Abstract
Sepsis, a leading cause of death in hospitalized patients, is characterized by lethal systemic inflammatory responses. JAK2 is an essential tyrosine kinase modulating immune responses. However, the implications of JAK2 in infectious disorders remain undetermined. Here, we report that JAK2 inhibitors rescue animals from polymicrobial sepsis in a clinically relevant time frame. JAK2 inhibition with AG490 prevents NF-κB activation, modulates macrophage activation, and restrains the production of inflammatory cytokines. The inhibition of JAK2 blunted TNF production in both macrophages and splenocytes in a concentration-dependent manner. JAK2 inhibition specifically prevents LPS-induced STAT3 tyrosine phosphorylation without affecting serine phosphorylation in macrophages. JAK2 inhibitor prevents the activation of the canonical p65RelA/p50NF-κB1 pathway but not the other NF-κB proteins. In vivo, JAK2 inhibition restrains serum TNF levels by modulating TNF production in the lung and the spleen and protects mice from lethal endotoxemia in a concentration-dependent manner. AG490 also inhibits extracellular release of HMGB1 from macrophages and prevents an increase in serum HMGB1 levels during sepsis. JAK2 inhibition started at 24 h after the onset of sepsis rescued the mice from polymicrobial sepsis. Our study is the first experimental evidence that JAK2 inhibitors may provide a pharmacological advantage for the treatment of sepsis in a clinically relevant time frame.
Keywords: Sepsis, Cytokines, TNF, HMGB1, Inflammation, Alpha7nAchR, Nicotinic receptors
Introduction
Sepsis remains a current challenge in modern medicine as it is the most common cause of death in hospitalized patients [1, 2]. Despite the recent advances in antibiotics and intensive care treatment, sepsis remains the third leading cause of death in developed countries [1, 3]. Albeit commonly originated by an infection, the pathogenesis of sepsis is driven by detrimental inflammatory responses produced by the host to the infection or trauma. Beneficial inflammatory cytokines are normally produced to confine and fight the infection. However, excessive cytokine production can be worse than the original stimuli and contribute to deleterious systemic inflammatory responses leading to cardiovascular collapse and multiple organ failure [3–5]. Tumor necrosis factor (TNF) is a characteristic inflammatory cytokine contributing to cardiovascular collapse in sepsis: recombinant TNF triggers the characteristic symptoms of “septic shock” and TNF neutralization can prevent septic shock in animals when administered before endotoxemia [4]. In addition to these “early” cytokines, other “late” inflammatory factors also contribute to the pathogenesis of sepsis. High mobility group B protein-1 (HMGB1) is an intracellular protein that functions as an inflammatory factor when released into the extracellular milieu by macrophages [6]. Extracellular HMGB1 is a characteristic “late” factor contributing to intestinal barrier dysfunction, vascular leakage, acute lung injury, and abrupt cardiac standstill in sepsis [7, 8]. These studies have increased the interest on the immunological mechanisms modulating cytokine production in infectious disorders.
We recently showed that nicotine can inhibit macrophages activation through a mechanism mediated by the alpha7 nicotinic acetylcholine receptor (alpha7nAChR) [9, 10]. Nicotine and acetylcholine inhibit the NF-κB pathway and prevent cytokine production via the alpha7nAChR [9]. In vivo, treatment with nicotine attenuates systemic inflammation, protects mice from lethal endotoxemia, and improves survival in polymicrobial sepsis [9]. Nicotinic agonists failed to improve survival in splenectomized animals, suggesting that they modulate systemic inflammation through a mechanism mediated by the spleen [11]. Since the clinical translation of this mechanism is limited by the collateral toxicity of nicotine, current efforts seeking selective strategies are directed to identify the specific antiinflammatory mechanism of this receptor. Recent studies suggested that activation of JAK2 mediates the antiinflammatory potential of nicotinic agonists in macrophages, and thus JAK2 activation may be beneficial in sepsis [12, 13]. Nicotine induces STAT3 tyrosine phosphorylation in macrophages via JAK2 because AG490, a well-established JAK2 inhibitor [14], prevented this effect [12]. However, other studies suggest that JAK2 activation contributes to “early” inflammatory responses in sepsis and AG490 can inhibit cytokine production in macrophage cell lines [15–19]. Here, we analyzed whether JAK2 contributes to the anti-inflammatory potential of the alpha7nAChR and whether JAK2 may provide pharmacological advantages for sepsis.
Material and methods
Chemicals and reagents
AG490 and LPS (Sigma-Aldrich, St. Louis, MO, USA) were dissolved, respectively, in 20 mM ethanol or PBS and were administered intra-peritoneally at the indicated concentrations and time points.
Animal experiments
All animal experiments conforms the National Institutes of Health Guidelines and were approved by the Institutional Animal Care and Use Committee of the UMDNJ–New Jersey Medical School. Six- to eight-week-old male wild-type C57BL/6J mice were purchased from The Jackson Laboratory. Animals were randomly grouped and investigators were blinded to the experimental treatment. Endotoxemia and cecal ligation and puncture were performed as we previously described in Wang et al. [9]. Briefly, Endotoxemia: endotoxin (Escherichia coli LPS 0111:B4; Sigma) was dissolved in sterile, pyrogen-free PBS, and sonicated for 30 min immediately before used intraperitoneally at the indicated concentration.
Cecal ligation and puncture
Animals were anesthetized with ketamine (75 mg/kg, i.m.; Fort Dodge, Fort Dodge, IA, USA) and xylazine (20 mg/kg, i.m.; Boehringer Ingelheim, St. Joseph, MO, USA) and subjected to abdominal incision and ligation of the cecum at 5.0 mm from the cecal tip away from the ileocecal valve. The ligated cecal stump was punctured once with a 22-gauge needle, and stool was extruded (approx. 1 mm) to ascertain patency of puncture. Abdominal wound was closed in two layers, peritoneum and fascia separately to prevent leakage of fluid. To mimic clinical standards, the animals received antibiotic (7 mg/kg enrofloxacine) dissolved in 0.9% normal saline (20 ml/kg, s.c.) immediately after surgery and every 12 h during 3 days as recommended by manufacturer (Bayer, Shawnee Mission, KS, USA). After collection, the organs were homogenized (Homogenizer; Omni International, GA, USA) in lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 0.5% NPO4, 50 mM NaF, 0.2 mM NaPO4, 25 ug/ml aprotonin, 25 ug/ml pepstatin A, 1 mM phenylmethylsulfonyl fluoride). The resulting suspension was centrifuged (10,000 g for 25 min at 4°C), and the supernatant was collected for analyses. The protein concentration was quantified by the Bradford method (Biorad, Hercules, CA, USA).
Cell cultures
Murine macrophages RAW264.7 and human peripheral blood monocytic THP1 cells (ATCC, Manassas, VA, USA) were cultured as we described in Wang et al [9]. Briefly, cells were grown in RMPI-1640 medium with L-glutamine (Gibco BRL, Rockville, MD, USA) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone Laboratories, Logan, UT, USA) at 37°C in a humidified incubator with 5% CO2. For experiments, cells were transferred to a 24-well polystyrene culture plates at 3×105 cells/well in 1-ml medium per well. After overnight incubation, the medium was removed and replaced with serum free OPTIMEM I medium. Cell cytotoxicity or survival was analyzed by using 10% of 5 mg/ml MTT solution in PBS, and the resulting solution was added to each well for 2 h. After removal of 800 ul, 200 ul of solubilizer solution (isopropanol, HCl, and Triton ×−100) was added to dissolve the formazan crystals. The absorbance at 540 nm was determined.
Cytokine, STAT3, and NF-κB analyses
Blood was collected at the indicated time points, allowed to clot for 2 h at room temperature and centrifuged for 15 min at 2,000×g. Organs were collected and immediately homogenized in 4°C PBS. The homogenates were normalized by protein concentration and TNF concentration was analyzed by using the TNF ELISA kit (eBioscience, San Diego, CA, USA). TNF in the culture cells were analyzed at 3 h after stimulation. TNF levels in the serum and organs were analyzed at 90 min after the septic challenge. TNF concentration in major organs was normalized by protein concentration. Serum or conditioned media were concentrated by ultrafiltration using Centricon-10 (Millipore, Billerica, MA, USA), and the retentive fraction was resolved on 4–20% gradient SDS-PAGE gels and analyzed by Western blot using anti-HMGB1 Ab18256 from Abcam Inc. (Cambridge, MA, USA). Specific NF-κB protein binding to DNA was analyzed using the TransAM DNA-Binding ELISA (Active Motif; Cambridge, MA, USA) following manufacturer’s instructions. STAT3 total protein and phosphorylation were analyzed using the antibodies STAT3 no. 9132, phospho STAT3 (Tyr705) and phospho STAT3 (Ser727) from Cell Signaling Technology, Danvers, MA, USA.
Statistical analyses
All experiments were repeated at least three times per condition and the data are expressed as mean±standard deviation (STD). Statistical analyses were performed using the one way analysis of variance (ANOVA) with multiple pairwise comparisons with the Bonferroni’s adjustment for multiple hypothesis testing. Normality and homogeneity of variance were confirmed. ANOVA was used to compare all treatments and specific pairwise comparisons as stated in the experiments. Statistical analyses of survival were determined using the logrank test. Kaplan–Meier product-limit method was used for survival graphs. P values <0.05 were considered statistically significant.
Results
JAK2 inhibition blunted LPS-induced TNF production
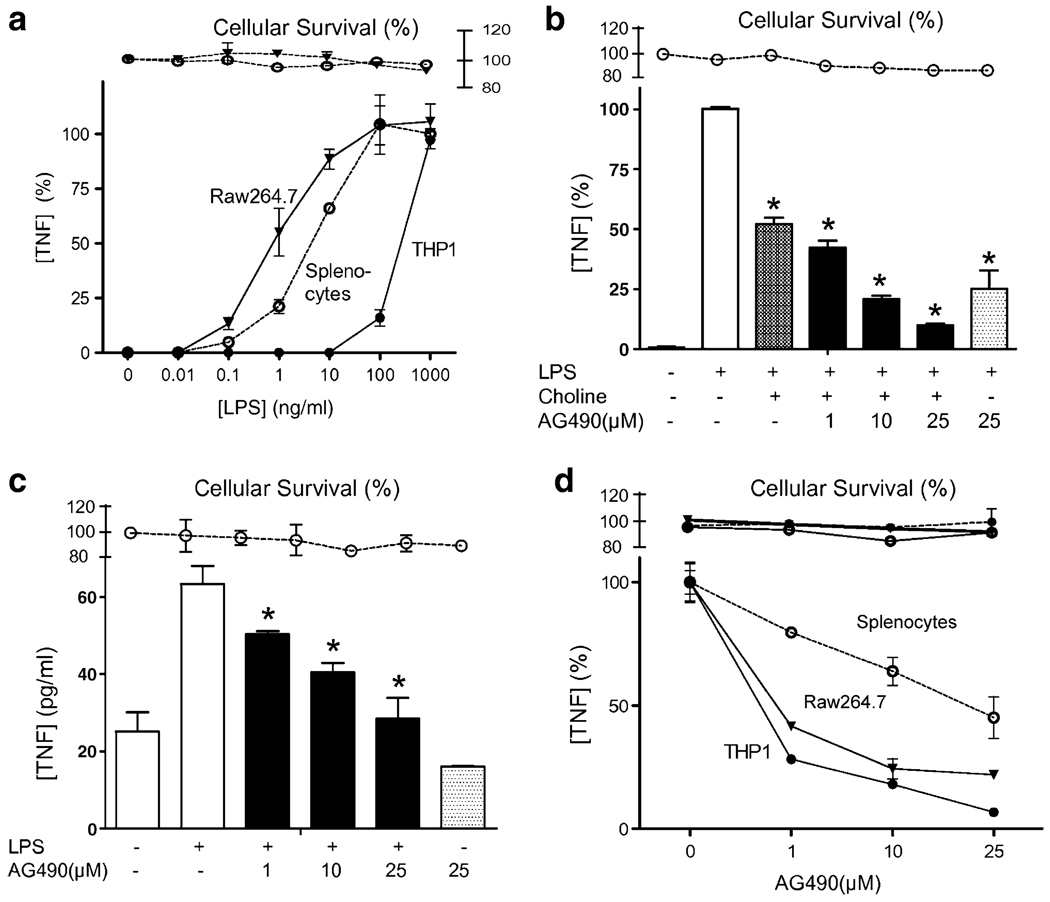
Raw264.7 macrophages and splenocytes showed higher sensitivity to LPS than THP1 cells (Fig. 1a). The JAK2 inhibitor, AG490 was used to determine whether JAK2 mediates the anti-inflammatory potential of the alpha7-nAChR to inhibit TNF production in macrophages. Primary cultures of splenocytes were treated with the alpha7nAChR-agonist, choline alone or with AG490. Choline inhibited LPS-induced TNF production, but JAK2 inhibition failed to reestablish TNF production in the presence of choline (Fig. 1b). Similar results were confirmed using other alpha7nAChR-agonists including nicotine and in other cell types including Raw264.7 and THP1 cells (data not shown). Those results suggested that JAK2 inhibition failed to prevent the anti-inflammatory potential of alpha7nAChR to inhibit TNF production in macrophages. Actually our results suggested that JAK2 inhibition prevented LPS-induced TNF production. To confirm that hypothesis, primary cultures of splenocytes were treated with AG490, and TNF production was analyzed in the conditioned media at 3 h after LPS stimulation. Trypan blue exclusion studies and MTT assay indicates that AG490 was not cytotoxic at the indicated concentrations. JAK2 inhibition prevented LPS-induced TNF production in a concentration-dependent manner in splenocytes (Fig. 1c). Similar results were also found in Raw264.7 and THP1 cells (Fig. 1d), yet AG490 appears to be more effective in these cell lines probably due to their cellular homogeneity as compared to primary culture of splenocytes. These results strongly suggested that JAK2 contributes to the innate immune responses induced during sepsis.
Fig. 1.
JAK2 inhibition blunted TNF production. a The LPS dose response to endotoxin was analyzed in RAW264.7, THP1, and primary culture of splenocytes. TNF in the conditioned supernatant was analyzed by ELISA at 3 h after stimulation. The graph depicts the results of three different experiments normalized as percent of the TNF levels found at the higher concentration of endotoxin. b Primary cultures of splenocytes were treated with choline (50 mM) alone or with AG490 30 min prior LPS stimulation (400 ng/ml). The graph depicts the results of three different experiments normalized as percent of the TNF levels induced by endotoxin alone. c Primary culture of splenocytes, d RAW264.7, or THP1 cells were pretreated with a concentration range of the JAK2 inhibitor AG490, 30 min prior LPS stimulation. Asterisk represents p<0.01 vs LPS. Survival represents the cellular cytotoxicity as determined by MTT assay. The graphs depict the results of three different experiments normalized as percent of the TNF levels induced by endotoxin alone
JAK2 inhibition prevented STAT3 tyrosine phosphorylation and NF-κB activation
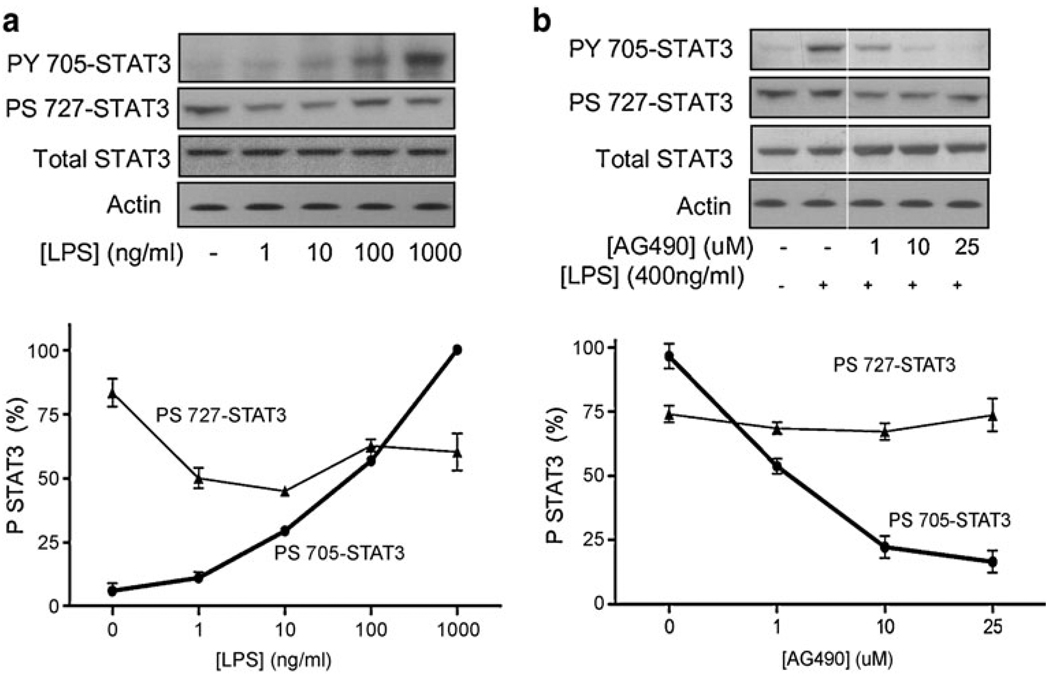
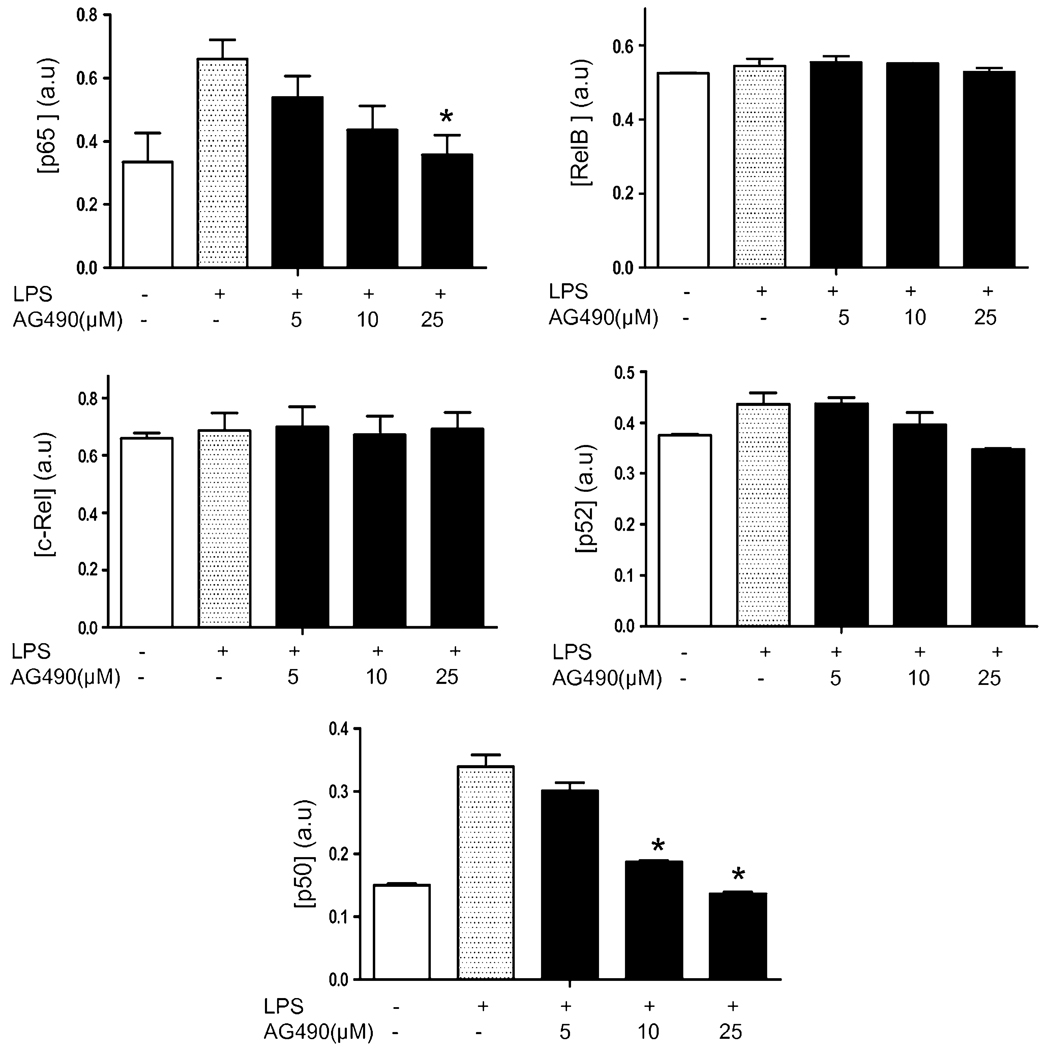
The implications of JAK2 in the LPS signaling were confirmed by analyzing STAT3 phosphorylation, the most characteristic substrate of JAK2 activation. In agreement with our previous results, LPS activated STAT3 tyrosine (Y705) phosphorylation in a concentration-dependent manner (Fig. 2a). LPS also induced STAT3 serine (S727) dephosphorylation in a concentration-dependent manner. Since STAT3 tyrosine 705 (Y705) is the most characteristic substrate for JAK2, we confirmed that our treatment with AG490 inhibits STAT3 phosphorylation. JAK2-inactivation with AG490 prevented the LPS-induced STAT3 tyrosine phosphorylation in a concentration-dependent manner. Treatment with 10 uM AG490 inhibited STAT3 tyrosine phosphorylation by over 60%, and this effect was specific since the same treatment did not affect significantly the STAT3 serine phosphorylation (Fig. 2b). In order to confirm the anti-inflammatory potential of JAK2 inhibition, we analyzed its effect on NF-κB, a critical pathway for macrophage activation and cytokine production. LPS stimulated the canonical p65RelA/p50NF-κB1 by activating both p65RelA and p50NF-κB1 in a similar proportion (Fig. 3). LPS failed to induce a statistical activation of the other NF-κB proteins including RelB, c-Rel, and p52NF-κB2. AG490 specifically prevented activation of the canonical NF-κB pathway in a concentration-dependent manner without affecting the alternative pathways.
Fig. 2.
JAK2 inhibition prevented STAT3 tyrosine phosphorylation. a THP1 cells were treated with LPS for 3 h, and STAT3 tyrosine and serine phosphorylation were analyzed by Western blot. Total STAT3 was used as a control for protein loading. The depicted gel is representative of results that were obtained in an experiment that was repeated three times. The graph represents the densitometric values of the scanned films, which were an approximate percent of the total STAT3 protein levels. b THP1 cells were pretreated with JAK2 inhibitor AG490, 30 min prior LPS stimulation. JAK2 inhibition prevented LPS-induced STAT3 tyrosine (Y705) phosphorylation in a concentration-dependent manner. Actin Western blots are used as loading control. The faint line indicates where lanes with samples not relevant to this article were cut out off the gel
Fig. 3.
JAK2 inhibition prevented p65 and p52 NF-κB activation. The effects of JAK2 inhibition were analyzed on the specific NF-κB pathways p65RelA, RelB, c-Rel, p50, and p52 by using the TransAM DNA-binding. Data represented in bar graphs are mean±STD of three experiments. Asterisk represents p<0.01 vs LPS
JAK2 inhibition prevented serum HMGB1 and rescued mice from polymicrobial sepsis
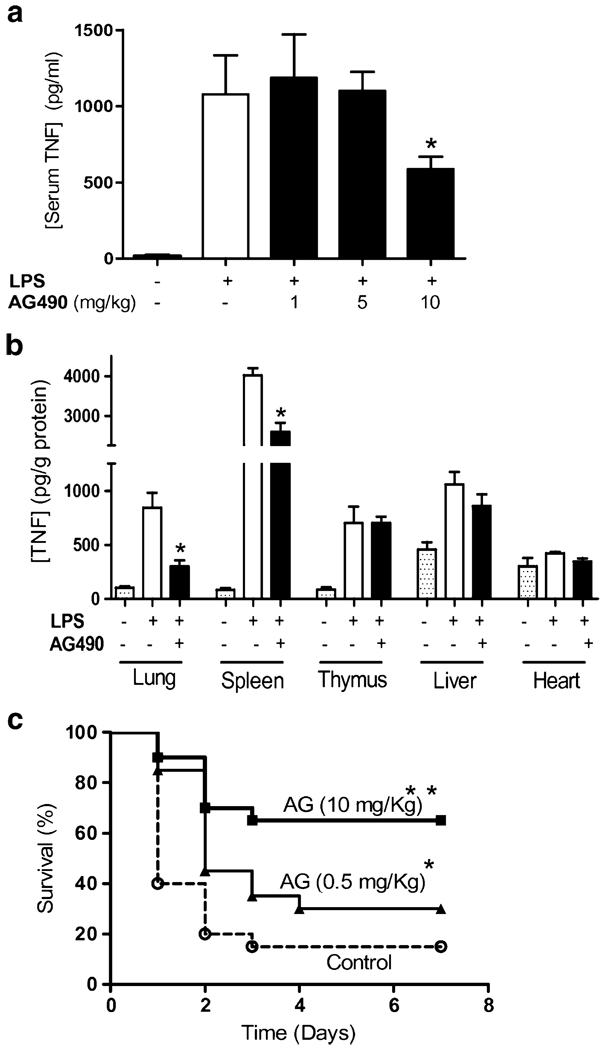
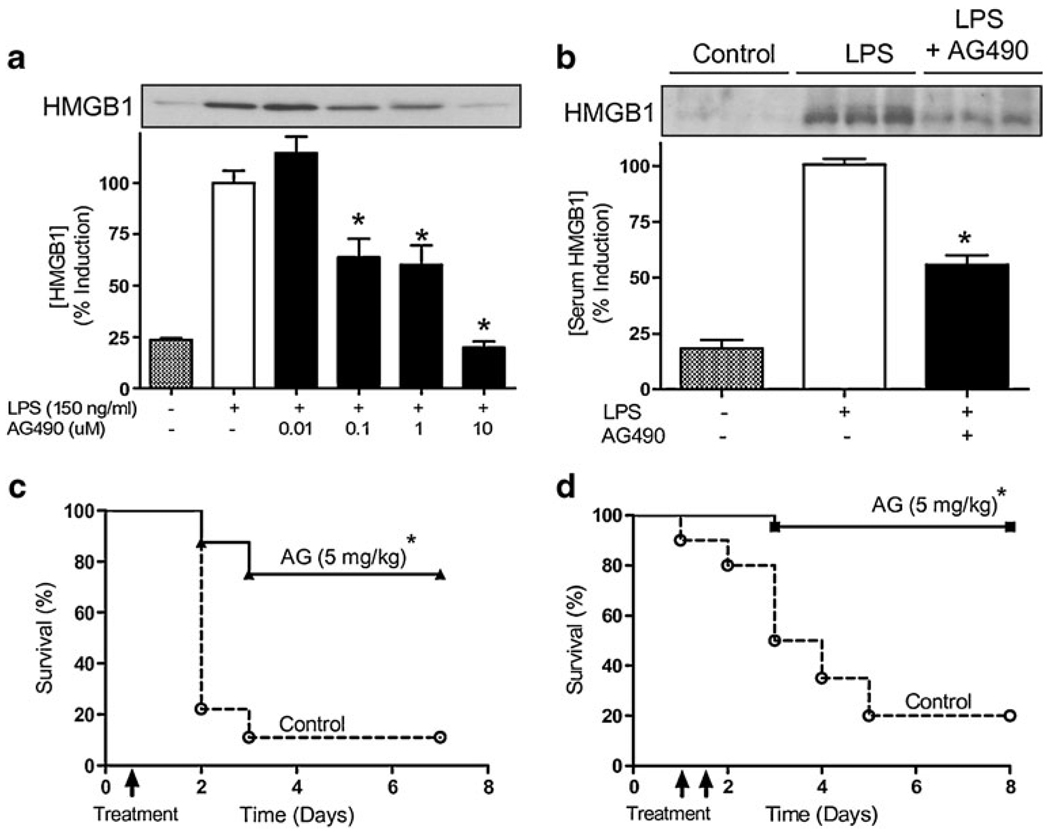
Pretreatment with AG490 30 min prior the LPS challenge prevented systemic inflammation and inhibited serum TNF levels in a concentration-dependent manner (Fig. 4a). The most significant effect was observed at 10 mg/kg AG490 inhibiting serum TNF levels by ~50%. The physiological mechanism of TNF regulation was analyzed in the major organs. Similar as we previously described [11], endotoxin induced a massive production of TNF in the spleen that is released to the bloodstream. JAK2 inhibition by AG490 inhibited TNF levels in the spleen and the lung but not in liver or heart (Fig. 4b). AG490 was more efficient in inhibiting TNF in the lung by ~70% than in the spleen with ~40% of inhibition. JAK2 inhibition improved survival in endotoxemic mice in a concentration-dependent manner (Fig. 4c). Animals were monitored for 2 weeks and no late deaths were observed, suggesting that JAK2 inhibition conferred a lasting effect and did not merely delay the onset of death. The anti-inflammatory potential of JAK2 inhibition with AG490 was also analyzed in “late” inflammatory mediators, which provide a wider therapeutic time window for the treatment of sepsis. Raw264.7 cells were pretreated with AG490, and extracellular HMGB1 was analyzed in the in the conditioned media at 24 h after LPS stimulation. JAK2 inhibition with AG490 was not cytotoxic based on trypan blue exclusion studies and MTT assay. JAK2 inhibition prevented LPS-induced HMGB1 secretion in a concentration dependent manner (Fig. 5a). The most significant effect was found at the higher concentration of AG490 (10 uM) with ~80% HMGB1 inhibition. The anti-inflammatory potential of AG490 to inhibit HMGB1 secretion was also analyzed in vivo. C57BL/6J mice were treated with vehicle or AG490 (10 mg/kg, i.p.), 30 min before the LPS challenge (6 mg/kg, i.p.). Serum HMGB1 was analyzed at 24 h after stimulation. AG490 inhibited the increase in serum HMGB1 levels of septic mice by ~50% (Fig. 5b). In addition to endotoxemia, we also studied the anti-inflammatory potential of AG490 in polymicrobial sepsis induced by cecal ligation and puncture, a more clinically relevant experimental model for human sepsis. Cecal ligation and puncture is an optimal experimental model to study inflammatory responses induced by both the polymicrobial peritonitis induced by the cecal puncture and the necrotic tissue induced by the cecal ligation. Together, our results suggest that JAK2 inhibition may provide a significant therapeutic time window to rescue animals from polymicrobial sepsis. To confirm this hypothesis, C57BL/6J mice were subjected to cecal ligation and puncture, and the AG490 treatment was delayed after the onset of the pathogenesis. AG490 treatment was started at 12 or 24 h after the septic challenge rescued mice from established sepsis and improved survival. A single dose of AG490 (5 mg/kg, i.p.) administered at 12 h after the onset of cecal ligation and puncture improved survival by over 65% (Fig. 5c). Likewise, AG490 (5 mg/kg, i.p.) started at 24 h after the onset of the cecal ligation and puncture rescued the mice from polymicrobial sepsis. In the later experiment, AG490 was administered twice at 24 and 36 h after the onset of the sepsis and rescued the mice by improving survival ~90% (Fig. 5d). Animals were monitored for 2 weeks, and no late deaths were observed, indicating that JAK2 inhibition confers lasting protection, rescues animal from established peritonitis, and reverses the clinical signs of sepsis.
Fig. 4.
JAK2 inhibition prevented systemic inflammation and improved survival in endotoxemia. Mice were treated with a a concentration range of AG490 or b AG490 (10 mg/kg, i.p.), 30 min before LPS challenge (6 mg/kg, i.p.). a Serum and b major organs were collected at 90 min after LPS to analyze TNF concentration. TNF concentration in major organs was normalized according to the total amount of protein (*p<0.05 vs LPS; n=6 mice/group). c Endotoxemic mice were treated with control vehicle or the indicated concentration of AG490, 30 min before LPS. Survival was analyzed for 2 weeks, and no late deaths were observed (*p<0.038 vs control, **p<0.0003 vs control, logrank survival test; n=20 animals/group)
Fig. 5.
JAK2 inhibition prevented systemic HMGB1 and rescued mice from polymicrobial sepsis. a Raw264.7 cells were treated with a concentration range of AG490 30 min prior LPS stimulation. Extracellular HMGB1 levels were analyzed in the media supernatant by Western blot at 24 h after LPS treatment (*p<0.01 vs LPS). The graph depicts the results of three different experiments normalized as percent of the TNF levels induced by endotoxin alone. b Endotoxemic mice were treated with control vehicle or AG490 (5 mg/kg, i.p.) 30 min before LPS challenge. Serum HMGB1 levels were analyzed by Western blot at 24 h after LPS challenge. Data was a representative of six animals per group (*p<0.01 vs LPS; n=3/group). c, d Septic mice subjected to cecal ligation and puncture were treated with control vehicle or AG490 (i.p.). Treatment with the JAK2 inhibitor AG490 was started c 12 h (n=10 animals/group) or d 24 h (n=22 animals/ group) after the onset of sepsis. Survival was analyzed for 2 weeks, and no late deaths were observed (*p<0.01 vs control, logrank survival test)
Discussion
JAK2 plays critical roles in the immune system, but its clinical implications in infectious disorders remain undetermined. Recent studies suggested that JAK2 activation may be beneficial in sepsis by mediating the anti-inflammatory potential of the alpha7nAChR [12]. These studies indicated that AG490 (100 uM) inhibits STAT3 tyrosine phosphorylation induced by nicotine [12]. However, our results indicate that AG490 failed to reestablish cytokine production or prevent the anti-inflammatory potential of selective alpha7nAChR-agonists. These differences can be explained by the specificity of the nicotinic agonists and the treatment of AG490. In our study, JAK2 inhibition was confirmed by showing specific inhibition of STAT3 tyrosine phosphorylation without inducing cellular cytotoxicity or affecting STAT3 serine phosphorylation. Nicotine is a general agonist whereas choline appears to be a specific alpha7nAChR-agonist in immune cells [10, 20, 21]. Furthermore, our studies indicate that AG490 can be administered at low concentrations (<25 uM) without producing deleterious effects, but it becomes cytotoxic at higher concentrations. These results concur with previous studies indicating that low concentrations of AG490 (<25 uM) did not alter hematopoietic progenitor cells [14]. Together, these results indicated that JAK2 activation is not required for the anti-inflammatory potential of alpha7nAChR.
Our results indicate that JAK2 plays a critical role in sepsis by leading innate immune responses against bacterial endotoxin. The mechanism by which JAK2 modulates innate immune responses in sepsis is unknown. Our results indicated that JAK2 contributes to activate NF-κB in response to bacterial endotoxin. AG490 specifically inhibited the activation of the canonical NF-κB without affecting the other NF-κB pathways. The crosstalk between JAK2 and the NF-κB pathway was previously proposed in neurons by showing that JAK2 modulates the phosphorylation of the inhibitor of NF-κB (IκB) [22]. This pathway crosstalk has multiple clinical implications. Indeed, JAK2 is essential for erythropoiesis as JAK2-deficient mice die at an early stage of development from fetal anemia [23]. Interestingly, preconditioning with erythropoietin protects neurons during ischemic and degenerative damage via a JAK2-NF-κB signaling [22]. JAK2 inhibition with AG490 (10 uM) prevents erythropoietin-induced tyrosine phosphorylation of the NF-κB inhibitor and subsequent NF-κB activation in neurons [22]. Together, these studies support our results and suggest that a similar JAK2-NF-κB signaling can contribute to sepsis. The specificity of this mechanism inhibiting the canonical NF-κB (without affecting the other NF-κB pathways) and also inhibiting STAT3 tyrosine phosphorylation (without affecting its serine phosphorylation) appears to have significant implications modulating cell death. STAT3 serine phosphorylation appears essential for macrophages survival [24] and p65RelA [25] and IKKβ [26] knockout mice exhibit embryonic death produced by extensive TNF-mediated fetal hepatocyte apoptosis. Consistently, disruption of the TNF signaling, either by removing TNF or TNF-R1, prevents hepatocyte apoptosis in real−/− mice and allows embryonic development to birth [27]. In agreement with these studies, NF-κB inhibition after partial hepatectomy results in massive hepatocyte apoptosis and impaired liver function, and NF-κB activation in the liver prevents hepatic injury during ischemia and reperfusion [28]. These studies suggest that unspecific NF-κB inhibition may not generate an overall beneficial effect especially in the liver, unless the therapy targets specific organs, immune cells or NF-κB isoforms [29]. Noteworthy, AG490 inhibited TNF responses in the lung and the spleen but not in the liver. These results suggest that our strategy allowed TNF responses in the liver to prevent necrosis, but prevented the overzealous responses of the spleen. These results concur with the implications of the spleen in sepsis [11, 30] and clinical studies indicating that splenectomy reduces hospitalization and improves the prognosis and outcome in severe trauma [31]. Since our studies indicated that JAK2 inhibition improves survival in experimental sepsis and we did not observe organ dysfunction or liver injury, AG490 specificity may provide pharmacologic advantages to modulate NF-κB in particular organs or cells types.
JAK2 inhibition with AG490 prevented the production of critical inflammatory factors contributing to sepsis including TNF and HMGB1. TNF is a characteristic immune cytokine produced under the regulation of the NF-κB. However, the inhibition of HMGB1 with AG490 has an additional value because of its clinical implications in sepsis and other infectious disorders. Unlike characteristic immune cytokines, HMGB1 is expressed in all cell types as a nuclear DNA-binding protein that functions as a structural cofactor. However, bacterial endotoxin activates macrophages to secrete HMGB1 into the extracellular milieu where HMGB1 becomes an inflammatory factor contributing to sepsis [7, 8]. Despite its clinical interest, the mechanism of HMGB1 secretion remains elusive and thereby the implications of JAK2. In vitro, LPS activates peritoneal macrophages appears to secrete HMGB1 through a mechanism mediated by JAK2 and both STAT1 and STAT3 [18] or the INF-JAK1-STAT1 pathway [19]. These results reveal the complexity of factors contributing to HMGB1 secretion in sepsis. Probably, one of the most interesting future questions is whether the JAK/STAT pathways are directly involved in the posttranslational modulation of HMGB1 (such as phosphorylation or acetylation) or whether they regulate HMGB1 secretion indirectly. Indeed, more recent studies indicate that HMGB1 can be “passively released” into the extracellular milieu from apoptotic or necrotic cells [30]. Giving these results, JAK2 and NF-κB may contribute to serum HMGB1 by modulating cell necrosis or survival during sepsis, as described above. This mechanism is clinically relevant because HMGB1 is a “late” mediator of sepsis that appears many hours after the onset of the disease. A major advantage of this mechanism is its potential to increase the therapeutic window for sepsis as it rescued animals from “established” lethal peritonitis even when the treatment with AG490 was started at 24 h after the septic challenge. By comparison with other targets, administration of anti-TNF antibodies increased mortality when administered after cecal perforation [32]. Anti-macrophage migration inhibitory factor antibodies are ineffective if administered more than 8 h after the induction of peritonitis [33]. The therapeutic window for alpha7nAChR in animals is also significantly wider than for lysophosphatidylcholine, which is only effective when treatment occurs within 10 h after cecal puncture [34]. Our results suggest that JAK2 may provide pharmacologic advantages for sepsis. However, further investigation is warranted to evaluate its potential clinical translation because as opposed to the experimental models using healthy animals, the clinical settings of sepsis typically involve elderly patients with previous disorders.
Acknowledgments
L.U. is supported by the faculty program of the Department of Surgery of the New Jersey Medical School and grants from the U.S. Army Medical Research Command [USAMRMC#05308004], the American Heart Association [AHA06352230N], and the NIH [RO1-GM084125].
Footnotes
Conflict of interest G.P, B. C., and L.U. are applying for patents as inventors on technology related to the topic.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ulloa L, Brunner M, Ramos L, Deitch EA. Scientific and clinical challenges in sepsis. Curr Pharm Des. 2009;15:1918–1935. doi: 10.2174/138161209788453248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulloa L, Tracey KJ. The "cytokine profile": a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 5.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 7.Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17:189–201. doi: 10.1016/j.cytogfr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 10.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 11.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 13.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, Levitzki A, Roifman CM. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 15.Ruetten H, Thiemermann C. Effects of tyrphostins and genistein on the circulatory failure and organ dysfunction caused by endotoxin in the rat: a possible role for protein tyrosine kinase. Br J Pharmacol. 1997;122:59–70. doi: 10.1038/sj.bjp.0701345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JY, Sullivan KE. Gamma interferon and lipopolysac-charide interact at the level of transcription to induce tumor necrosis factor alpha expression. Infect Immun. 2001;69:2847–2852. doi: 10.1128/IAI.69.5.2847-2852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui L, Yao Y, Wang S, Yu Y, Dong N, Li H, Sheng Z. Inhibition of Janus kinase 2 and signal transduction and activator of transcription protect against cecal ligation and puncture-induced multiple organ damage. J Trauma. 2009;66:859–865. doi: 10.1097/TA.0b013e318164d05f. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Yao YM, Yu Y, Dong N, Yin HN, Sheng ZY. Role of Janus kinase/signal transducer and activator of transcription pathway in regulation of expression and inflammation-promoting activity of high mobility group box protein 1 in rat peritoneal macrophages. Shock. 2007;27:55–60. doi: 10.1097/01.shk.0000233197.40989.31. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Kim SJ, Lee IS, Lee MS, Uematsu S, Akira S, Oh KI. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J Immunol. 2009;182:2458–2466. doi: 10.4049/jimmunol.0801364. [DOI] [PubMed] [Google Scholar]
- 20.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X, Patel N, Johnson SM, Chavan S, Goldstein RS, Czura CJ, Miller EJ, Al-Abed Y, Tracey KJ, Pavlov VA. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15:195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 23.Ghaffari S, Kitidis C, Fleming MD, Neubauer H, Pfeffer K, Lodish HF. Erythropoiesis in the absence of janus-kinase 2: BCR-ABL induces red cell formation in JAK2(−/−) hematopoietic progenitors. Blood. 2001;98:2948–2957. doi: 10.1182/blood.v98.10.2948. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Ma Y, Cole SM, Zander C, Chen KH, Karras J, Pope RM. Serine phosphorylation of STAT3 is essential for Mcl-1 expression and macrophage survival. Blood. 2003;102:344–352. doi: 10.1182/blood-2002-11-3396. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 26.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 27.Doi TS, Marino MW, Takahashi T, Yoshida T, Sakakura T, Old LJ, Obata Y. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc Natl Acad Sci USA. 1999;96:2994–2999. doi: 10.1073/pnas.96.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 29.Mantell LL, Parrish WR, Ulloa L. HMGB1 as a therapeutic target for infectious and inflammatory disorders. Shock. 2006;25:4–11. doi: 10.1097/01.shk.0000188710.04777.9e. [DOI] [PubMed] [Google Scholar]
- 30.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crandall M, Shapiro MB, West MA. Does splenectomy protect against immune-mediated complications in blunt trauma patients? Mol Med. 2009;15:263–267. doi: 10.2119/molmed.2009.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 33.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Mannel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 34.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]