fst<0.01) to Sagalassos has been observed.
Figure 2.
Two-dimensional MDS plot of pairwise Fst values from HVS-I/HVS-II showing relationships among the 77 populations from Eurasia and Africa. The stress value for the MDS plot is 0.204. White diamond represents the Byzantine Sagalassos population. Population codes and the matrix of Fst values are reported in Supplementary Tables 3 and 6.
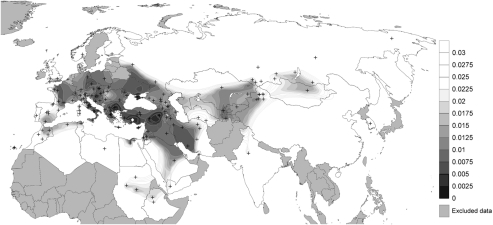
The maternal gene pool of Sagalassos was then compared with a higher number of populations (ie 157 populations) but from a shorter mtDNA fragment (HVS-I) and the Fst values obtained (Supplementary Table 7) have been graphically plotted on a map of Eurasia and North Africa (Figure 3). Results are consistent with those from the HVS-I/HVS-II comparisons, showing the genetic proximity of Sagalassos with its neighbouring populations, particularly some Eastern Mediterranean groups (Turkey/Anatolia), the Balkans (mainland Greece, Bulgaria and Croatia) and Crimea.20, 34, 37, 38, 39
Figure 3.
Spatial distribution of the Fst values calculated from the pairwise comparison of the Sagalassos mtDNAs (HVS-I) with those of 157 populations from Eurasia and North Africa (Supplementary Table 2). Fst values as reported in the Supplementary Table 7, follow the gray scale. Black crosses indicate the geographic location of the populations surveyed.
Overall, it is remarkable that the Fst values point to a proximity of Sagalassos with the samples from Turkey, Crimea, Iran and Italy (Campania and Puglia), Cyprus and the Balkans (Bulgaria, Croatia and Greece), which show the lowest nonsignificant Fst values (Fst<0.005, Supplementary Table 8).
Haplogroup composition in Sagalassos was investigated by means of the PCA (Supplementary Table 9). In the two-dimensional plot (Supplementary Figure S1), which explains 32% of the total variation, Sagalassos is located in a marginal position, close to a cluster of populations from the Caucasus (the Georgian sample being the closest to Sagalassos), the East Mediterranean (Cyprus and Rhodes) and the samples from the Near/Middle East. In this pool of populations, contribution of haplogroups U3, N1, U1 and HV to the first and second PC was found to be particularly high (Supplementary Figure S2).
A total of 20 HVS-I/HVS-II haplotypes from Sagalassos were found to match modern sequences (Supplementary Table 10 and Supplementary Text). In agreement with the results of the Fst-based analyses, we detected relevant haplotype matches with the populations that historically may have contributed to the makeup of the Sagalassos mtDNA pool, particularly the Balkan area (eg Northern Greece, Macedonia, Bosnia and Bulgaria), Italy and Iran. Of particular note is the presence of haplotype LA35 exclusively in Macedonia (frequency 0.5%), and AK41a and LA20 exclusively in Italy (both at 0.25%), as well as the high frequency of AK15a in Iran (15%). Shared haplotypes were also found in the Near/Middle East (eg AK62 and AK56-69_2, 2.33% in Saudi Arabia), in particular LA54 found only in Jordan (1%). Finally, two matches with Central Asian samples, one with Tajiks (the haplotype AK15a, assigned to haplogroup N1b) and one with Uygurs (AK60, assigned to haplogroup T2b) were detected.
Discussion
The high rate of successful genetic analyses (ie 62% of the total individuals sampled) indicates the good state of preservation of the aDNA in the Byzantine samples from Sagalassos. This is likely related to the high altitude of the archaeological site (ie, approximately 1500 m), which provides suitable thermal conditions for the preservation of DNA even at the low latitudes of South-West Turkey. Among the samples resulting in unsuccessful analyses, a large fraction (91%) is from the Lower Agora site. This site was excavated 10–15 years ago, and differently from Apollo Klarios, skeletons from the Lower Agora were unearthed without the necessary precautions for avoiding contamination, this confirming how crucial the post-excavations procedures are for aDNA investigations.40
Within the context described in the introduction, the mtDNA pool of the Byzantine population of Sagalassos brings new elements to estimate the genetic signature associated to the historic/demographic events, which took place in this region since the Bronze Age. The results of our study point to a West-Eurasian composition of the mtDNA gene pool of the 11th–13th-century Byzantine population. This genetic pattern is in agreement with results from previous studies investigating Anatolian/Turkish populations history and obtained from different biological markers, eg proteins, Alu sequences, mtDNA, Y chromosome and morphological data.2, 20, 21, 26, 34, 41, 42
More particularly, a genetic affinity of Sagalassos with Anatolian and Balkan populations has been revealed by our study (Figure 2, Figure 3, Supplementary Tables 8 and 10), suggesting a relatively similar background of the maternal gene pool in these populations. The affinity with current Anatolian populations, suggests that the same historic and demographic events that shaped the mtDNA pool of Sagalassos, might have left a genetic signature in the modern Turkish populations.
The genetic proximity with Balkan populations may probably be the result of the long-term interactions between Anatolia and Balkan regions since at the latest the Early Iron Age, as attested by linguistic, archaeological, mythological and biological studies.9, 10, 26, 32 This might be a genetic signature of the Phrygians originating from the Balkans, who filled the political hiatus in Central Anatolia left by the Hittites from the 10th century BC onwards. Yet, it is uncertain whether domination of the Phrygians ever extended as far as Sagalassos, although Phrygian influences are present in its territory. Most likely, the Balkan genetic signature might date back to the 2nd–3rd century BC, when the Macedonian Seleucid dynasty ruling over Pisidia enlisted local mercenaries in their armies, this leading to mixed marriages. Just north of Sagalassos, which seemingly also received Macedonian settlers, a whole series of Seleucid colonies populated by Macedonian veterans and Jews were created.43
Our analyses also detected an affinity between Sagalassos and populations from Iran and the Italian peninsula. Within the historical context described above, this pattern may result from historical events that facilitated the installation of Persian and Roman settlers. When ruling over Sagalassos, the Persian Achaemenids (546-333/332 BC) enlisted many Pisidians in their armies, but also acquired estates near the city of Sagalassos and even must have had settlers in the city itself. As for the Italian affinity, this can easily be explained by the fact that Augustus, after his confiscation of the Kingdom of Galatia, to which Sagalassos had belonged from 39 to 25 BC, established at least eight colonies of Roman veterans from Italy within a radius of a 100 km around Sagalassos.16
A Levantine component has been also detected in Sagalassos, which is likely related to the high frequencies of haplogroups U3 (5.66%) and N1 (11.32%) (Supplementary Table 9, Supplementary Figures S1 and S2). This result may be explained by an intense trade system that was established with the Levantine populations from Archaic, over Hellenistic and Roman Imperial to Early Byzantine time,44 when Southwest Anatolia formed a kind of cultural koinè in its material culture with Western Cyprus, Pamphylia and the Levant itself. This is further supported by the proximity of the sample from Cyprus in the PCA (Supplementary Figure S1).
As an alternative scenario, it should be mentioned that the introduction of Levantine mtDNAs may result from the allocation of Pisidian mercenaries by the Macedonian dynasty of the Ptolemies in the Levantine coasts, where they may have married local women with whom they eventually returned to their home cities.45
It is worth noting that as far as the U3 lineages are concerned, the hypothesis of a Caucasian origin cannot be totally ruled out.6, 46 The presence of this haplogroup in the Sagalassos territory might represent an ancient genetic legacy of the Indo-European tribes from Trans-Caucasus who occupied Anatolia at the latest around the beginning of the 2nd millennium BC. In particular, the Hittites and the Luwians settled in Central and Southern Anatolia, whereby the territory of Sagalassos became part of a Luwian state.14
Overall, the analyses that we performed point to the absence of a significant Central Asian genetic signature in Sagalassos, at least at the maternal side. With this regard, the apparent affinity detected with some Central Asian populations (eg, the Uzbeks in Figure 2, Turkmen and Kurds in Supplementary Figure S1, Tajiks and Uygurs in Supplementary Table 10) likely result from the high frequencies of West-Eurasian specific haplogroup in these samples (up to 42% in Uygurs and Uzbeks47). As far as evidenced by the archaeological data, presence of the Seljuk Turks, a nomadic tribe from Central Asia that invaded Anatolia in the late 11th century AD, is not attested in Sagalassos before the first half of the 13th century AD, when they built a hamam and according to Arabic sources also a caravansary in the neighbour site of Ağlasun.48 Even if it is not excluded that they had already occupied part of the former territory of Sagalassos before, the 11th–13th century AD cemetery, which is analysed here shows a clearly homogeneous Christian population. Remarkably, a 12th–13th century AD fortified site on the southern part of Sagalassos was destroyed and erased by the Seljuks in the course of the 13th century AD, making a definitive end to the occupation of the site and showing that the relations between the two population groups seem to have been hostile.
In conclusion, despite the limitations of investigating a single maternally inherited locus, and the impossibility to perform statistical methods to test genealogical relationships between past and present populations (within the framework of the Sagalassos project, research is restricted to the period preceding the capture of Constantinople by the Ottoman Turks in 1453 AD), our study provides new clues to pinpoint the impact of historical events, and correspondingly of the related population groups, on the maternal gene pool of the Byzantine population from Sagalassos.
The analysis of the Fst genetic distances and the shared haplotypes highlighted a genetic proximity of Sagalassos with populations from the Balkan and Italian peninsulas and the Levant. Notably, no signature from Central Asia has been detected, whereas an ancient genetic legacy of the Indo-European tribes from Trans-Caucasus who occupied Anatolia at the beginning of the 2nd millennium BC cannot be excluded. Whether this relatively good agreement between female history (mtDNA) and historical/archaeological records is also found in the paternal lineages, will have to be explored through investigation of Y-chromosome markers.
Acknowledgments
We thank Maarten Larmuseau for critical reading and comments on earlier drafts of the present manuscript. This research was supported by the Belgian Programme on Interuniversity Poles of Attraction (IAP 6/22) and the Research Fund of the K.U.Leuven (BOF-GOA 07/02), next to projects G.0286.00 and G.0421.06 of the Fund for Scientific Research, Flanders (FWO). M Waelkens is one of the people who received a ‘Methusalem grant' from the Flemish Ministry for Science Policy. The mtDNA sequences reported in this paper have been deposited in GenBank (Accession Numbers: HQ007946–HQ008051).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Kuhn S. Paleolithic archeology in Turkey. Paleolithic archeology in Turkey. 2002;11:198–210. [Google Scholar]
- Cinnioglu C, King R, Kivisild T, et al. Excavating Y-chromosome haplotype strata in Anatolia. Hum Genet. 2004;114:127–148. doi: 10.1007/s00439-003-1031-4. [DOI] [PubMed] [Google Scholar]
- Vermeersch P, Öztürk I, Ekinci H, et al. Late Palaeolithic at the Dereköy Karain Cavein Waelkens M, Poblome J (eds): Sagalassos V Report on the Survey and Excavation Campaigns of 1996 and 1997 Leuven: Leuven University Press; 2000451–462. [Google Scholar]
- Ammerman A, Cavalli-Sforza LL. The Neolithic transition and genetics of populations in Europe. Princeton, NJ: Princeton University Press; 1984. [Google Scholar]
- Renfrew C.Archaeogenetics: Towards a population prehistory of Europein Renfrew C, Boyle K (eds): Archaeogenetic: DNA and the Population Prehistory of Europe Cambridge, UK: McDonald Institute for Archaeological Research; 2000 [Google Scholar]
- Richards M, Macaulay V, Hickey E, et al. Tracing European founder lineages in the Near Eastern mtDNA pool. Am J Hum Genet. 2000;67:1251–1276. [PMC free article] [PubMed] [Google Scholar]
- Bellwood R, Renfrew C. Examining the Farming/Language Dispersal Hypothesis. Cambridge, UK: McDonald Institute for Archaeological Research; 2002. [Google Scholar]
- Zeder MA. Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact. Proc Natl Acad Sci USA. 2008;105:11597–11604. doi: 10.1073/pnas.0801317105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards IES, Gadd CJ, Hammond NGL, Sollberger E.The Middle East and the Aegean Region, c.1800–1380 BC3 edn, Vol. 2.Cambridge, UK: Cambridge University Press; 2000 [Google Scholar]
- Sahoglu V.The Anatolian Trade Network and the Izmir region During the Early Bronze AgeVol. 24.Oxford, ROYAUME-UNI: Blackwell; 2005 [Google Scholar]
- Waelkens M, Vanhaverbeke H, Martens F, et al. The late antique to early Byzantine city in Southwest Anatolia. Sagalassos and its territory: a case studyin J. K, C. W (eds): Die Stadt in der Spätantike—Niedergang oder Wandel? Stuttgart; 2006. Vol 190, pp199–255. [Google Scholar]
- King RJ, Ozcan SS, Carter T, et al. Differential Y-chromosome Anatolian influences on the Greek and Cretan Neolithic. Ann Hum Genet. 2008;72:205–214. doi: 10.1111/j.1469-1809.2007.00414.x. [DOI] [PubMed] [Google Scholar]
- Cruciani F, La Fratta R, Trombetta B, et al. Tracing past human male movements in northern/eastern Africa and western Eurasia: new clues from Y-chromosomal haplogroups E-M78 and J-M12. Mol Biol Evol. 2007;24:1300–1311. doi: 10.1093/molbev/msm049. [DOI] [PubMed] [Google Scholar]
- Waelkens M.Sagalassos and Pisidia during the Late Bronze Agein Waelkens M, Loots L (eds): Sagalassos V: report on the survey and excavation campaigns of 1996 and 1997 Leuven: Leuven university press; 2000509–549. [Google Scholar]
- Oettinger R.Indo-germanische Sprachträger lebten schon im 3. Jahrtausend v. ChrIn Kleinasien (ed): Die Ausbildung der anatolischen Sprachen: Die Hethiter und ihr Reich Volk der 1000 Götter Bonn; 200250–55. [Google Scholar]
- Waelkens M. Romanization in the East: A case study. Sagalassos and Pisidia (SW Turkey) Istanbuler Mitteilungen. 2002;52:311–368. [Google Scholar]
- Waelkens M. Ein Blick von der Ferne: Seleukiden und Attaliden in Pisidien. Istanbuler Mitteilungen. 2004;54:435–471. [Google Scholar]
- Russell J. Late ancient and medieval population. Transactions of the American Philosophical Society. 1958;48:1–152. [Google Scholar]
- Mitchell S. Anatolia:land, men, and gods in Asia Minor I, The Celts in Anatolia and the Impact of Roman Rule. New York: Oxford University Press; 1994. [Google Scholar]
- Di Benedetto G, Erguven A, Stenico M, et al. DNA diversity and population admixture in Anatolia. Am J Phys Anthropol. 2001;115:144–156. doi: 10.1002/ajpa.1064. [DOI] [PubMed] [Google Scholar]
- Berkman CC, Dinc H, Sekeryapan C, Togan I. Alu insertion polymorphisms and an assessment of the genetic contribution of Central Asia to Anatolia with respect to the Balkans. Am J Phys Anthropol. 2008;136:11–18. doi: 10.1002/ajpa.20772. [DOI] [PubMed] [Google Scholar]
- Zalloua PA, Platt DE, El Sibai M, et al. Identifying genetic traces of historical expansions: Phoenician footprints in the Mediterranean. Am J Hum Genet. 2008;83:633–642. doi: 10.1016/j.ajhg.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalloua PA, Xue YL, Khalife J, et al. Y-chromosomal diversity in Lebanon is structured by recent historical events. Am J Hum Genet. 2008;82:873–882. doi: 10.1016/j.ajhg.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sibai M, Platt DE, Haber M, et al. Geographical structure of the Y-chromosomal genetic landscape of the Levant: a coastal-inland contrast. Ann Hum Genet. 2009;73:568–581. doi: 10.1111/j.1469-1809.2009.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelkens M, Paulissen E, Vermoere M, et al. Man and environment in the territory of Sagalassos, a classical city in SW Turkey. Quaternary science reviews. 1999;18:697–709. [Google Scholar]
- Ricaut FX, Waelkens M. Cranial discrete traits in a Byzantine population and eastern Mediterranean population movements. Hum Biol. 2008;80:535–564. doi: 10.3378/1534-6617-80.5.535. [DOI] [PubMed] [Google Scholar]
- Cooper A, Poinar HN. Ancient DNA: do it right or not at all. Science. 2000;289:1139. doi: 10.1126/science.289.5482.1139b. [DOI] [PubMed] [Google Scholar]
- Hofreiter M, Serre D, Poinar HN, Kuch M, Pääbo S. Ancient DNA. Nat Rev Genet. 2001;2:353–359. doi: 10.1038/35072071. [DOI] [PubMed] [Google Scholar]
- Pääbo S, Poinar H, Serre D, et al. Genetic analyses from ancient DNA. Annu Rev Genet. 2004;38:645–679. doi: 10.1146/annurev.genet.37.110801.143214. [DOI] [PubMed] [Google Scholar]
- Yang DY, Eng B, Waye JS, Dudar JC, Saunders SR. Improved DNA extraction from ancient bones using silica-based spin columns. Am J Phys Anthropol. 1998;105:539–543. doi: 10.1002/(SICI)1096-8644(199804)105:4<539::AID-AJPA10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Van Oven M, Kayser M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum Mutat. 2009;30:E386–E394. doi: 10.1002/humu.20921. [DOI] [PubMed] [Google Scholar]
- Vanhaverbeke H, Waelkens M.(eds): The Chora of Sagalassos: The Evolution of the Settlement Pattern on the Territory of Sagalassos (Pisidia, SW Turkey) from Prehistory Until Recent Times Turnhout, Belgium: Brepols; 2003 [Google Scholar]
- Gilbert MTP, Bandelt H-J, Hofreiter M, Barnes I. Assessing ancient DNA studies. Trends Ecol Evol. 2005;20:541–544. doi: 10.1016/j.tree.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Calafell F, Underhill P, Tolun A, Angelicheva D, Kalaydjieva L. From Asia to Europe: mitochondrial DNA sequence variability in Bulgarians and Turks. Ann Hum Genet. 1996;60:35–49. doi: 10.1111/j.1469-1809.1996.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Comas D, Plaza S, Wells RS, et al. Admixture, migrations, and dispersals in Central Asia: evidence from maternal DNA lineages. Eur J Hum Genet. 2004;12:95–504. doi: 10.1038/sj.ejhg.5201160. [DOI] [PubMed] [Google Scholar]
- Derenko M, Malyarchuk B, Grzybowski T, et al. Phylogeographic analysis of mitochondrial DNA in northern Asian populations. Am J Hum Genet. 2007;81:1025–1041. doi: 10.1086/522933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster P, Cali F, Rohl A, et al. Continental and subcontinental distributions of mtDNA control region types. Int J Legal Med. 2002;116:99–108. doi: 10.1007/s00414-001-0261-z. [DOI] [PubMed] [Google Scholar]
- Kouvatsi A, Karaiskou N, Apostolidis A, Kirmizidis G. Mitochondrial DNA sequence variation in Greeks. Hum Biol. 2001;73:855–869. doi: 10.1353/hub.2001.0085. [DOI] [PubMed] [Google Scholar]
- Quintana-Murci L, Chaix R, Wells RS, et al. Where west meets east: the complex mtDNA landscape of the southwest and Central Asian corridor. Am J Hum Genet. 2004;74:827–845. doi: 10.1086/383236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruvost M, Schwarz R, Correia VB, et al. Freshly excavated fossil bones are best for amplification of ancient DNA. Proc Natl Acad Sci USA. 2007;104:739–744. doi: 10.1073/pnas.0610257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brega A, Scacchi R, Cuccia M, Kirdar B, Peloso G, Corbo RM. Study of 15 protein polymorphisms in a sample of the Turkish population. Hum Biol. 1998;70:715–728. [PubMed] [Google Scholar]
- Tambets K, Kivisild T, Metspalu E, et al. The topology of the maternal lineages of the Anatolian and Trans-Caucasus populations and the peopling of Europe: Some preliminary considerationsin: Renfrew C, Boyle K (eds): Archaeogenetic: DNA and the Population Prehistory of Europe Cambridge, UK: McDonald Institute for Archaeological Research; 2000219–236. [Google Scholar]
- Kosmetatou E, Waelkens M.The ‘Macedonian Shields of Sagalassos', In Sagalassos IV. Report of the Survey and Excavation Campaigns of 1994 and 1995in: Waelkens M, Poblome J (eds).Leuven: Leuven University Press; 1997 [Google Scholar]
- Arndt A, Van Neer W, Hellemans B, Robben J, Volckaert F, Waelkens M. Roman trade relationships at Sagalassos (Turkey) elucidated by ancient DNA of fish remains. J Archaeol Sci. 2003;30:1095–1105. [Google Scholar]
- Bracke H.Pisidia in Hellenistic times (334-25 B.C.)in: Waelkens M (ed): Sagalassos I. First General Report on the Survey (1986–1989) and Excavations (1990–1991) Leuven: Leuven University Press; 199315–29. [Google Scholar]
- Bermisheva MA, Kutuev IA, Korshunova T, Dubova NA, Villems R, Khusnutdinova EK. Phylogeografic analysis of mitochondrial DNA Nogays: the high level of mixture of maternal lineages from Eastern and Western Eurasia. Mol Biol (Mosk) 2004;38:617–624. [PubMed] [Google Scholar]
- Yao YG, Kong QP, Wang CY, Zhu CL, Zhang YP. Different matrilineal contributions to genetic structure of ethnic groups in the silk road region in China. Mol Biol Evol. 2004;21:2265–2280. doi: 10.1093/molbev/msh238. [DOI] [PubMed] [Google Scholar]
- Vanhaverbeke H, Başağaç Ö, Paul K. A Selçuk Hamam at Ağlasun (Burdur province) Turcica. 2005;37:309–336. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.