Abstract
In a French national cohort of unaffected females carriers/non-carriers of a BRCA1/2 mutation, long-term preventive strategies and breast/ovarian cancer risk perceptions were followed up to 5 years after test result disclosure, using self-administered questionnaires. Response rate was 74%. Carriers (N=101) were younger (average age±SD=37±10) than non-carriers (N=145; 42±12). There were four management strategies that comprised 88% of the decisions made by the unaffected carriers: 50% opted for breast surveillance alone, based on either magnetic resonance imaging (MRI) and other imaging (31%) or mammography alone (19%); 38% opted for either risk reducing salpingo-oophorectomy (RRSO) and breast surveillance, based on MRI and other imaging (28%) or mammography alone (10%). The other three strategies were: risk reducing mastectomy (RRM) and RRSO (5%), RRM alone (2%) and neither RRM/RRSO nor surveillance (6%). The results obtained for various age groups are presented here. Non-carriers often opted for screening despite their low cancer risk. Result disclosure increased carriers' short-term high breast/ovarian cancer risk perceptions (P⩽0.02) and decreased non-carriers' short- and long-term perceptions (P<0.001). During follow-up, high breast cancer risk perceptions increased with time among those who had no RRM and decreased in the opposite case; high ovarian cancer risk perceptions increased further with time among those who had no RRSO and decreased in the opposite case; RRSO did not affect breast cancer risk perceptions. Informed decision-making involves letting women know whether opting for RRSO and breast MRI surveillance is as effective in terms of survival as RRM and RRSO.
Keywords: risk perception, BRCA1/2 genetic testing, preventive management, breast/ovarian cancer
Introduction
Many surveys have addressed the short-term impact of BRCA1/2 genetic mutation testing on perceived risks and behavioural outcomes in heterogeneous samples.1, 2 In three prospective studies, unaffected women were followed for 2 years3, 4 and 3 years5 after disclosure of their test results in a routine context, and only one study has followed a selected sample of 23 women (21 of whom underwent risk reducing mastectomy (RRM)) up to 5 years.6 The largest samples of unaffected BRCA1/2 carriers studied so far included 53 (Foster et al5) and 68 women.3 In these studies, the behaviour of cohorts of female non-carriers from BRCA1/2 families was followed simultaneously. Another study was specifically designed to describe the behaviour of this group.7 A large Canadian survey retrospectively selected a large number of unaffected BRCA1/2 carriers (N=342) and followed their preventive behaviours until 4 years after their test results on average.8
A decrease in breast and ovarian cancer risk perception has been described in non-carriers 12 months after genetic testing, and a lower breast cancer risk perception in non-carriers compared with carriers. Only one study has shown that carriers' risk perceptions were higher 6 months after disclosure than before testing.9 It has been concluded that risk perception decreases with time.2
Behavioural outcomes have been described as rates of surveillance or prophylactic surgery undergone by carriers/non-carriers: RRM is undergone by most carriers in the Netherlands (55%) (Meijers-Heijboer et al3) and Denmark,10 in a third of the UK series studied (34%) (Foster et al5) and less frequently elsewhere.4, 8, 11 Although the rates of risk reducing salpingo-oophorectomy (RRSO) reported were higher, most of these studies made no reference to the age at which the intervention should be proposed according to current guidelines. Rates of compliance with mammographic surveillance have been studied in women who were screened for 2 years after test disclosure, but no information is available about the number of investigations carried out. Women's decision-making processes have been described individually but no information has been published so far about the follow-up of various age groups or about the whole series of decisions on which their medical management depends. In particular, the uptake of magnetic resonance imaging (MRI) by these cohorts as a means of surveillance has not yet been documented.
The aim of this study was first to describe the sequences of preventive decisions made by women up to 5 years after disclosure of their test results and the surveillance/surgical options chosen by various age groups. It was also proposed to examine the impact of BRCA1/2 genetic testing and preventive strategies on these women's cancer risk perceptions with time. Women included in this prospective national study were unaffected carriers/non-carriers who underwent a baseline assessment before test result disclosure and regular follow-up for the next 5 years. BRCA1/2 carriers' and non-carriers' long-term risk perceptions were expected to depend not only on their carrier/non-carrier status but also on the series of preventive strategies chosen.
Materials and methods
Population
In the framework of the ongoing French ‘Genepso' project managed by the French Cancer Genetic Network, BRCA1/2 carriers attending 29 participating centres were registered between 2000 and 2006. Only epidemiological data have been published so far on this cohort, although other studies are under way.12, 13, 14 In this study on ‘Psychosocial and Preventive Behaviour', non-carriers from families where these BRCA1/2 mutations had been identified were included along with carriers.
The behavioural characteristics of unaffected female carriers and non-carriers of the deleterious BRCA1/2 mutation were therefore compared. For this purpose, all the cases who reached the 5-year follow-up endpoint were selected. In France, the cost of cancer genetic consultations, BRCA1/2 testing and any subsequent medical management of people at risk is covered by the national healthcare system.
Questionnaires
Process
Consultees were asked to complete six self-administered questionnaires. The first questionnaire, which was designed to collect baseline information, was completed before disclosure of the BRCA1/2 genetic test results by all the women. The women who agreed to participate to the follow-up were then given a second questionnaire at disclosure, to be returned 15 days later. The four other questionnaires were mailed to the women's homes 6 months, 1 year, 2 years and 5 years later. If no answer had been received 1 month after mailing a questionnaire, a reminder letter and a copy of the questionnaire were sent out. All the questionnaires were mailed back to the coordinating centre. The procedure used was approved by the ‘Comité National Informatique et Libertés'.
The cancer geneticists also completed a questionnaire describing the women's family and medical characteristics at inclusion and follow-up.
Variables
Preventive behaviour
At the 5-year follow-up, information was collected about the occurrence, frequency and number of mammography, MRI, and ovarian ultrasound examinations undergone during the previous 3 years. The same questions were also asked about mammography and ovarian ultrasound at the 1- and 2-year follow-ups but the question about MRI was asked only at the 5-year follow-up, since the medical guidelines only recommended this type of investigation for the first time in 2004 (Eisinger et al15, 16). The occurrence of prophylactic surgery was cross-checked with the medical records. Details about prophylactic surgery (RRM and RRSO) were collected before disclosure of results and then again at the 1-, 2- and 5-year follow-ups. Baseline preventive behaviours had been asked in the pre-disclosure questionnaire.
Perception of personal risk of cancer
Perception of the personal risk of developing breast cancer was measured on a six-point Likert scale before disclosure and at follow-up by asking the same question: ‘Do you think your risk of developing breast cancer is: ‘very high', ‘high', ‘moderate', ‘low', ‘very low' and ‘don't know'. The same questions were asked about respondents' perception of their risk of developing ovarian cancer and about their perception of the risk of developing cancer of other kinds.
Sociodemographic, psychological and medical characteristics
Sociodemographic variables such as age, number of children, level of education, occupation, previous preventive behaviour and psychological variables were collected at baseline for all the women included and updated during follow-up.
The medical characteristics included the number of first- and second-degree relatives affected with breast/ovarian cancer at inclusion, and the deleterious BRCA1/2 mutation involved.
Statistical analysis
Chi-square tests, t-tests and ANOVA were used to make univariate comparisons on categorical and continuous variables. McNemar's χ2 tests were used to compare respondents' cancer risk perceptions at different times. The level of statistical significance (α-error) was taken to be P≤0.05.
Data analysis was conducted in three stages.
To check how representative the final sample was after 5 years, the respondents' and non-respondents' baseline sociodemographic, psychosocial and medical characteristics were compared.
The preventive strategies used by carriers/non-carriers were recorded at the 5-year follow-up. They are presented in a decision tree and the two groups were then compared after stratifying the results by age group. The sequences of preventive options chosen by BRCA1/2 carriers were analysed at the 5-year follow-up.
High/very high breast, ovarian and other cancer risk perceptions documented at various times were studied, depending on the preventive strategies chosen (or not) by carriers/non-carriers. High- and very high-risk perception levels were grouped together and denoted ‘high' perceptions.
The Statistical Package for Social Science (SPSS/PC released 17.0) was used to perform all the statistical analyses (SPSS Inc., Chicago, IL, USA).
Results
Respondents vs non-respondents
Among the 345 women who completed the first questionnaire before test result disclosure and who should have reached the 5 years follow-up endpoint, 14 were excluded because they had developed breast cancer (N=12) or ovarian cancer (N=2) by the fifth year of follow-up; all 14 were BRCA1/2 mutation carriers. Among the unaffected women at 5 years (N=331), 246 (74%) completed all five questionnaires and were included in this analysis.
Respondents and non-respondents did not differ significantly in their baseline sociodemographic, psychological or medical characteristics. However, respondents had reported doing breast self-examination significantly more frequently than non-respondents (P≤0.05) in the baseline questionnaire.
Sample characteristics
Among these 246 women, 41% (N=101) were carriers of a familial BRCA1/2 mutation, whereas 59% (N=145) were non-carriers. The mean age of the BRCA1/2 carriers at the time of disclosure was 37.2 years (SD=10.2), which differed significantly from that of the non-carriers (41.7±11.8) (±SD) (P=0.002). The level of education, marital status and number of children did not differ significantly between BRCA1/2 mutation carriers and non-carriers. Medical characteristics, such as the number of first-degree relatives affected were also similar between carriers and non-carriers. Respondents' characteristics are given in Table 1. The 246 respondents belonged to 166 different families: 68.1% (N=113) of these families were represented in the sample by a single woman, whereas the remaining, 31.9% (N=53) were represented by several members, numbering 2.5 on average (median 2; range 2–6). Among the 18 centres who reported carriers, 3 had reported 56.5% of the cases analysed (N=57) and 15 had reported the remaining 44.5% (N=44). The average number of carriers by centre was 5.6 (range 1–31).
Table 1. Baseline medical and sociodemographic characteristics of study population (N=246).
| Carriers (N=101) | Non-carriers (N=145) | ||||
|---|---|---|---|---|---|
| n | % | n | % | P* | |
| Having first-degree relatives with breast cancer (n=218) | |||||
| 0 | 29 | 30.9 | 35 | 28.2 | 0.212 |
| 1 | 50 | 53.2 | 57 | 46.0 | |
| ≥2 | 15 | 16.0 | 32 | 25.8 | |
| Having first-degree relatives with ovarian cancer (n=220) | |||||
| 0 | 58 | 61.1 | 73 | 58.4 | 0.691 |
| ≥1 | 37 | 39.0 | 52 | 41.6 | |
| Married (n=243) | |||||
| Yes | 58 | 58.6 | 97 | 67.4 | 0.162 |
| No | 41 | 41.4 | 47 | 32.6 | |
| Educational background (n=242) | |||||
| >High school | 59 | 58.4 | 74 | 52.5 | 0.360 |
| ≤High school | 42 | 41.6 | 67 | 47.5 | |
| Children (n=220) | |||||
| Yes | 67 | 81.7 | 110 | 79.7 | 0.718 |
| No | 15 | 18.3 | 28 | 20.3 | |
*χ2 test.
Proportions do not always add up to 100% because of rounding.
Preventive strategies in mutation carriers/non-carriers after disclosure of the BRCA1/2 test results
Carriers (N=101)
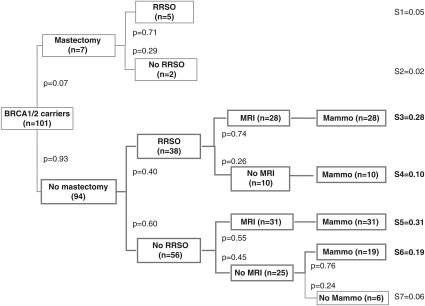
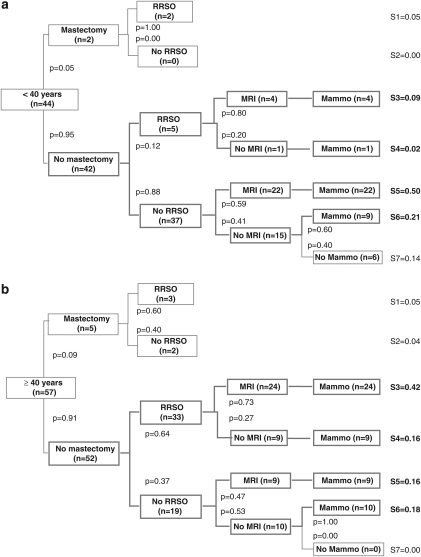
A decision tree was drawn up describing the seven strategies documented at the 5-year follow-up, including prophylactic surgery and breast imaging (Figure 1). As transvaginal ultrasound is not thought to be an effective17 means of improving ovarian cancer prognosis and is not officially recommended in France,15, 16 it was not included in these decision trees but noted separately. Four strategies accounted for 88% of the preventive options chosen by the carriers at the end of the 5-year follow-up: 50% had opted for breast surveillance alone, based on either MRI and other imaging methods in 31% of cases (N=31; strategy S5), or based on mammography alone in 19% (N=19; S6); 38% had opted for RRSO and breast surveillance based on MRI and/or other imaging methods in 28% of cases (N=28; S3) or mammography alone in 10% (N=10; S4). The other three strategies documented were RRM and RRSO (5%) (N=5; S1), RRM alone (N=2; 2% S2), and neither RRM/RRSO nor surveillance (N=6; 6% S7). One 45-year-old woman who had opted for S2 planned to undergo RRSO 5 weeks after completing the final questionnaire; the other one was 48 years old and had no family history of ovarian cancer. In the S7 group, two women were under 30 years of age; the others were aged between 31 and 39 years. These strategies were stratified by age, above and below 40 years of age, as shown in Figures 2a and b.
Figure 1.
Preventive management strategies observed in BRCA1/2 mutation carriers (N=101) 5 years after test result disclosure.
Figure 2.
(a) Preventive management strategies observed in BRCA1/2 mutation carriers <40 years (N=44) 5 years after test result disclosure. (b) Preventive management strategies observed in BRCA1/2 mutation carriers ≥40 years (N=57) 5 years after test result disclosure.
Among the carriers
RRM was undergone by 7%, and this option was not correlated with the women's age. RRSO was undergone by 43% of the carriers, and this option was chosen significantly more frequently with age (P<0.001): the rate of uptake increased from 18% in the 30–39-year olds to 65% in those ≥40 years of age. During the last 3 years, the women underwent 2.8 mammograms on average and 1.7 MRI on average (Table 2). MRI was undergone by 63% (in 59/94 cases without RRM), and no correlations were observed with age in this respect. A high rate of ovarian ultrasound screening was still observed during the last 3 years among those who underwent no RRSO. The results obtained on each age group are presented in Table 2.
Table 2. Preventive strategies adopted by BRCA1/2 carriers and non-carriers (all unaffected) 5 years after test result disclosure (N=246).
| Carriers (N=101) | Non-carriers (N=145) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age category | Age category | |||||||||
| Total | <30 n=6 | 30–39 n=38 | 40–49 n=36 | ≥50 n=21 | Total | <30 n=10 | 30–39 n=32 | 40–49 n=44 | ≥50 n=59 | |
| Preventive strategies | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Bilateral prophylactic mastectomy after test result delivery | 7 (6.9) | 0 | 2 (5.3) | 4 (11.1) | 1 (4.8) | 0 | 0 | 0 | 0 | 0 |
| Bilateral prophylactic oophorectomy | 43 (42.6) | 0 | 7 (18.4) | 20 (55.6) | 16 (76.2) | 17 (11.7) | 0 | 0 | 3 (6.8) | 14 (23.7) |
| Before test result delivery | 2 | 1 | 1 | 0 | 14 | 1 | 13 | |||
| After test result delivery | 41 | 6 | 19 | 16 | 3 | 2 | 1 | |||
| m (SD) a | m (SD) a | m (SD) a | m (SD) a | m (SD) a | m (SD) a | m (SD) | m (SD) | m (SD) | m (SD) | |
| Mammograms during the previous 3 yearsb | 2.8 (1.4) | 1.8 (2.3) | 2.6 (1.4) | 3.1 (1.4) | 3.2 (1.1) | 1.7 (1.2) | 0.5 (1.3) | 0.9 (1.0) | 1.7 (1.1) | 2.2 (1.1) |
| MRI during the previous 3 yearsb | 1.7 (1.7) | 1.5 (2.1) | 1.5 (1.5) | 2.2 (2.0) | 1.2 (1.4) | 0.1 (0.4) | 0.2 (0.7) | 0.0 (0.2) | 0.1 (0.5) | 0.1 (0.3) |
| Ovarian ultrasoundc | ||||||||||
| Transvaginal US | 1.7 (1.7) | 0.5 (1.2) | 1.4 (1.7) | 2.3 (1.6) | 2.6 (2.3) | 0.7 (1.4) | 0.6 (1.9) | 1.0 (1.6) | 0.5 (1.3) | 0.6 (1.1) |
| Abdominal US | 0.6 (1.2) | 0.7 (1.2) | 0.8 (1.5) | 0.2 (0.8) | 0 (0) | 0.4 (1.2) | 0.1 (0.3) | 0.2 (0.8) | 0.8 (1.8) | 0.3 (0.8) |
Mean number of investigations undergone during the last 3 years (means and SD).
Apart from RRM.
Apart from RRSO.
Non-carriers (N=145)
No RRM was performed on non-carriers after disclosure of the results, but three salpingo-oophorectomies were undergone by non-carriers for other medical reasons (fibroma or cystic ovaries) (Table 2). In all, 53% of the women aged between 30 and 39 years (20% of the <30 age group) reported at the final follow-up that they had continued to undergo annual or 2-yearly mammography during the previous 3 years; only 7 (4.8%) had undergone MRI; 43.2% stated that they had undergone an ovarian ultrasound examination during the last 3 years.
High perceptions of breast/ovarian cancer risks vs preventive strategies and mutation carrier/non-carrier status
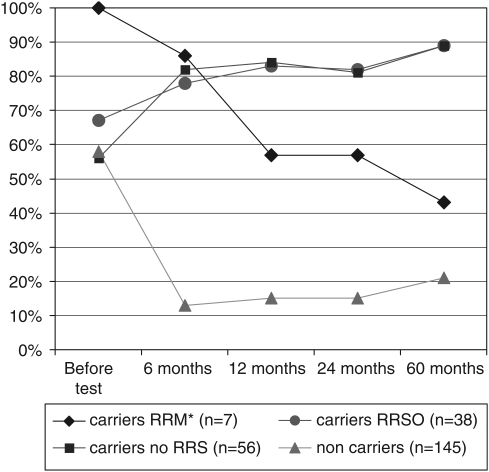
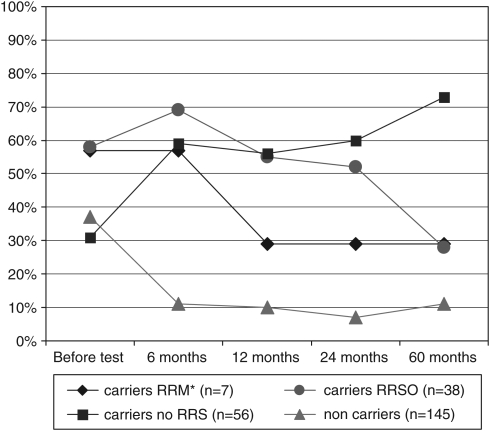
Disclosure of the BRCA1/2 status significantly affected the carriers and non-carriers' high perceptions of breast/ovarian cancer risks. Among the carriers, it significantly increased the proportion of carriers with high/very high breast cancer risk perceptions (from 63% before disclosure to 81% 6 months after disclosure, McNemar's χ2, P=0.016) and some carriers' high ovarian cancer risk perceptions (from 42% before disclosure to 62% 6 months after disclosure, McNemar's χ2 P=0.007); whereas it decreased high breast cancer risk perceptions (N=145) (Figure 3; P<0.001) and high ovarian cancer risk perceptions (Figure 4; P<0.001) in non-carriers. High perception of other cancer risks was relatively infrequent and not affected by disclosure (12.1 and 9.5% before disclosure and 6 months after disclosure, respectively, McNemar's χ2 P=0.248 and 11.2, 7.9 and 15.5% after 1, 2 and 5 years, respectively).
Figure 3.
High perceptions of breast cancer risks before/after BRCA1/2 test result disclosure, depending on prophylactic surgery undergone during follow-up (N=246). *RRM+RRSO (n=5) or RRM alone (n=2).
Figure 4.
High perceptions of ovarian cancer risks before/after BRCA1/2 test result disclosure, depending on prophylactic surgery undergone at follow-up (N=246). *RRM+RRSO (n=5) or RRM alone (n=2).
All those who eventually opted for RRM by the fifth year of follow-up (strategies S1 and S2 in Figure 1; N=7) had high breast cancer risk perceptions before disclosure; these high perceptions decreased with time after disclosure and after undergoing RRM (Figure 3). High breast cancer risk perceptions increased steadily with time among all the other carriers, regardless of whether or not they opted for RRSO (strategies S3 and S4 in Figure 1; N=38) or not (strategies S5–S7, Figure 1; N=56).
High ovarian cancer risk perceptions with time showed the same pattern as with breast cancer. Among carriers who did not opt for RRM, those who opted for RRSO (strategies S3–S4 in Figure 1) had higher ovarian cancer risk perceptions before disclosure than those who did not (Figure 4; P=0.014). Between 6 and 60 months after disclosure of the results, these high perceptions decreased significantly after RRSO (McNemar; P=0.002), whereas a significant increase (McNemar; P=0.049) occurred among those who did not opt for surgery.
Discussion
This is the first time the preventive strategies used by a French national cohort of unaffected female BRCA1/2 carriers and non-carriers have been documented for 5 years after routine genetic test result disclosure. The preventive strategies reported by respondents 5 years after disclosure show that in France, regular MRI surveillance (along with other breast imaging methods) is the most frequently preferred strategy among unaffected female BRCA1/2 carriers under 40 years of age, whereas RRSO tends to be combined with regular MRI breast imaging among older women (Figures 2a and b). When the decision to undergo RRM has been made, RRSO is generally undergone as well (Figure 1). Transvaginal ultrasound investigations were undergone regularly by carriers without RRSO, and non-carriers appeared to favour opting for screening despite their low cancer risk (Table 2). In France, this screening is possible when prescribed by a medical practitioner. High breast/ovarian cancer risk perceptions were significantly affected in both the short- and long-term among carriers and non-carriers alike. In the long run, high breast cancer risk perceptions increased steadily with time among those without RRM (and decreased among the small sample with RRM), and a similar pattern was observed in the case of ovarian cancer risk perception and RRSO. RRSO had no effect on breast cancer risk perceptions.
It was reported some time ago that there is little enthusiasm in France for RRM, either among health professionals or women attending cancer genetic clinics.18, 19 The present results confirm the reluctance previously observed about these issues, although the rate of uptake of RRSO in France is comparable to that of other countries in the relevant age groups. Health professionals in France seem to expect MRI to be a highly effective means of detecting early forms of breast cancer, resulting in a good prognosis, whereas those in other countries prefer the highly effective surgical strategies available. It would be interesting to know the survival rates associated with each combination of preventive strategies, which would help to give women all the information required for their decision making to be autonomous and well informed.
It is worth discussing the increase observed here in the high breast/ovarian cancer risk perceptions of those who did not undergo risk reducing surgery, as well as the fact that RRSO did not decrease high breast cancer risk perceptions although this intervention is known to significantly decrease the risk of breast cancer.20 Finch et al21 retrospectively assessed the impact of genetic counselling and RRSO on breast and ovarian cancer risk knowledge and concluded that most women accurately perceived their breast cancer risks. Our results mean either that the information was not delivered by health-care professionals or that RRSO had no impact on a priori lay beliefs about breast cancer risks. Cancer risk perception is a complex and subjective issue, because risk perception seems to have nothing to do with ‘risk knowledge'. Risk perception is known to determine behaviour, whereas people's ‘risk knowledge' is not predictive of their health-related behaviour.22 Risk perception may decrease when the main target organ of cancer has been removed surgically, whereas the situation is not the same when another organ is taken out, and the preventive action obtained through the suppression of hormonal effect. Previous studies have shown that women's psychological distress decreases after undergoing prophylactic mastectomy,23 and that their fear of developing cancer also decreases,6 which is similar to what occurs with high-risk perception or affective risk perception.24 However, other investigators have reported that the levels of distress are still high after these interventions, especially RRSO,25 possibly because these women's perception of the remaining risk of breast cancer has not changed significantly.
Our study had several limitations. First, respondents always differ from non-respondents, even if they have the same baseline characteristics. Only baseline breast self-examination practices differed here between respondents and non-respondents; therefore, we must remember that our results may not be completely representative of the whole cohort or of the whole French population of BRCA1/2 carriers, although we benefited from the advantages of multicentre, nationwide recruitment. Second, the same bias occurred as in previous studies, as following the same group of people with questionnaires may affect their spontaneous decisions during follow-up and hence the methods of surveillance and prevention they choose. We also have to take the time factor into account, since preventive recommendations evolve with time and while the people in this study were being tested, new preventive recommendations were issued in 2004, especially as regards prophylactic surgery, which was more strongly recommended16 than previously.15
In conclusion, women's cancer risk perceptions 5 years after disclosure of their BRCA1/2 status depend on decisions about preventive interventions more than simply on the outcomes of mutation tests. Women need to know more these days about the probability of survival associated with all the strategies available, and in particular whether opting for RRSO and breast MRI surveillance is likely to be as effective as undergoing both RRM and RRSO in terms of survival.
Acknowledgments
All the members of the Genepso cohort investigators including, Claude Adenis, Yves-Jean Bignon, Annie Chevrier, Agnès Chompret, Odile Cohen-Haguenauer, Isabelle Coupier, Liliane Demange, Capucine Delnatte, Hélène Dreyfus, Catherine Dugast, François Eisinger, Laurence Faivre, Marc Frenay, Paul Gesta, Laurence Gladieff, Christine Lasset, Michel Longy, Tan Dat NGuyen, Claude Picard, Irwin Piot, Hagay Sobol, Dominique Stoppa-Lyonnet, Laurence Venat-Bouvet, Philippe Vennin, Hélène Zattara-Cannoni. Roxane Fabre and Anne-Deborah Bouhnik are acknowledged for their statistical expertise. This work was supported by grant from the ‘Institut National du Cancer' (National Cancer Institute) R08097AA/RPT08011AAA INCA.
The authors declare no conflict of interest.
References
- Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008;299:1320–1334. doi: 10.1001/jama.299.11.1320. [DOI] [PubMed] [Google Scholar]
- Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med. 2008;10:19–32. doi: 10.1097/GIM.0b013e31815f524f. [DOI] [PubMed] [Google Scholar]
- Meijers-Heijboer EJ, Verhoog LC, Brekelmans CT, et al. Presymptomatic DNA testing and prophylactic surgery in families with a BRCA1 or BRCA2 mutation. Lancet. 2000;355:2015–2020. doi: 10.1016/s0140-6736(00)02347-3. [DOI] [PubMed] [Google Scholar]
- Botkin JR, Smith KR, Croyle RT, et al. Genetic testing for a BRCA1 mutation: prophylactic surgery and screening behavior in women 2 years post testing. Am J Med Genet A. 2003;118A:201–209. doi: 10.1002/ajmg.a.10102. [DOI] [PubMed] [Google Scholar]
- Foster C, Watson M, Eeles R, et al. Predictive genetic testing for BRCA1/2 in a UK clinical cohort: three-year follow-up. Br J Cancer. 2007;96:718–724. doi: 10.1038/sj.bjc.6603610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oostrom I, Meijers-Heijboer H, Lodder LN, et al. Long-term psychological impact of carrying a BRCA1/2 mutation and prophylactic surgery: a 5-year follow-up study. J Clin Oncol. 2003;21:3867–3874. doi: 10.1200/JCO.2003.10.100. [DOI] [PubMed] [Google Scholar]
- Dawson SJ, Price MA, Jenkins MA, et al. Cancer risk management practices of noncarriers within BRCA1/2 mutation positive families in the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer. J Clin Oncol. 2008;26:225–232. doi: 10.1200/JCO.2007.11.0262. [DOI] [PubMed] [Google Scholar]
- Metcalfe KA, Ghadirian P, Rosen B, et al. Variation in rates of uptake of preventive options by Canadian women carrying the BRCA1 or BRCA2 genetic mutation. Open Med. 2007;1:e92–e98. [PMC free article] [PubMed] [Google Scholar]
- Watson M, Foster C, Eeles R, et al. Psychosocial impact of breast/ovarian (BRCA1/2) cancer-predictive genetic testing in a UK multi-centre clinical cohort. Br J Cancer. 2004;91:1787–1794. doi: 10.1038/sj.bjc.6602207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skytte AB, Gerdes AM, Andersen MK, et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: uptake and timing. Clin Genet. 2010. [DOI] [PubMed]
- Lynch HT, Snyder C, Lynch JF, et al. Patient responses to the disclosure of BRCA mutation tests in hereditary breast-ovarian cancer families. Cancer Genet Cytogenet. 2006;165:91–97. doi: 10.1016/j.cancergencyto.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Andrieu N, Goldgar DE, Easton DF, et al. Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS) J Natl Cancer Inst. 2006;98:535–544. doi: 10.1093/jnci/djj132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou AC, Rookus M, Andrieu N, et al. Reproductive and hormonal factors, and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers: results from the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol Biomarkers Prev. 2009;18:601–610. doi: 10.1158/1055-9965.EPI-08-0546. [DOI] [PubMed] [Google Scholar]
- Julian-Reynier C, Bouhnik AD, Mouret-Fourme E, et al. Time to prophylactic surgery in BRCA1/2 carriers depends on psychological and other characteristics. Genet Med. 2010;12:801–807. doi: 10.1097/GIM.0b013e3181f48d1c. [DOI] [PubMed] [Google Scholar]
- Eisinger F, Alby N, Bremond A, et al. Recommendations for medical management of hereditary breast and ovarian cancer: the French National Ad Hoc Committee. Ann Oncol. 1998;9:939–950. doi: 10.1023/A:1008389021382. [DOI] [PubMed] [Google Scholar]
- Eisinger F, Bressac B, Castaigne D, et al. Identification and management of hereditary predisposition to cancer of the breast and the ovary (update 2004) Bull Cancer. 2004;91:219–237. [PubMed] [Google Scholar]
- Evans DG, Gaarenstroom KN, Stirling D, et al. Screening for familial ovarian cancer: poor survival of BRCA1/2 related cancers. J Med Genet. 2009;46:593–597. doi: 10.1136/jmg.2008.058248. [DOI] [PubMed] [Google Scholar]
- Julian-Reynier C, Eisinger F, Moatti JP, Sobol H. Physicians' attitudes towards mammography and prophylactic surgery for hereditary breast/ovarian cancer risk and subsequently published guidelines. Eur J Hum Genet. 2000;8:204–208. doi: 10.1038/sj.ejhg.5200418. [DOI] [PubMed] [Google Scholar]
- Julian-Reynier CM, Bouchard LJ, Evans DG, et al. Women's attitudes toward preventive strategies for hereditary breast or ovarian carcinoma differ from one country to another: differences among English, French, and Canadian women. Cancer. 2001;92:959–968. doi: 10.1002/1097-0142(20010815)92:4<959::aid-cncr1406>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch A, Metcalfe K, Lui J, et al. Breast and ovarian cancer risk perception after prophylactic salpingo-oophorectomy due to an inherited mutation in the BRCA1 or BRCA2 gene. Clin Genet. 2009;75:220–224. doi: 10.1111/j.1399-0004.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- Julian-Reynier C, Welkenhuysen M, Hagoel L, Decruyenaere M, Hopwood P. Risk communication strategies: state of the art and effectiveness in the context of cancer genetic services. Eur J Hum Genet. 2003;11:725–736. doi: 10.1038/sj.ejhg.5201037. [DOI] [PubMed] [Google Scholar]
- Metcalfe KA, Esplen MJ, Goel V, Narod SA. Psychosocial functioning in women who have undergone bilateral prophylactic mastectomy. Psychooncology. 2004;13:14–25. doi: 10.1002/pon.726. [DOI] [PubMed] [Google Scholar]
- van Dooren S, Rijnsburger AJ, Seynaeve C, et al. Psychological distress in women at increased risk for breast cancer: the role of risk perception. Eur J Cancer. 2004;40:2056–2063. doi: 10.1016/j.ejca.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Bresser PJ, Seynaeve C, Van Gool AR, et al. The course of distress in women at increased risk of breast and ovarian cancer due to an (identified) genetic susceptibility who opt for prophylactic mastectomy and/or salpingo-oophorectomy. Eur J Cancer. 2007;43:95–103. doi: 10.1016/j.ejca.2006.09.009. [DOI] [PubMed] [Google Scholar]