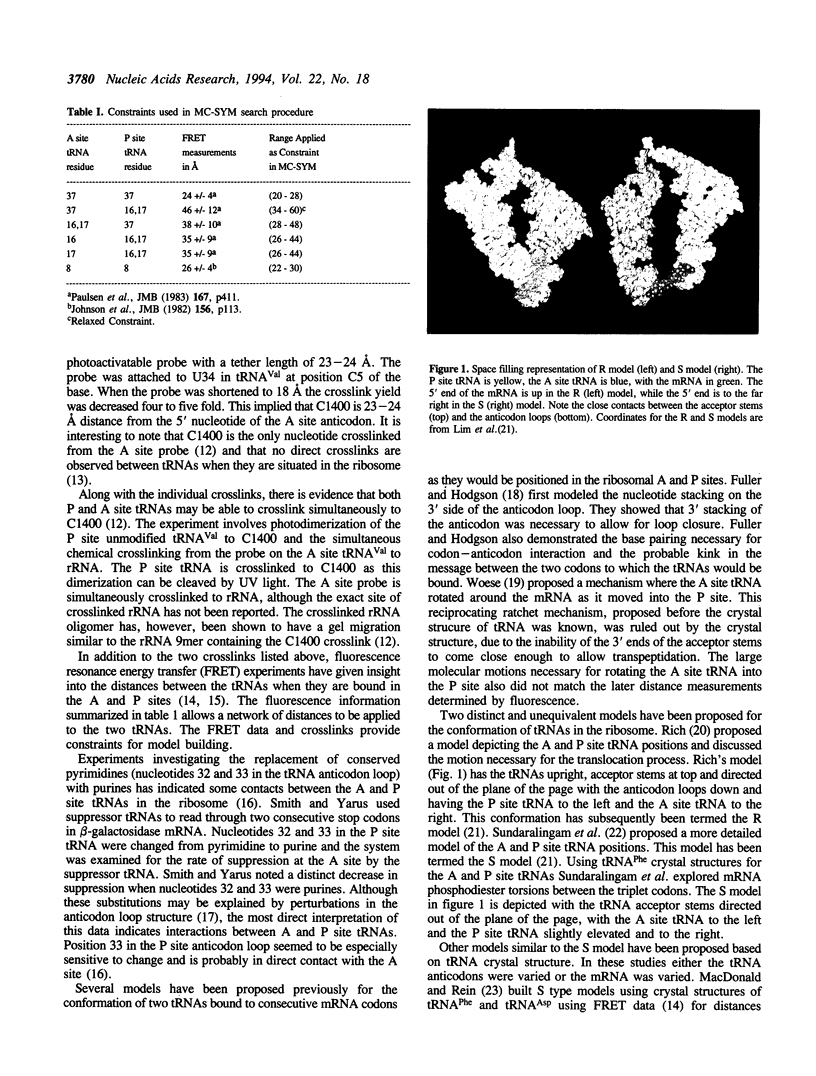
Abstract
In protein synthesis, peptide bond formation requires that the tRNA carrying the amino acid (A site tRNA) contact the tRNA carrying the growing peptide chain (P site tRNA) at their 3' termini. Two models have been proposed for the orientations of two tRNAs as they would be bound to the mRNA in the ribosome. Viewing the tRNA as an upside down L, anticodon loop pointing down, acceptor stem pointing right, and calling this the front view, the R (Rich) model would have the back of the P site tRNA facing the front of the A site tRNA. In the S (Sundaralingam) model the front of the P site tRNA faces the back of the A site tRNA. Models of two tRNAs bound to mRNA as they would be positioned in the ribosomal A and P sites have been created using MC-SYM, a constraint satisfaction search program designed to build nucleic acid structures. The models incorporate information from fluorescence energy transfer experiments and chemical crosslinks. The models that best answer the constraints are of the S variety, with no R conformations produced consistent with the constraints.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. S., Dewan J. C., Klug A. Crystallographic and biochemical investigation of the lead(II)-catalyzed hydrolysis of yeast phenylalanine tRNA. Biochemistry. 1985 Aug 27;24(18):4785–4801. doi: 10.1021/bi00339a012. [DOI] [PubMed] [Google Scholar]
- Ciesiolka J., Gornicki P., Ofengand J. Identification of the site of cross-linking in 16S rRNA of an aromatic azide photoaffinity probe attached to the 5'-anticodon base of A site bound tRNA. Biochemistry. 1985 Aug 27;24(18):4931–4938. doi: 10.1021/bi00339a031. [DOI] [PubMed] [Google Scholar]
- Döring T., Mitchell P., Osswald M., Bochkariov D., Brimacombe R. The decoding region of 16S RNA; a cross-linking study of the ribosomal A, P and E sites using tRNA derivatized at position 32 in the anticodon loop. EMBO J. 1994 Jun 1;13(11):2677–2685. doi: 10.1002/j.1460-2075.1994.tb06558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough R. H., Cantor C. R., Wintermeyer W., Zachau H. G. Fluorescence studies of the binding of a yeast tRNAPhe derivative to Escherichia coli ribosomes. J Mol Biol. 1979 Aug 25;132(4):557–573. doi: 10.1016/0022-2836(79)90374-7. [DOI] [PubMed] [Google Scholar]
- Fuller W., Hodgson A. Conformation of the anticodon loop intRNA. Nature. 1967 Aug 19;215(5103):817–821. doi: 10.1038/215817a0. [DOI] [PubMed] [Google Scholar]
- Gautheret D., Major F., Cedergren R. Modeling the three-dimensional structure of RNA using discrete nucleotide conformational sets. J Mol Biol. 1993 Feb 20;229(4):1049–1064. doi: 10.1006/jmbi.1993.1104. [DOI] [PubMed] [Google Scholar]
- Gornicki P., Ciesiolka J., Ofengand J. Cross-linking of the anticodon of P and A site bound tRNAs to the ribosome via aromatic azides of variable length: involvement of 16S rRNA at the A site. Biochemistry. 1985 Aug 27;24(18):4924–4930. doi: 10.1021/bi00339a030. [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Adkins H. J., Matthews E. A., Cantor C. R. Distance moved by transfer RNA during translocation from the A site to the P site on the ribosome. J Mol Biol. 1982 Mar 25;156(1):113–140. doi: 10.1016/0022-2836(82)90462-4. [DOI] [PubMed] [Google Scholar]
- Lim V., Venclovas C., Spirin A., Brimacombe R., Mitchell P., Müller F. How are tRNAs and mRNA arranged in the ribosome? An attempt to correlate the stereochemistry of the tRNA-mRNA interaction with constraints imposed by the ribosomal topography. Nucleic Acids Res. 1992 Jun 11;20(11):2627–2637. doi: 10.1093/nar/20.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig F., Borén T., Claesson C., Simonsson C., Barciszewska M., Lagerkvist U. The nucleotide in position 32 of the tRNA anticodon loop determines ability of anticodon UCC to discriminate among glycine codons. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3343–3347. doi: 10.1073/pnas.90.8.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major F., Gautheret D., Cedergren R. Reproducing the three-dimensional structure of a tRNA molecule from structural constraints. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9408–9412. doi: 10.1073/pnas.90.20.9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major F., Turcotte M., Gautheret D., Lapalme G., Fillion E., Cedergren R. The combination of symbolic and numerical computation for three-dimensional modeling of RNA. Science. 1991 Sep 13;253(5025):1255–1260. doi: 10.1126/science.1716375. [DOI] [PubMed] [Google Scholar]
- Matzke A. J., Barta A., Kuechler E. Mechanism of translocation: relative arrangement of tRNA and mRNA on the ribosome. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5110–5114. doi: 10.1073/pnas.77.9.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. J., Rein R. A stereochemical model of the transpeptidation complex. J Biomol Struct Dyn. 1987 Apr;4(5):729–744. doi: 10.1080/07391102.1987.10507675. [DOI] [PubMed] [Google Scholar]
- Nierhaus K. H. Solution of the ribosome riddle: how the ribosome selects the correct aminoacyl-tRNA out of 41 similar contestants. Mol Microbiol. 1993 Aug;9(4):661–669. doi: 10.1111/j.1365-2958.1993.tb01726.x. [DOI] [PubMed] [Google Scholar]
- Nierhaus K. H. The allosteric three-site model for the ribosomal elongation cycle: features and future. Biochemistry. 1990 May 29;29(21):4997–5008. doi: 10.1021/bi00473a001. [DOI] [PubMed] [Google Scholar]
- Odom O. W., Craig B. B., Hardesty B. A. The conformation of the anticodon loop of yeast tRNAPhe in solution and on ribosomes. Biopolymers. 1978 Dec;17(12):2909–2931. doi: 10.1002/bip.1978.360171212. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Liou R., Kohut J., 3rd, Schwartz I., Zimmermann R. A. Covalent cross-linking of transfer ribonucleic acid to the ribosomal P site. Mechanism and site of reaction in transfer ribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4322–4332. doi: 10.1021/bi00587a010. [DOI] [PubMed] [Google Scholar]
- Paulsen H., Robertson J. M., Wintermeyer W. Topological arrangement of two transfer RNAs on the ribosome. Fluorescence energy transfer measurements between A and P site-bound tRNAphe. J Mol Biol. 1983 Jun 25;167(2):411–426. doi: 10.1016/s0022-2836(83)80342-8. [DOI] [PubMed] [Google Scholar]
- Prabahakaran M., Harvey S. C. Models for two tRNAs bound to successive codons on mRNA on the ribosome. J Biomol Struct Dyn. 1989 Aug;7(1):167–179. doi: 10.1080/07391102.1989.10507758. [DOI] [PubMed] [Google Scholar]
- Prince J. B., Taylor B. H., Thurlow D. L., Ofengand J., Zimmermann R. A. Covalent crosslinking of tRNA1Val to 16S RNA at the ribosomal P site: identification of crosslinked residues. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5450–5454. doi: 10.1073/pnas.79.18.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinberger H. J., Sternbach H., Nierhaus K. H. Three tRNA binding sites on Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5310–5314. doi: 10.1073/pnas.78.9.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D., Yarus M. tRNA-tRNA interactions within cellular ribosomes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4397–4401. doi: 10.1073/pnas.86.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner G., Lührmann R., Kuechler E. Crosslinking transfer RNA and messenger RNA at the ribosomal decoding region: identification of the site of reaction on the messenger RNA. Nucleic Acids Res. 1984 Nov 12;12(21):8181–8191. doi: 10.1093/nar/12.21.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. Molecular mechanics of translation: a reciprocating ratchet mechanism. Nature. 1970 May 30;226(5248):817–820. doi: 10.1038/226817a0. [DOI] [PubMed] [Google Scholar]