Abstract
Obliterative bronchiolitis (OB) is the key impediment to the long term survival of lung transplant recipients and the lack of a robust pre-clinical model precludes examining OB immunopathogenesis. In the current study, lungs from C57BL/10 H-2b mice that are MHC compatible, but minor histocompatability antigen incompatible, were transplanted into C57BL/6 mice. Histological features and cytokine profiles of OB were assessed. Moderate rejection (grade A3) developed by day 14, with evidence of OB at that time point. At 21 days, OB was present in 55% of grafts and moderate to severe rejection (Grade A3-A4) was present in all mice. At 28 days, OB was present in 44% of mice and severe rejection (Grade A4) was present in all. IL-17A, but not IL-17F, splenic mRNA transcripts and serum protein levels were increased only in mice that developed OB, whereas IL-10 transcripts and protein were increased only in non-OB mice. Neutralizing IL-17 prevented OB, down regulated acute rejection, and upregulated systemic IL-10. Collectively, these data show that transplantation of minor histoincompatible lungs from C57BL/10 mice into C57BL/6 mice results in a highly reproducible pre-clinical model of OB. In addition, these data indicate that neutralizing IL-17A or augmenting IL-10 could be therapeutic interventions to prevent OB.
Keywords: Lung transplantation, IL-17, mouse, obliterative bronchiolitis
INTRODUCTION
Orthotopic lung transplantation in rats were first reported by Asimacopoulos and colleagues in 1971 (1). Widely utilized by many investigators, the rat model, and more recently the murine model, became the accepted approach to study the immunopathology of lung transplant rejection. As expected, the tempo and histology of the rejection response varied depending on the degree of histoincompatibilty between donor and recipient. For example, fully MHC incompatible rat or mouse lung allografts undergo severe acute rejection within five days post-transplant with evidence of necrosis on the seventh day, but no evidence of obliterative bronchiolitis (OB) at any time point (2).
Due to no effective therapies for OB, the leading cause of death in lung transplant patients, there is a need for pre-clinical models that replicate OB. The tracheal allograft model may reproduce some of the histopathologic features of OB (3). However, incomplete pathophysilogic features of OB limit the utility of this model (4). Hirt et al, reported OB in a rat model of orthotopic lung transplantation utilizing donors and recipients that were MHC-matched but disparate at minor histoincompatible antigens (5). Occurring by the 14th week post-transplant, and confirmed by the Wilkes laboratory (6), OB histopathology was very similar to the human condition, but unpredictable in onset. While certain minor antigens may be immunodominant, the full range of murine minor histocompatibility antigens is not known. In addition, unlike MHC, it is possible that minor antigen expression may vary even within a specific strain of in bred mice (7). Obliterative bronchiolitis is believed to be immunologically mediated due to recognition of transplantation antigens that could include MHC and/or minor histocompatibility antigens. Since as yet determined minor antigens could be expressed variably within in bred mice, then we hypothesized that if OB was minor antigen dependent then it may it may occur variably in lung allografts, i.e., not all lung allografts may develop OB.
Lung transplant rejection is associated with the production of a multitude of cytokines. IL-17, involved in both innate and adaptive immunity, is a family of six isoforms and IL-17A has a key role in the pathogenesis of alloimmune-induced autoimmunity post lung transplant (8). In addition, a recent report from Vanaudenaerde and colleagues demonstrated that IL-17 was associated with bronchiolitis obliterans syndrome (BOS) (9). However, there is no direct evidence that IL-17 induces OB or that IL-17 blockade will prevent OB.
Noting the onset of OB in the minor histocompatible antigen mismatch rat model, in the current study we utilized lungs from two different strains of murine mice (SJL and C57BL/10, H2s and H2b) for transplantation into minor histoincompatible C57BL/6 mice (H2b). While severe acute rejection was manifest in the SJL donor lungs, OB did not develop occur at any time point. In contrast, OB was manifest as early as 14 days post-transplantation in C57BL/10 lungs and progressed through the 28th day post transplantation. High expression of IL-17A transcripts in the spleen and serum protein levels were only observed in mice that developed OB, and neutralizing IL-17A prevented OB.
MATERIALS AND METHODS
Animals
Specific pathogen-free male inbred mice C57BL/6 (H2b), C57BL/10(H2b), and SJL (H2S) were purchased from Harlan Sprague-Dawley (Indianapolis, IN) and housed in the Laboratory Animal Resource Center at Indiana University School of Medicine in accordance with institutional guidelines. All mice which were 8–12 weeks of age and 24–32g were used as both donors and recipients. All studies were approved by the Laboratory Animal Resource Center at the Indiana University School of Medicine.
Surgical Technique
Orthotopic lung transplantation
All surgical procedures were performed by L.F. utilizing sterile techniques. A Prescott's operating microscope (Zeiss 6SFC, Monument, USA) with 20–40X magnification was used for both donor and recipient operations. Both donor and recipient were anesthetized with Isoflurane.
Donor Procedure
After intubation with a 20-guage catheter, the donor was ventilated (Harvard Rodent Ventilator, Harvard Apparatus, Holliston, MA) using 100% oxygen at 125 breaths/minute and 0.5 ml tidal volume. Anesthesia was maintained with inhaled 1-2% Isoflurane. After preparing with Betadine solution (Purdue Products L.P. Stamford, CT), a laparosternotomy was performed as a combined midline and transverse incision thus exposing the thoracic cavity. Heparin (APP Pharmaceuticals, LLC, Schaumburg, IL, 100u/kg) was injected into the IVC, and the lungs flushed with 2 mL of cooled (4°C) Perfadex solution (Vitrolife, Inc, Englewood, CO) and 0.1 ml of Heparin via the root of the PA (pulmonary artery) trunk. The heart–lung block was excised after arresting ventilation and stored on ice (4°C).
The donor left lung was then prepared for the recipient by the attachment of cuffs. In brief, PA cuff was made from a 24-gauge I.V. catheter (Terumo Medical Corporation, Somerset, NJ) (10). The bronchus (Br) cuff was a 20-gauge catheter and the Pulmonary Vein (PV) cuffs varied with the weight of the donor mouse (10). Specifically, for mice 24-27 grams the cuff size was 22 gauge. For mice weighing 27-32 grams, the studies utilized a 20 gauge catheter. The cuffs were inserted into the distal ends of the PA, PV and bronchus and secured with a 9-0 silk suture (Ethicon, JOHNSON & JOHNSON Company, Piscataway, NJ). The prepared donor lung was wrapped in Perfadex-soaked gauze and placed on ice (4°C) until transplantation.
Recipient Procedure
After anesthesia induction, the left chest wall was shaved and sterilized, a thoracotomy incision made in the left fourth intercostal space, and pulmonary vessels and bronchus clamped, and the left lung was resected. After dissecting the PA and PV completely from their adventitial sheath, a small transverse incision of approximately one fourth of the vessel's circumference was made into the anterior wall. The donor lung, wrapped in cold saline was positioned in the thoracic cavity, cuffs inserted into the recipient Br, PV and PA and secured with 9-0 silk suture. The hilar cross clamp was removed and the thoracotomy incision was closed using a 5-0 silk suture, with a 30 gauge blunt tip needle attached to a syringe inserted into the thoracotomy site to aspirate any remaining air. The mouse was allowed to recover from anesthesia. No mice received any immunosuppressive agents.
Histology
Mice were euthanized by ketamine (50 mg/kg)/xylazine (10 mg/kg), native and donor lungs harvested, glutaraldehyde-fixed, and paraffin embedded. A portion of the lower lobe of each lung was sectioned and stained with hematoxylin/eosin or Masson's trichrome stain (11). Four sections from the lower lobes of transplanted lungs were examined and grading of rejection pathology conducted in a blinded fashion utilizing standard criteria for clinical lung transplantation (12). Scoring was focused on the severity of vascular lesions (“A” scores). A0= no acute rejection, A1= minimal acute rejection, A2= mild acute rejection, A3= moderate acute rejection, A4= severe acute rejection. Since the airway involvement during the acute rejection response paralleled that observed in the vasculature, then “B scores” (airway) were not reported. The presence of absence of OB was determined.
IL-17R Fusion Protien
IL-17A:Fc fusion protein (AdIL-17R:Fc) was a generous gift from Dr. Jay K. Kolls, (New Orleans, LA) (13), the control vector, adenovirus vector encoding the firefly luciferase gene (AdLuc) was purchased from (Welgen, Inc, Worcester, MA). AdIL-17R:Fc, an adenoviral vector encoding a soluble murine IL-17A:Fc fusion protein (AdIL-17R:Fc), neutralizes IL-17A and IL-17F activity (13). C57BL/6 (recipient) mice were injected via tail vein with AdlL-17R:Fc or AdLuc at 109 plaque forming units (pfu) per animal. Seventy two hours later, C57BL10 (donor) lungs were transplanted into AdlL-17R:Fc or AdLuc treated (recipient) C57BL/6 mice. Twenty one days post transplant, mice were euthanized by ketamine (50 mg/kg)/xylazine (10 mg/kg), and the lungs were harvested. Vector construction has been described (14).
Cytokine profiling by Quantitative RT-PCR
Total RNA was isolated from spleen of transplant recipient mice using RNeasy RNA extraction kit (Qiagen, Inc., Valencia, CA), and mRNA expression levels detected with PerfeCTa™ SYBR Green FastMix, Low ROX (Quanta Biosciences, Gaithersburg, MD) on a Applied Biosystems 7500 according to the manufacturer's instructions. Each isograft and allograft sample was normalized to murine β-actin. Isograft samples were then averaged according to days post transplant. Allograft samples were normalized to the isograft average corresponding to days post transplant. Primer sequences: β-actin-{F:CAATAGTGATGACCTGGCCGT, R:AGAGGGAAATCGTGCGTGAC}, IL-17A-{F:CTGTGTCTCTGATGCTGTTG, R:ATGTGGTGGTCCAGCTTTC}, IL-17F-{F:CTGTTGATGTTGGGACTTG, R:GTTCATGGTGCTGTCTTCCTG}, IL-10-{F:GGTTGCCAAGCCTTATCGGA, R:ACCTGCTCCACTGCCTTGCT}, IFN-γ-{F:TGGCTCTGCAGGATTTTCATG, R:TCAAGTGGCATAGATGTGGAAGAA}, IL-4-{F:ACAGGAGAAGGGACGCCAT, R:GAAGCCCTACAGACGAGCTCA}, TGF-β-{F:TGACGTCACTGGAGTTGTACGG, R:GGTTCATGTCATGGATGGTGC} (Integrated DNA Technologies, Coralville, IA).
Cytokine profiling by cytometric bead array (CBA)
Serum from isograft and allograft transplant C57BL/6 recipients was collected and cytokine protein levels of IL-17A, IL-10, TNF-α, IFN-γ, IL-6, IL-4, and IL-2 were measured using the Mouse Th1/Th2/Th17 Cytokine Kit (BD Biosciences, San Jose, CA) according to manufacturer's instructions.
ELISPOT assay
Splenocytes from lung transplant recipient mice were harvested at post-operative day (POD) 7, 14, and 21. Cells were seeded in HL-1 media [2 mmol/l glutamine, 50 μg/ml penicillin-streptomycin] at 2.3 × 105 − 5 × 105 per well. Mouse IL-17A (eBiosciences, San Diego, CA) and IFN-γ (BD, San Jose, CA) purified and biotinylated pairs were used to detect cytokine-producing cells. Recipient cells were stimulated with donor antigen (irradiated splenocytes, POD 7 or cell lysate, POD 14) or type V collagen [col(V)] as reported (8). Cells were incubated for 48 h at 37°C/5% CO2. Spots were developed with streptavidin-AP and BCIP substrate (R&D Systems) and counted using the AID ELISpot Reader System to give a total spots/million cytokine producing cells.
Statistical analysis
Data expressed as means ± Standard Deviation. The significance of differences between means in figure 2J and figure 6E was determined by one-way ANOVA. Lung transplant recipients with and without OB were compared using two tailed Fisher's exact test (Table 2). Figure 4 was analyzed using two-way ANOVA with Bonferroni post-tests. Figure 5 was analyzed using two-tailed unpaired t tests. A “p value” of <0.05 was considered significant. All analyses were performed using a statistics software package (GraphPad Prism 4).
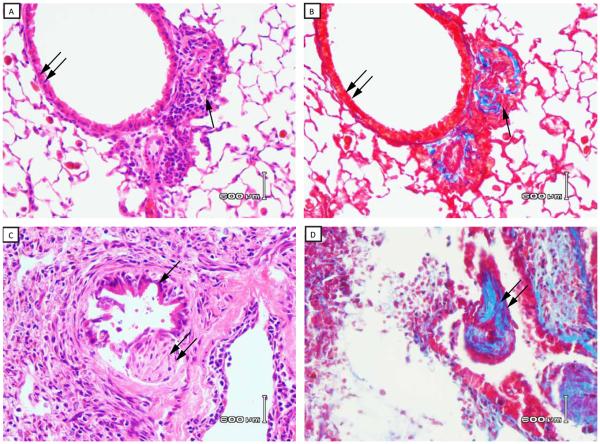
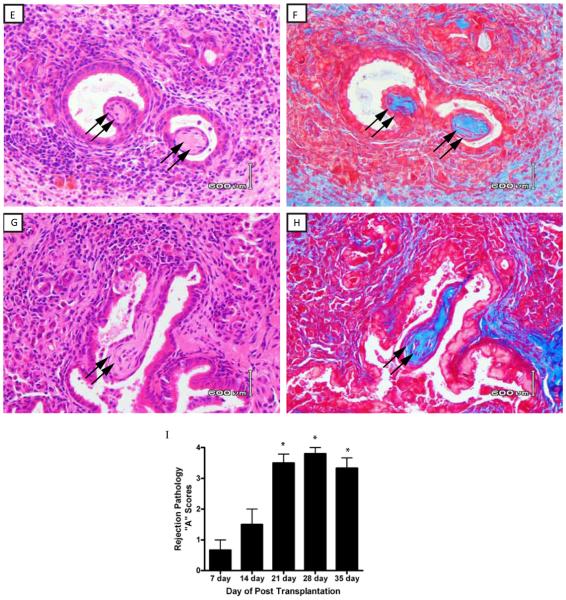
Figure 2. Histopathology of C57BL/10 lung allografts transplanted into C57BL/6 mice – days 7, 14, 21, 28 and 35.
Lung transplantation was performed as described in Methods and transplanted lungs harvested at 7, 14, 21, 28 and 35 days post transplant. Panels 2A and 2C represent H&E stained lung sections from allografts harvested at days 7 and 14, respectively. Panels 2B and 2D represent trichrome stains (blue color) of the same sections shown in 2A and 2C, respectively. Double arrows in 2A and 2C identify airway epithelium and single arrow identifies an early area of peribronchiolar fibrosis in the same sections (day 7). The single arrow in 2C (day 14) indentifies residual airway epithelium, and lesions of OB are identified by the double arrows in 2C and 2D (day 14). Panels 2E and 2F represent H&E stained lung sections from allografts harvested at days 21 and 28, respectively. Panels 2F and 2H represent trichrome stains of the same sections shown in 2E and 2H, respectively. Double arrow shows lesions of OB in each panel. Data are representative three mice transplanted at day 7 and day 14, five of nine mice harvested at 21 days (Figure 2E and 2F) and four of nine mice harvested at 28 days (Figure 2G and 2H) with similar pathology. (40x magnification). I) Histological score of acute rejection (“A” scores) by standard criteria as described in Methods. Data represent the mean + SD of “A” scores for three mice each at 7, 14 and 35 days, and nine mice each at 21 and 28 days (*p < 0.05 compared to day 7 or day 14 groups).
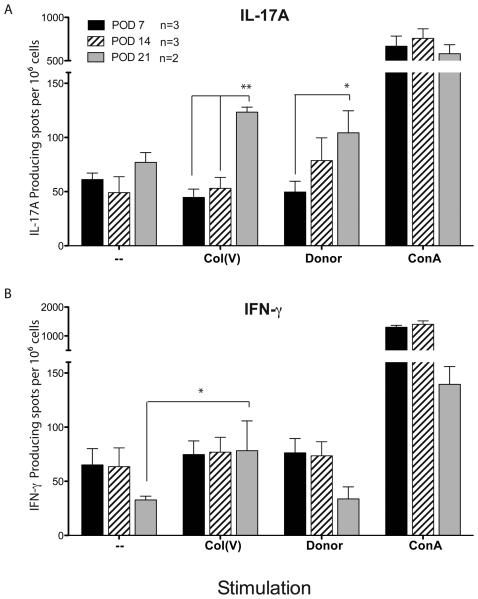
Figure 6. IL-17 production in response to Col(V) and B6 donor antigens.
Splenocytes from lung transplant recipient mice were harvested at 7, 14, and 21 days post transplant. Cells were seeded at 2.3 × 105 − 5 × 105 per well. Recipient cells were stimulated with donor antigen (irradiated splenocytes, 7 days post transplant or cell lysate, 14 days post transplant) or col(V). Cells cultured without antigen or with concanavalin A (Con A) were used as negative and positive controls, respectively. Cells were incubated for 48 h at 37°C. Spots were developed to give a total spots/million IL-17 cytokine producing cells. Data are representative of two-three mice in each group. Response of recipient cells were compared using 2-way repeated measures ANOVA analysis. ** p<0.01, * p<0.05
Table 2.
Prevalence of Obliterative Bronchiolitis Relative to Time Post Transplant.
| Day of post- transplantation |
OB / Total Mice in Group | ||
|---|---|---|---|
| C57BL/6 → C57BL/6 | SJL→ C57BL/6 | C57BL/10 → C57BL/6 | |
| 7 | 0/3 | 0/3 | 0/3 |
| 14 | 0/4 | 0/4 | 1/3 |
| 21 | 0/4 | 0/4 | 5/9* |
| 28 | 0/4 | N/A | 4/9* |
| 35 | 0/4 | N/A | 0/3 |
Isograft (C57BL/6→C57BL/6) or allograft (SJL→C57BL/6 or C57BL/10→C57BL/6) lung tissues were harvested and examined microscopically for OB at days 7, 14, 21, 28 and 35 post transplantation. Data represent the quantity of mice in each group that developed OB at each time point (p<0.05 compared to other groups at the same time point).
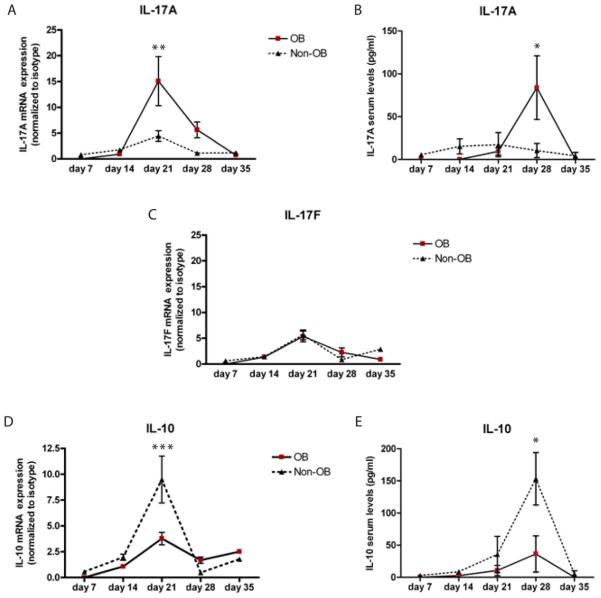
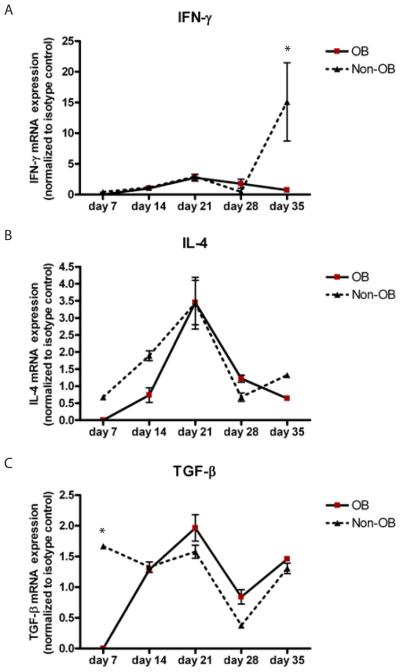
Figure 4. IL-17A/F and IL-10 cytokine profile of OB v.s. Non-OB relative to time post transplantation.
Spleen tissue and serum from C57BL/6 transplant mice were collected and A) IL-17A, C) IL-17F and D) IL-10 mRNA expression levels were measured by quantitative RT PCR. B) IL-17A and E) IL-10 protein expression was measured by cytometric bead assay. Data are representative of three mice harvested at 14 days, nine mice harvested at 21 days, nine mice harvested at 28 days and three mice harvested at 35 days. *p<0.05, **p<0.01, ***p<0.001
Figure 5. IFN-γ, IL-4 and TGF-β cytokine profile of OB v.s. Non-OB relative to time post transplantation.
Spleen tissue from C57BL/6 transplant mice were collected and A) IFN-γ, B) IL-4 and C) TGF-β mRNA expression levels were measured by quantitative RT PCR. Data are representative of three mice harvested at 14 days, nine mice harvested at 21 days, nine mice harvested at 28 days and three harvested at 35 days. *p<0.001,
RESULTS
Surgical Outcomes
Preliminary surgeries revealed that ventilation and perfusion of the transplanted lung was highly dependent on the size of PV anastomotic cuffs relative to the weight of donor and recipient mice. Specific PV anastomotic cuff sizes were utilized to match donor and recipients as reported (10). Forty transplants were performed to determine the optimal cuff sizes. Improper cuff sizing resulted in either atelectasis of the transplanted lung or dehiscence of the PV anastomosis. Once the optimal cuff sizes were established we conducted 60 transplants with a 96% technical success rate, i.e., 58 of 60 mice transplanted survived with no evidence of infarction, mucous plugging, or anastomotic dehiscence at the time of sacrifice at days 7, 14, 21, 28 and 35 post-transplantation. The warm ischemia time was 17.74 ± 3.19 minutes; cold ischemia time was 76.26 ± 8.50 minutes for the group of 58 mice.
Histologic Features
Except for isografts (C57BL/6 lungs →C57BL/6), all groups were major histocompatibility antigen matched (H-2b) but had varying degrees of minor histoincompatibilty (Table 1). Using standard rejection response criteria, we examined the histology in isograft recipients (C57BL/6 →C57BL/6), or two groups of minor histoincompatible allografts (SJL→C57BL/6 or C57BL/10→C57BL/6). As expected, no histologic lesions were routinely detectable in the isograft lungs at all times post-transplant (not shown). Although the SJL→C57BL/6 is considered a “intermediate” minor mismatch, all transplanted lungs had undergone severe acute rejection (Grade A4) within seven days post transplantation (Figure 1). By the 14th day all lungs were completely destroyed and, therefore, were not able to be scored histologically at that time point or later. Obliterative bronchiolitis was not detected in the SJL→C57BL/6 group. In contrast, mild rejection, on average Grade A1, was detected by the seventh day post-transplant in the C57BL/10→C57BL/6 model which progressed to severe rejection by the 21st and 28th day (on average Grade A3-A4, Figure 2J). Whereas OB was not detected by the 7th post-transplant day (Table 2, Figure 2A), lesions of OB were detectable in one of three mice at day 14 (Figure 2C), and were manifest in nearly 60% C57BL/10 lungs by day the 21st day (Table 2, Figure 2E), and 44% of mice at 28 days (Table 2, Figure 2G).
Table 1.
Degree of Minor Histoincompatibility
| Group | Strain Combination | Degree of Minor Histoincompatibility |
|---|---|---|
| Isograft | C57BL/6(H2b)→C57BL/6(H2b) | None |
| Allograft | C57BL/10(H2b)→C57BL/6(H2b) | Weak |
| Allograft | SJL /(H2S)→C57BL/6(H2b) | Intermediate |
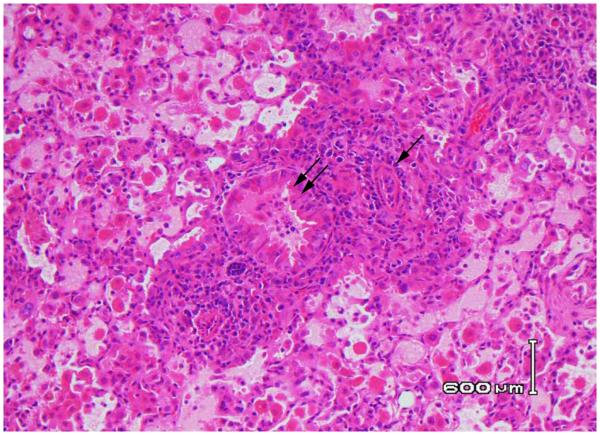
Figure 1. Histopathology of SJL lung allograft transplanted into a C57BL/6 mouse recipient.
Lung transplantation was performed as described in Methods and transplanted lungs harvested at seven days post transplant. H&E stained section reveals extensive mononuclear cell infiltration with destruction of airways and vessels, Grade A4 rejection. Double arrows reveal an infiltrated bronchiole, and the single arrow identifies severe vasculopathy. Data are representative of three mice with similar lesions (40x magnification).
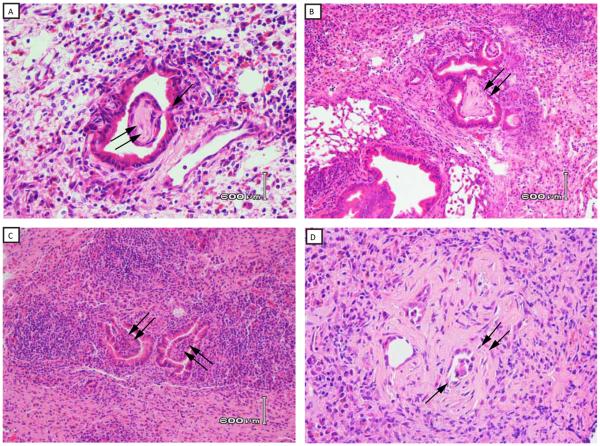
Peribronchiolar fibrosis and fibrotic plugs are histologic hallmarks of OB (12). Therefore, trichrome stains were used to determine the presence of fibrosis in OB lesions. Fibrotic changes were seen in peribronchiolar tissues and airway plugs of mice with OB (Figure 2D, 2F, 2H). Small bronchioles were associated with a mild peribronchiolar mononuclear infiltrate. In some, the overlying epithelium was attenuated with distortion of the airway lumen. The lesions of OB appeared to progress over time, and were manifest as subepithelial or polypoid fibrotic lesions that impinged on the bronchiolar lumen (day 14, Figure 3A), to large lesions nearly obstructing the airways at days 21 and 28 (Figure 3B and 4C, respectively). Another hallmark of OB is depithelialization of the airway in the area of the fibrotic plug. Indeed, figures 2C and 3B show loss of epithelium overlying the fibrous plug. An additional finding of OB is the near obliteration or complete obliteration of airways as observed in figure 3D. In many OB lesions there was direct evidence of subepithelial connective tissues in the form of a “polyp” erupting through the bronchiolar epithelium that was attached to the airway by a stalk of granulation tissue (Figure 3A).
Figure 3. Progression of OB relative to time post transplantation.
Lungs from C57BL10 mice were transplanted into C57BL/6 mice as described in methods. A) Polypoid lesion in bronchiole at 14 days post transplantation. Note the stalk of connective tissue erupting through the bronchial wall and extending into the lumen (single arrow) that is attached to an intraluminal fibrotic polyp of OB (double arrow). B) OB at 21 days post transplant. Double arrow identifies OB lesion nearly occluding bronchiolar lumen. C. OB at 28 days post transplant. D. Obliterative bronchiolitis at 28 days. Double arrow identifies OB lesions occluding bronchiolar lumens. Data are representative of one of three mice harvested at 14 days (Figure 3A), five of nine mice harvested at 21 days (Figure 3B), and four of nine mice harvested at 28 days (Figure 3C, 3D) with similar pathology. (20x magnification).
Systemic cytokine profiles
With previous reports demonstrating a possible link between IL-17 and acute rejection/BOS, and with data demonstrating that the progression of OB lesions occur over time, we next determined IL-17A/F splenic mRNA and serum protein expression patterns in OB versus non-OB mice. Compared to isografts, IL-17A and IL-17F transcript expression peaked at 21 days. However, IL-17A reached a nearly 20 fold increase as compared to a five-fold increase observed for IL-17F. Moreover, mice with OB exhibited significantly elevated IL-17A transcript levels compared to non-OB mice (Figure 4A, p<0.01). IL-17F transcript expression was not different between OB and non-OB mice (Figure 4C). These data suggest a temporal sequence of IL-17A expression that corresponds to the presence of OB. Serum IL-17A protein levels were increased in OB, compared to non-OB mice (Figure 4B, p <0.05), and peaked one week (28 day) after the peak in transcript levels. IL-10 mRNA transcripts were eleveated in non-OB mice 21 days post transplantation (Figure 4D, p<0.001). IL-10 serum protein peaked in non-OB mice at day 28 (Figure 4E, p<0.05), which was pattern similar to IL-17A transcript/protein. We did not observe any temporal relationship to the presence or absence of OB and IL-4, IFN-γ, or TGF-β transcript expression (Figure 5). In fact, IFN-γ, often associated with rejection responses, was upregulated in non-OB mice at day 35 and not in those mice that developed OB (Figure 5A).
Col(V) and donor antigens induce IL-17 secretion
With data demonstrating elevated IL-17A protein in mice that developed OB compared to non-OB mice, and our reports demonstrating col(V) as an inducer of IL-17 in clinical OB (8), we next determined if donor-derived antigens or col(V) were responsible for the induction of IL-17 secretion in mice that developed OB. ELISPOT data revealed few IL-17 producing cells from mice at 7, 14 or 21 days post transplant when cultured in the absence of an antigenic stimulus (figure 6A). In response to donor-derived antigen or col(V), there was a significant time dependent increase in IL-17 producing cells, peaking at day 21 (Figure 6A,** p<0.01, * p<0.05). Although Th1 (T helper 1) responses have been linked to allograft rejection, neither donor antigens nor col(V) induced IFN-γ production (Figure 6B).
IL-17 receptor fusion protein prevents the development of OB
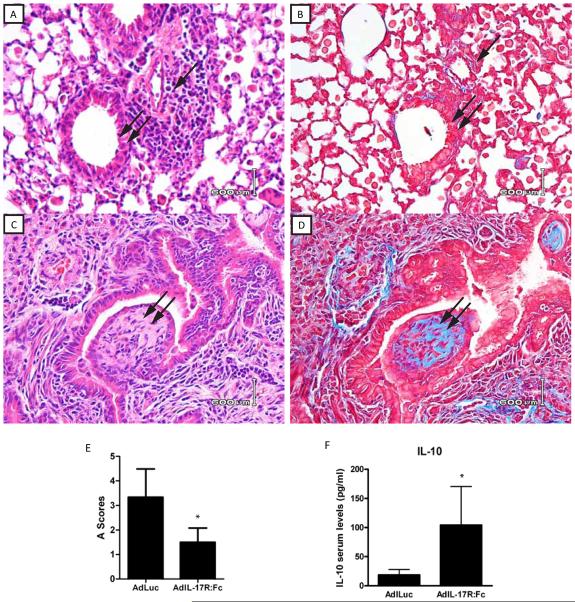
To further confirm the role of IL-17A in the development of OB, we utilized a vector encoding a soluble murine IL-17R:Fc fusion protein (AdIL-17R:Fc) that neutralizes circulating IL-17A and IL-17F by preventing their binding to their receptors and downstream signaling events. Thus, we hypothesized that blocking IL-17A/F from binding to their receptors, will afford protection and prevent OB in the C57BL/10→C57BL/6 transplant model. C57BL/6 recipient mice were injected intravenously with AdIL-17R:Fc or an adenovirus empty vector encoding firefly luciferase gene (AdLuc) on day 0 pre-transplant. Seventy-two hours later, C57BL10 lungs were transplanted into these AdIL-17R:Fc or AdLuc treated C57BL/6 recipient mice. Since our previous data demonstrated that day 21 post transplantation yielded such a significant increase in IL-17A expression, and OB was also manifest at this time, we focused on day 21 in these studies. As shown in Figure 7A, treatment with AdIL-17R:Fc, the IL-17 receptor fusion protein, prevented the development of OB as compared with treatment with AdLuc, the empty vector, which developed OB as expected (Figure 7C). Furthermore, the severity of acute rejection was also diminished, as shown by the 50% decrease in “A” scores (Figure 7E). Indeed, trichrome staining confirmed that the IL-17 receptor fusion protein prevented the fibrotic changes seen in the mice with OB (Figure 7B and 7D respectively). Additionally, protein analysis revealed elevated levels of serum IL-10 protein in the mice treated with the IL-17 fusion protein (Figure 7F, p=0.05).
Figure 7. Histopathology of C57BL/10 lung allografts transplanted into AdIL-17R:Fc or AdLuc treated C57BL/6 mice – day 21.
Lung transplantation was performed as described in Methods and transplanted lungs harvested at 21 days post transplant. Panels A and C represent H&E stained lung sections from allografts harvested at day 21 and panels B and D represent trichrome stains of the same sections shown in A and C, respectively. Single arrow orients to a small vein in the lung corresponding to site of rejection. Double arrow shows lesions of OB in each panel. E) Histologic rejection scores for Adluc and AdIL-17R:Fc treated mice. Data represent the mean + SD of “A” scores for seven mice of AdLuc treated and seven mice of AdIL-17R:Fc at 21 days (*p < 0.05 compared to AdIL-17R:Fc treated and the AdLuc treated mice. F) IL-10 serum protein expression in AdIL-17R:Fc treated as compared to the AdLuc treated mice. *p=0.05. Data are representative of seven mice in each group harvested at 21 days with similar pathology. (40x magnification).
DISCUSSION
OB is the major impediment to the long term survival in lung transplant recipients. Since the pathogenesis of OB remains poorly understood and there are no effective therapies, there is a critical need for animal models that can facilitate understanding of this devastating disease. To the best of our knowledge, the current study is the first report of OB that occurs reproducibly in a clinically relevant orthotopic murine model of lung transplantation.
The recent development of the orthotopic murine lung transplant model is a major development in the attempt to gain greater insights into the immunopathogenesis of the rejection response (2, 15). However, these models, utilizing strains that are disparate at MHC antigen loci, develop severe acute rejection within seven days post-transplant, and destruction of the graft without developing OB (2, 16). Allan et al (17) reported the OB development in a minor histocompatibility antigen mismatched swine orthotopic lung transplant model. Our laboratory and others identified OB in orthotopic rat lung transplants that were MHC-matched but minor histocompatible antigen mismatched (5, 6, 18). However, limitations of the swine model include its expense, and the rat model is limited by inconsistent onset of OB and the 12 to 14 week period required for the lesion to develop. The vigorousness of the rejection response and the rapid onset of graft destruction in the MHC mismatch models may account for the inability to detect OB. In other words, the graft may be destroyed before OB develops. Similar to Allan's group (17), our prior reports of OB utilized minor histoincompatible strains as donors and recipients (6). Due to a less vigorous alloimmune response, we hypothesized that OB may develop prior to allograft destruction in a MHC-matched but minor antigen mismatched model. Indeed, the current study shows OB developed in the murine minor histoincompatible mismatch model. A key question that remains is why OB is not observed uniformly in all mice. In fact, OB was noted in approximately 50% of all mice studied at 21 and 28 days. Since the lesions appeared to progress over time then we hypothesized that later time points would allow for more uniform development of OB. However, the prevalence of OB remained at 50% even at 35 days while the allograft was becoming generally fibrotic with loss of recognizable lung structures (data not shown).
Obliterative bronchiolitis pathology may vary in clinical lung transplantation. In the human transplant setting, obliterative bronchiolitis has been primarily described as constrictive rather than proliferative. The proliferative lesions have been best described in bronchiolitis obliterans organizing pneumonia (BOOP), which can result from alloimmune-mediated injury and contain some features of OB. In this minor mismatch model, although we did not see features of BOOP, the OB lesions had some evidence of a proliferative appearance that may have been ascribed to BOOP. However, the presence of fibrous plugs, evidence of depithelialization, and airway obliteration are very consistent with OB. These differences in observation in our murine model may be due to the fact that murine airways may exhibit different characteristics causing them to take on more of a proliferative appearance, rather than the a constrictive appearance seen in human airways. Altenatively, the histopathological differences of OB manifestation may also be a consequence of the murine model driven by minor MHC differences rather than the minor and major differences that occur in humans. While it is important to acknowledge the potential histopathologial differences between murine OB and human OB, it is important to highlight the fact that this model was able to generate OB lesions, something not previously describled in the mouse orthotopic lung transplant model.
Obliterative bronchiolitis is believed to be mediated via immune activation. The nature of the alloimmune response, and therefore pathology of rejection, is not uniform in clinical transplant due to multiple variables including the polymorphic nature of MHC molecules. In the current study the pathology of the rejection response should be uniform due to the use of in inbred mice. Therefore, if OB pathogenesis is due strictly to immune activation, then one would expect OB to occur in a uniform fashion in all mice studied in the C57BL/10→C57BL/6 combination in the current study. Although the donor and recipient mice were MHC matched (H2b), they differed in the expression of minor histocompatibility antigens H-9, Igh-2, and Lv (7). While certain minor antigens may be immunodominant, the full range of murine minor histocompatibility antigens is not known. Unlike MHC, minor antigen expression may vary within a specific strain of in bred mice (7). Therefore, the recognition of an, as yet, unidentified minor antigen(s) expressed in either donor or recipient mice could have contributed to rejection, OB and accounted for the variable OB expression observed. Furthermore, immune recognition of non-histocompatibility antigens, such as the autoantigens type V collagen and tubulin, implicated in the pathogenesis of OB in patients and pre-clinical models (8, 19), may have contributed to the onset of OB in the current study. Indeed, data in the current study showed that both donor antigens and col(V) were able to induce IL-17 production. These data highlight further the role of alloantigens and autoantigens in OB pathogenesis.
Variable OB expression in the current study is an opportunity to identify the key genes involved in its pathogenesis. Although our data suggest that IL-17A in OB development, the process appears time dependent. In OB mice, IL-17A transcript and protein expression patterns exhibited a time course, with transcript levels and serum protein peaking at day 21 and 28 days, respectively; a sequence that matched time to onset of OB lesions. Rejection severity increased during this time and progressed to OB in nearly 60% of mice by day 21. In contrast to OB-mice, IL-17A was lower and IL-10 increased systemically in non-OB mice. These data suggested that IL-10 may be protective, and that blocking IL-17A/F would prevent OB. Indeed, blocking IL-17 prevented OB at 21 days post transplant, and these mice had lower acute rejection scores and upregulated systemic IL-10. We and others have reported the protective effects of IL-10 in rat lung allografts (28, 29). We previously reported in a rat model of lung allograft rejection that concomitant treatment with cyclosporine A (CsA) and collagen V (Col V) exhibited a higher frequency of alloantigen-induced IL-10+ T cells which was associated with preventing graft rejection (28). Peron et al, reported in a murine model of EAE, that oral tolerance to IL-17 reduced IL-17 expression in the periphery and CNS and lead to elevated levels of IL-10 expression (30). The current study suggests that IL-17A may promote while IL-10 prevents OB. Since OB is a fibrotic lesion, then data showing IL-17A blockade prevents OB are consistent with studies of non-transplant-related lung disease that showed blocking IL-17A prevented fibrosis (31).
In summary, the data strongly suggest a role for minor, and not major, histocompatibility antigens in OB pathogenesis. This model should be helpful in understanding molecular mechanisms involved in OB, the specific minor antigens involved, and suggest additional study of the role of IL-17 and IL-10 in OB induction and prevention, respectively.
Acknowledgements/ Funding sources
This work as supported by National Institutes of Health grants RO1 HL067177 and PO1 AI084853 to D.S.W. RO1-AI 066219 and RO1-AI 066219-S2 to W.J.B.
List of Abbreviations
- OB
Obliterative Bronchiolitis
- MHC
Major Histocompatibility Complex
- IVC
Inferior Vena Cava
- PA
Pulmonary Artery
- PV
Pulmonary Vein
- Br
Bronchus
- I.V.
Intravenous
- SD
Standard Deviation
- OTT
Orthotopic Tracheal Transplantation
- HTT
Heterotopic Tracheal Transplantation
- BA
Bronchial Artery
- BOS
Bronchiolitis Obliterans Syndrome
- AdLuc
the firefly luciferase gene
- AdIL-17R:Fc
IL-17A:Fc fusion protein
Footnotes
Disclosure: This manuscript was not prepared in any part by a commercial organization, including educational grants. D.S.W. is co-founder of ImmuneWorks, a biotech company developing therapeutics for various forms of lung diseases. No other authors have any disclosures to report as described by the American Journal of Transplantation.
REFERENCES
- 1.Asimacopoulos PJ, Molokhia FA, Pegg CA, Norman JC. Lung transplantation in the rat. Transplant Proc. 1971;3(1):583–585. [PubMed] [Google Scholar]
- 2.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7(6):1672–1679. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 3.McDyer JF. Human and murine obliterative bronchiolitis in transplant. Proc Am Thorac Soc. 2007;4(1):37–43. doi: 10.1513/pats.200605-107JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato M, Keshavjee S, Liu M. Translational research: animal models of obliterative bronchiolitis after lung transplantation. Am J Transplant. 2009;9(9):1981–1987. doi: 10.1111/j.1600-6143.2009.02770.x. [DOI] [PubMed] [Google Scholar]
- 5.Hirt SW, You XM, Moller F, Boeke K, Starke M, Spranger U, et al. Development of obliterative bronchiolitis after allogeneic rat lung transplantation: implication of acute rejection and the time point of treatment. J Heart Lung Transplant. 1999;18(6):542–548. doi: 10.1016/s1053-2498(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 6.Yasufuku K, Heidler KM, Woods KA, Smith GN, Jr., Cummings OW, Fujisawa T, et al. Prevention of bronchiolitis obliterans in rat lung allografts by type V collagen-induced oral tolerance. Transplantation. 2002;73(4):500–505. doi: 10.1097/00007890-200202270-00002. [DOI] [PubMed] [Google Scholar]
- 7.Slingsby JH, Hogarth MB, Simpson E, Walport MJ, Morley BJ. New microsatellite polymorphisms identified between C57BL/6, C57BL/10, and C57BL/KsJ inbred mouse strains. Immunogenetics. 1996;43(1-2):72–75. doi: 10.1007/BF00186607. [DOI] [PubMed] [Google Scholar]
- 8.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, Meyts I, Bullens DM, Reynders V, et al. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8(9):1911–1920. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 10.Jungraithmayr WM, Korom S, Hillinger S, Weder W. A mouse model of orthotopic, single-lung transplantation. J Thorac Cardiovasc Surg. 2009;137(2):486–491. doi: 10.1016/j.jtcvs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Cohen AH. Masson's trichrome stain in the evaluation of renal biopsies. An appraisal. Am J Clin Pathol. 1976;65(5):631–643. doi: 10.1093/ajcp/65.5.631. [DOI] [PubMed] [Google Scholar]
- 12.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflammatory Bowel Diseases. 2006;12(5):382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 14.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of Interleukin 17 Receptor Signaling for Lung Cxc Chemokine and Granulocyte Colony-Stimulating Factor Expression, Neutrophil Recruitment, and Host Defense. The Journal of Experimental Medicine. 2001;194(4):519–528. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelman AE, Li W, Richardson SB, Zinselmeyer BH, Lai J, Okazaki M, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182(7):3969–3973. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okazaki M, Gelman AE, Tietjens JR, Ibricevic A, Kornfeld CG, Huang HJ, et al. Maintenance of airway epithelium in acutely rejected orthotopic vascularized mouse lung transplants. Am J Respir Cell Mol Biol. 2007;37(6):625–630. doi: 10.1165/rcmb.2007-0257RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allan JS, Wain JC, Schwarze ML, Houser SL, Madsen JC, Sachs DH. Obliterative bronchiolitis develops in miniature swine transplanted across a minor histocompatibility barrier. Transplant Proc. 2001;33(1-2):358–359. doi: 10.1016/s0041-1345(00)02047-9. [DOI] [PubMed] [Google Scholar]
- 18.Romaniuk A, Prop J, Petersen AH, Wildevuur CR, Nieuwenhuis P. Expression of class II major histocompatibility complex antigens by bronchial epithelium in rat lung allografts. Transplantation. 1987;44(2):209–214. doi: 10.1097/00007890-198708000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180(7):4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husain S, Singh N. Bronchiolitis obliterans and lung transplantation: evidence for an infectious etiology. Semin Respir Infect. 2002;17(4):310–314. doi: 10.1053/srin.2002.36442. [DOI] [PubMed] [Google Scholar]
- 21.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant. 1998;17(12):1255–1263. [PubMed] [Google Scholar]
- 22.Husain AN, Siddiqui MT, Holmes EW, Chandrasekhar AJ, McCabe M, Radvany R, et al. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 1999;159(3):829–833. doi: 10.1164/ajrccm.159.3.9607099. [DOI] [PubMed] [Google Scholar]
- 23.Fiser SM, Kron IL, Long SM, Kaza AK, Kern JA, Tribble CG. Influence of graft ischemia time on outcomes following lung transplantation. J Heart Lung Transplant. 2001;20(2):206–207. doi: 10.1016/s1053-2498(00)00444-7. [DOI] [PubMed] [Google Scholar]
- 24.Hosenpud JD, Bennett LE, Keck BM, Boucek MM, Novick RJ. The Registry of the International Society for Heart and Lung Transplantation: seventeenth official report-2000. J Heart Lung Transplant. 2000;19(10):909–931. doi: 10.1016/s1053-2498(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 25.Huang HJ, Yusen RD, Meyers BF, Walter MJ, Mohanakumar T, Patterson GA, et al. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008;8(11):2454–2462. doi: 10.1111/j.1600-6143.2008.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroshus TJ, Kshettry VR, Savik K, John R, Hertz MI, Bolman RM., 3rd Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg. 1997;114(2):195–202. doi: 10.1016/S0022-5223(97)70144-2. [DOI] [PubMed] [Google Scholar]
- 27.Dhillon GS, Zamora MR, Roos JE, Sheahan D, Sista RR, van der Starre P, et al. Lung Transplant Airway Hypoxia: A Diathesis to Fibrosis? Am J Respir Crit Care Med. doi: 10.1164/rccm.200910-1573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada Y, Sekine Y, Yoshida S, Yasufuku K, Petrache I, Benson HL, et al. Type V Collagen-Induced Oral Tolerance Plus Low-Dose Cyclosporine Prevents Rejection of MHC Class I and II Incompatible Lung Allografts. J Immunol. 2009;183(1):237–245. doi: 10.4049/jimmunol.0804028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stammberger U, Murat B, Mathias G, Amiq G, Jürg H, Stephen CH, et al. Prolonged Amelioration of Acute Lung Allograft Rejection by Overexpression of Human Interleukin-10 Under Control of a Long Acting Ubiquitin C Promoter in Rats. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2006;25(12):1474–1479. doi: 10.1016/j.healun.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Peron JPS, Yang K, Chen M-L, Brandao WN, Basso AS, Commodaro AG, et al. Oral tolerance reduces Th17 cells as well as the overall inflammation in the central nervous system of EAE mice. Journal of Neuroimmunology. 2010;227(1-2):10–17. doi: 10.1016/j.jneuroim.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, et al. Bleomycin and IL-1β–mediated pulmonary fibrosis is IL-17A dependent. The Journal of Experimental Medicine. 2010;207(3):535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]