Abstract
3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) is a potent psychedelic drug inducing euphoria and hyper-sociability in humans, as well as hyperactivity and anxiety in rodents. Adult zebrafish (Danio rerio) have become a widely used species in neurobehavioral research. Here, we explore the effects of a wide range (0.25–120 mg/L) of acute MDMA doses on zebrafish behavior in the novel tank test. While MDMA was inactive at lower doses (0.25–10 mg/L), higher doses reduced bottom swimming and immobility (40–120 mg/L) and impaired intra-session habituation (10–120 mg/L). MDMA also elevated brain c-fos expression, collectively confirming the utility of zebrafish models for screening of hallucinogenic compounds.
Keywords: MDMA, psychedelic hallucinogenic drugs, zebrafish, anxiety, locomotion, novelty-based paradigms
Introduction
3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) is a popular recreational drug that modulates brain monoamines by inhibiting their reuptake (White et al., 1996; Kalant, 2001; de la Torre et al., 2004; Nagai et al., 2007; Doly et al., 2009) and degradation (Leonardi & Azmitia, 1994). The serotonergic system appears to be the primary target of MDMA action, although dopamine also plays an important role (Benturquia et al., 2008; NIDA, 2010; Stove et al., 2010).
Typical clinical effects of MDMA include euphoria, elation and sociability (Parrott, 2007; Bedi et al., 2009; Stove et al., 2010). MDMA can evoke adverse effects, including anxiety, depression, psychoses and cognitive deficits (Hall & Henry, 2006; Control, 2009; NIDA, 2010).
The behavioral effects of acute MDMA have been extensively investigated in various animal models. In rodents, MDMA induces robust hyperlocomotion (Benturquia et al., 2008; Colussi-Mas & Schenk, 2008; Stove et al., 2010) and anxiety (Lin et al., 1999; Ho et al., 2004).
Previous research has utilized the zebrafish as a model sensitive to various pharmacological manipulations, including hallucinogens lysergic acid diethylamide (LSD) and salvinorin A (Braida et al., 2007; Grossman et al., 2010). Since MDMA has not yet been tested in zebrafish, we examined its behavioral effects on this species. Finally, published rodent data shows that psychedelic agents (e.g., LSD and MDMA), elevate the expression of c-fos, serving as a marker of neuronal activation that correlates with behavioral alterations (Salzmann et al., 2003; Benturquia et al., 2008; Reissig et al., 2008). Based on earlier studies validating brain c-fos analyses in zebrafish (Baraban et al., 2005; Wong et al., 2010b), we also examined the effects of MDMA on c-fos expression.
Methods
Subjects and housing
A total of 142 adult (5–7 month-old) male and female wild-type (short-fin) zebrafish were obtained from a local commercial distributor (50 Fathoms, Metairie, LA). All fish were housed in groups of 20–30 fish per 40-L tank. The tanks were filled with filtered system water and maintained at 25–27◦C on a 14:10-h cycle. All fish used in this study were experimentally naïve, and fed Tetramin Tropical Flakes twice daily. Animal experiments and care adhered to Institutional and National regulations.
Behavioral testing
Behavioral testing was performed between 11.00 and 15.00 h using tanks with water adjusted to the holding room temperature. To avoid the test battery effect, each experiment was performed on a separate cohort. Zebrafish behavior was recorded by two trained observers (inter-rater reliability >0.85) blinded to the treatments, and analyzed using Ethovision XT7 (Noldus IT, Netherlands).
Experiment 1 tested zebrafish behavior in the standard, 6-min novel tank test following a 20-minute pre-treatment with varying doses (0.25–120 mg/L) of MDMA. Experiment 2 used mild-to-high effective doses (40, 80 and 120 mg/l, established in Experiment 1) and assessed rapid MDMA action, exposing zebrafish to a 30-minute novel tank filled with drug-treated water, similar to the protocol described previously for LSD (Grossman et al., 2010).
The novel tank test, used to assess zebrafish anxiety and locomotion (Levin et al., 2007; Bencan et al., 2009; Egan et al., 2009), was a 1.5-L trapezoidal tank (15 height × 28 top × 23 bottom × 7 cm width; Aquatic Habitats, Apopka, FL; Fig. 1A) maximally filled with water and divided into two equal virtual horizontal portions (top and bottom; (Egan et al., 2009)). The following endpoints were assessed in this paradigm: latency (s), entries to and time spent (s) in the upper portion, the frequency of erratic movements (sharp changes in direction, and unorganized spontaneous darting), as well as the frequency and duration (s) of freezing bouts (absence of movement, except for eyes and gills, for >2 s; (Egan et al., 2009)). Once manual data were generated for each minute of the test in Experiment 1, we examined intra-session habituation of zebrafish behaviors by comparing behavioral scores for the first and last minutes of the test (Wong et al., 2010a).
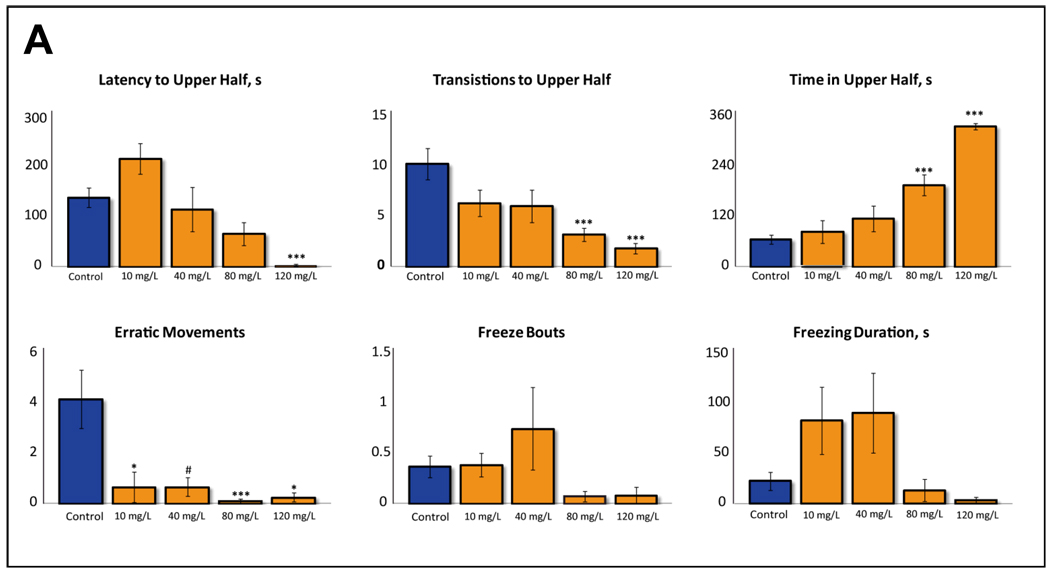
Figure 1. Behavioral effects of acute 20-min exposure to 3,4-Methylenedioxymethamphetamine (MDMA) on zebrafish tested in the standard 6-min novel tank test (Experiment 1; n = 27 (controls), 28 (10 mg/L), 12 (40 mg/L), 27 (80 mg/L) and 12 (120 mg/L).
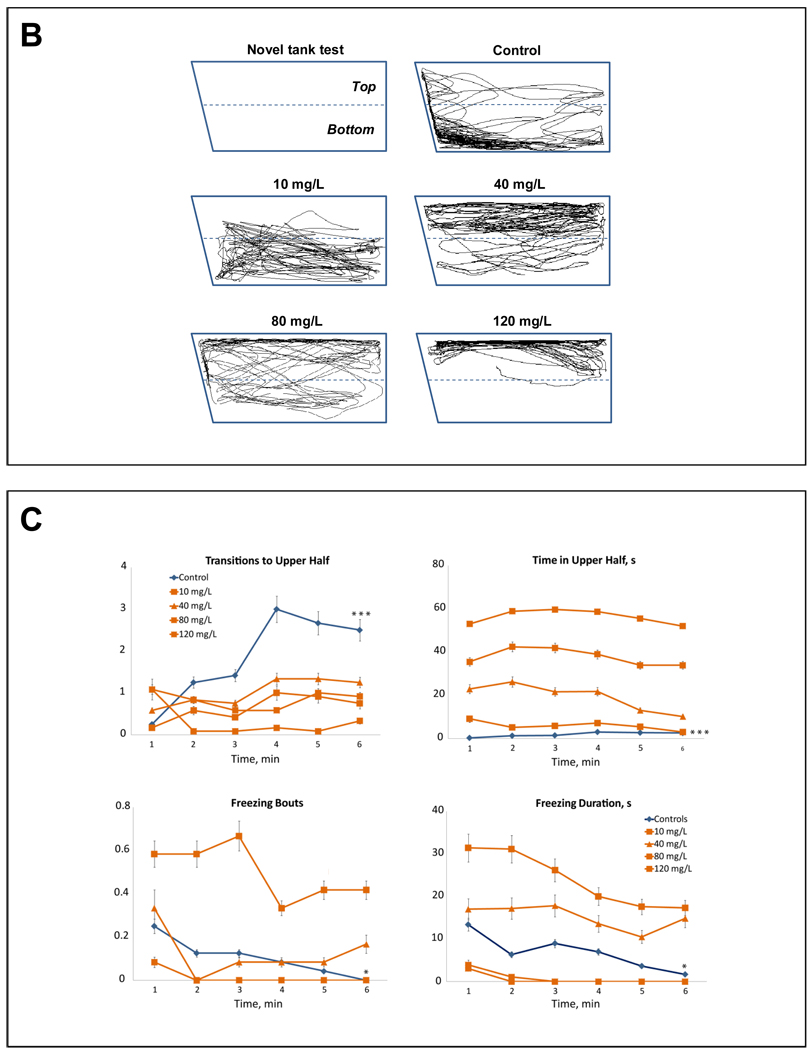
A – Manually recorded behavioral endpoints (*P<0.05, *** P<0.001, # P=0.05–0.01 (trend) vs. control; post-hoc Tukey test for significant ANOVA data). B – Two-dimensional (2D) traces generated using Ethovision XT7 software and a side-view camera. 2D traces were examined for each experimental cohort, rated from 1 to n (based on similarity to each other), and the middle trace was selected as representative, to best illustrate the typical patterns of zebrafish locomotion. C – Habituation (per-minute distribution) of zebrafish behavioral activity (* P<0.05, *** P<0.001, min 1 vs. min 6 of the respective drug group, U-test).
Video-tracking
Recorded videos were analyzed using Ethovision XT7, as described previously (Cachat et al., 2010a; Grossman et al., 2010; Wong et al., 2010a). The exported “side view” 2D traces were independently rated on a consensus basis from 1 to n (based on similarity to each other) by three trained observers blinded to the treatments. The middle trace was selected as representative for the group, to illustrate the 2D spatial pattern of swimming activity (Grossman et al., 2010).
Pharmacological manipulations
MDMA for this study was obtained through NIDA Drug Supply Program. MDMA doses (40–120 mg/L) were chosen based on our pilot studies with a wide range of doses (0.25 – 120 mg/L). A standard 20-minute pre-treatment time was chosen here based on our pilot experiments with MDMA and other similar hallucinogenics (Grossman et al., 2010; Stewart et al., 2011a). Pilot testing of the dose range in the novel tank showed effects of MDMA at doses between 40 and 120 mg/L (Fig. 1), but absence of effects at smaller doses (data not shown). Drug exposure was performed by immersing individual zebrafish in a 1-L plastic beaker for 20 min prior to the testing (Experiment 1) or into a 1.5-L novel tank for 30 min during the testing (Experiment 2). Control fish were exposed to drug-free facility water for the same treatment time.
C-fos expression assay
RT-PCR was performed for zebrafish c-fos mRNA in separate cohorts of animals exposed for 20 min to either drug-free water or to a behaviorally active dose (40 and 80 mg/L) of MDMA. The brains were dissected, with 2 brains combined per sample (6 samples per group) for RNA extraction. cDNA was synthesized using random primers and iScript Select cDNA Synthesis Kit (Bio Rad, CA). For QT-PCR, cDNA was amplified with c-fos forward and reverse primers (Tang et al., 2007).
Statistical analysis
Data were analyzed using one-way ANOVA (factor: dose) followed by post-hoc Tukey testing for significance. Intra-session habituation (min 1 vs. min 6) data were tested using the paired U-test. Experiment 2 data were analyzed using one-way ANOVA (factor: dose) with repeated measures (test minutes 1–30) followed by post-hoc Tukey testing (vs. respective minute in the control group) for significance. C-fos expression data were assessed using non-paired U-test (control vs. respective drug-treated group). Data were expressed as mean ± SEM. Significance was set at P < 0.05.
Results
In Experiment 1, acute exposure to MDMA dose-dependently effected novel tank behavior, modulating latency to the top, top transitions, time spent in top and the number of erratic movements (F(4, 105) = 7.9, 6.9, 23.3 and 5.9, respectively; P<0.001) as well as freezing bouts and duration (F(4, 105) = 2.8 and 3.5, P<0.05; Fig. 1a). There was a dose-dependent reduction in latency to top, top transitions and erratic movements, as well as an increase in time spent in top (Fig. 1a). These behavioral profiles were also evident in computer-generated 2D traces of zebrafish swimming, showing a dose-dependent increase in top dwelling in MDMA-treated fish (Fig. 1b).
Interesting effects were observed for per-minute distribution of zebrafish activity that reflects intra-session habituation, particularly sensitive to various psychotropic drugs (Wong et al., 2010a). Analysis of fish behavior in Experiment 1 showed robust intra-session habituation in control zebrafish, with significant minute 1 vs. minute 6 differences for the number of top entries and time in top (P<0.0005, U-test), as well as freezing bouts and duration (P<0.05, U-test). There was no difference between min 1 vs. min 6 for erratic movements in control fish, consistent with prior studies on the lack of intra-session habituation of this behavior (Wong et al., 2010a). In contrast, MDMA exposure at 40, 80 and 120 mg/L markedly impaired zebrafish habituation, yielding no significant differences for min 1 vs. min 6 data for the number of top entries, time in top, freezing bouts and freezing duration (Fig. 1c). Erratic movements, unaffected in controls, showed no habituation in any of the MDMA-treated fish cohorts (data not shown).
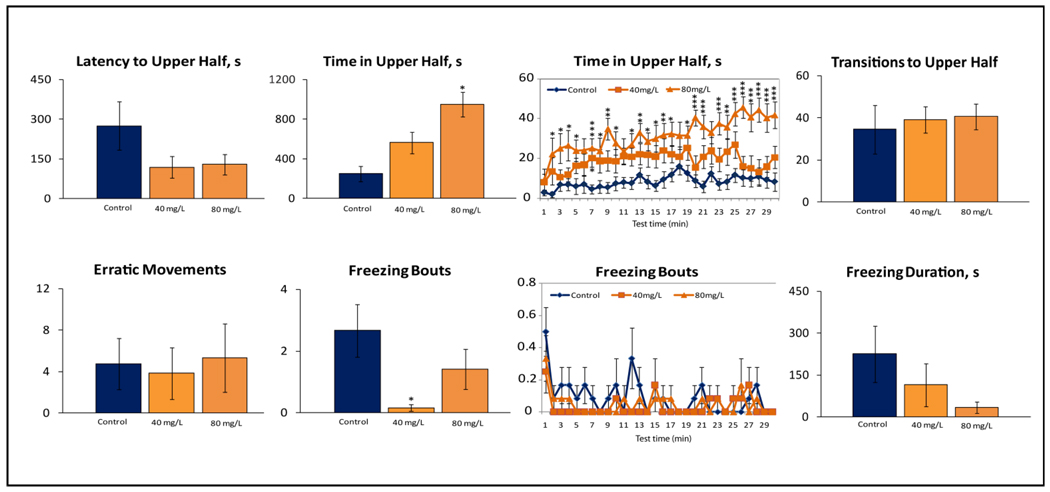
Experiment 2 examined the immediate effects of MDMA using a 30-min novel tank test filled with drug-treated water. While most behaviors were similar to those observed in Experiment 1, Fig. 2 shows that MDMA at 40 and 80 mg/L rapidly affected zebrafish behavior, within 5–10 min evoking typical top dwelling responses reported in Experiment 2. Similarly, there were no anxiogenic effects or behavioral inhibition, as the drug-exposed fish displayed top dwelling and lower immobility throughout this test.
Figure 2. Immediate behavioral effects of 40 and 80 mg/L MDMA on zebrafish tested in the 30-min novel tank containing drug-treated water (Experiment 2; n = 12 in each group).
ANOVA analyses revealed significant dose effect for time in top (F(2,35) = 11.2, P<0.0001) and freezing bouts (F(2,35) = 11.2, P<0.05) but not for latency to top (F(2,35) = 1.9, NS), transitions to top (F(2,35) = 0.9, NS), freezing duration (F(2,35) = 1.6, NS) or erratic movements (F(2,35) = 0.1, NS). Line diagrams with per-minute distribution of activity are presented only for two endpoints with significant dose effect. Post-hoc Tukey test for significant ANOVA data: for bar diagrams *P<0.05 vs. respective controls; for line diagrams *P<0.05, *** P<0.0001 vs. the respective minute’s control value. Note marked behavioral effects (increased time in top) starting to appear in zebrafish within the first 5–10 min of the drug treatment.
Finally, acute 20-min exposure to moderate behaviorally active doses of MDMA affected brain c-fos expression, causing 12.3-fold (NS) elevation at 40 mg/L and a significant 26.6-fold increase at 80 mg/L (P< 0.01, U-test).
Discussion
This study is the first report on the effects of MDMA in zebrafish, showing increased top dwelling, reduced immobility, impaired intra-session habituation and elevated brain c-fos expression. Although MDMA exerts positive effects in humans (Liechti et al., 2000; Parrott, 2007; Bedi et al., 2009), it evokes hyperlocomotion and anxiety in rodents (Gurtman et al., 2002; Navarro et al., 2004; Sumnall et al., 2004; Faria et al., 2006). In the zebrafish novel tank paradigm, increased top dwelling typically implies reduced anxiety (Levin et al., 2006; Egan et al., 2009; Cachat et al., 2010b). Similar to MDMA action in humans, our study did not find anxiety-like behavior in zebrafish (Fig. 1–2). It is possible that other factors play a role in the reduced apparent anxiety of our zebrafish. For example, anxiolytic manipulations usually increase several “top” behaviors, including both time spent and the number of entries to top (Levin et al., 2006; Egan et al., 2009; Cachat et al., 2010b). In the present study, the number of top entries was significantly reduced (Fig. 1), implying that top dwelling observed here may differ from a typical zebrafish anxiolytic response.
One possibility for this can be a serotonin syndrome-like state induced by serotonergic drugs in zebrafish (Stewart et al., 2010). Similar “surfacing” behavior was induced by other serotonergic drugs in this (Egan et al., 2009; Grossman et al., 2010; Sackerman et al., 2010; Stewart et al., 2011b) and other aquatic species (Abramson et al., 1962). Alternatively, given the known properties of MDMA, increased top dwelling may also represent zebrafish disorientation and/or hallucinogenic-like states. For example, similar phenotypes were induced in zebrafish by other hallucinogens, such as LSD (Grossman et al., 2010), salvinorin A (Braida et al., 2007) and ketamine (Zakhary et al., 2010), lending indirect support to this notion.
The behavioral effects of MDMA strikingly resemble the effects of LSD, another psychedelic drug previously tested in zebrafish (Grossman et al., 2010). Effective doses of MDMA identified in this study (40–120 mg/L) were 450–800 times higher than the effective doses of LSD (50–250 µg/L) in zebrafish (Grossman et al., 2010; Stewart et al., 2011b). In humans, ~0.4–1 µg/kg LSD is generally sufficient to produce strong behavioral effects, compared to ~0.4–2 mg/kg (1000–2000 times higher) doses of a less potent MDMA (Control, 2007; Control, 2009). Similar relative efficacy of these two drugs in zebrafish was close to that observed in humans, strongly supporting the translational value of zebrafish models for drug abuse research.
In line with previous studies on zebrafish habituation (Wong et al., 2010a), MDMA also altered zebrafish habituation, maintaining locomotion at a constant level throughout the test (Fig. 1). While the ability of MDMA to impair habituation has also been reported in rodents (Gold & Koob, 1989; Kehne et al., 1992; Scearce-Levie et al., 1999), other factors (e.g., drug-induced hyperlocomotion) may also contribute to this phenotype.
Mounting experimental evidence links MDMA behavioral effects to altered expression of brain c-fos. For example, MDMA increases c-fos and Fos in multiple areas of rodent brain (Stephenson et al., 1999; Salzmann et al., 2003; Colussi-Mas & Schenk, 2008). Similar effects have been reported for LSD (Gresch et al., 2002; Reissig et al., 2008). In general, elevated zebrafish c-fos following acute MDMA here parallels rodent c-fos evidence, and zebrafish LSD data (own unpublished observations).
In summary, our study showed that 40–120 mg/L MDMA evokes robust behavioral responses in zebrafish (Fig. 1–2), paralleling some animal and clinical effects of this drug. MDMA also induced physiological responses in zebrafish, elevating brain c-fos expression, similar to its effects in rodents. Expanding previous zebrafish reports using various psychotropic agents, such as LSD, salvinorin A, ketamine and dizocilpine (Swain et al., 2004; Braida et al., 2007; Grossman et al., 2010; Seibt et al., 2010; Zakhary et al., 2010), our present MDMA study supports high sensitivity of this aquatic model to hallucinogenic drugs.
Acknowledgements
The study was supported by Tulane University Intramural funds, Zebrafish Neuroscience Research Consortium (ZNRC) and LA Board of Regents P-Fund award. MDMA for this study was obtained through the NIDA Drug Supply Program. The authors thank Dr. M. Shultz, D. Carlos, V. Piet and R. Razavi for their help with this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest.
References
- Abramson HA, Gettner HH, Hewitt MP, Dean G. Effect of lysergic acid diethylamide on the surfacing behaviour of large carp. Nature. 1962;193:320–321. doi: 10.1038/193320a0. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 2005;131:759–768. doi: 10.1016/j.neuroscience.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berl) 2009;207:73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benturquia N, Courtin C, Noble F, Marie-Claire C. Involvement of D1 dopamine receptor in MDMA-induced locomotor activity and striatal gene expression in mice. Brain Res. 2008;1211:1–5. doi: 10.1016/j.brainres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Pegorini S, Zani A, Guerini-Rocco C, Gori E, Sala M. Hallucinatory and rewarding effect of salvinorin A in zebrafish: kappa-opioid and CB1-cannabinoid receptor involvement. Psychopharmacology (Berl) 2007;190:441–448. doi: 10.1007/s00213-006-0639-1. [DOI] [PubMed] [Google Scholar]
- Cachat J, Canavello P, Elegante M, Bartels B, Hart P, Bergner C, Egan R, Duncan A, Tien D, Chung A, Wong K, Goodspeed J, Tan J, Grimes C, Elkhayat S, Suciu C, Rosenberg M, Chung KM, Kadri F, Roy S, Gaikwad S, Stewart A, Zapolsky I, Gilder T, Mohnot S, Beeson E, Amri H, Zukowska Z, Soignier RD, Kalueff AV. Modeling withdrawal syndrome in zebrafish. Behav Brain Res. 2010a;208:371–376. doi: 10.1016/j.bbr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Min Chung K, Wu N, Wong K, Roy S, Suciu C, Goodspeed J, Elegante M, Bartels B, Elkhayat S, Tien D, Tan J, Denmark A, Gilder T, Kyzar E, DiLeo J, Frank K, Chang K, Utterback E, Hart P, Kalueff A. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nature Protocols. 2010b;5:1786–1799. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- Colussi-Mas J, Schenk S. Acute and sensitized response to 3,4-methylenedioxymethamphetamine in rats: different behavioral profiles reflected in different patterns of Fos expression. Eur J Neurosci. 2008;28:1895–1910. doi: 10.1111/j.1460-9568.2008.06467.x. [DOI] [PubMed] [Google Scholar]
- Control OoD. Drugs and Chemicals of Concern: D-Lysergic Acid Diethylamide. US Dept. of Justice, DEA; 2007. [Google Scholar]
- Control OoD. Drugs and Chemicals of Concern: 3,4-Methylenedioxymethamphetamine. US Dept. of Justice, DEA; 2009. [Google Scholar]
- de la Torre R, Farre M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, Cami J. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Doly S, Bertran-Gonzalez J, Callebert J, Bruneau A, Banas SM, Belmer A, Boutourlinsky K, Herve D, Launay JM, Maroteaux L. Role of serotonin via 5-HT2B receptors in the reinforcing effects of MDMA in mice. PLoS One. 2009;4:e7952. doi: 10.1371/journal.pone.0007952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria R, Magalhaes A, Monteiro PR, Gomes-Da-Silva J, Amelia Tavares M, Summavielle T. MDMA in adolescent male rats: decreased serotonin in the amygdala and behavioral effects in the elevated plus-maze test. Ann N Y Acad Sci. 2006;1074:643–649. doi: 10.1196/annals.1369.062. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF. MDMA produces stimulant-like conditioned locomotor activity. Psychopharmacology (Berl) 1989;99:352–356. doi: 10.1007/BF00445556. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Strickland LV, Sanders-Bush E. Lysergic acid diethylamide-induced Fos expression in rat brain: role of serotonin-2A receptors. Neuroscience. 2002;114:707–713. doi: 10.1016/s0306-4522(02)00349-4. [DOI] [PubMed] [Google Scholar]
- Grossman L, Utterback U, Stewart A, Gaikwad S, Wong K, Elegante M, Tan J, Gilder T, Wu N, DiLeo J, Cachat J, Kalueff AV. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav Brain Res. 2010;214:277–284. doi: 10.1016/j.bbr.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Gurtman CG, Morley KC, Li KM, Hunt GE, McGregor IS. Increased anxiety in rats after 3,4-methylenedioxymethamphetamine: association with serotonin depletion. Eur J Pharmacol. 2002;446:89–96. doi: 10.1016/s0014-2999(02)01820-4. [DOI] [PubMed] [Google Scholar]
- Hall AP, Henry JA. Acute toxic effects of 'Ecstasy' (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006;96:678–685. doi: 10.1093/bja/ael078. [DOI] [PubMed] [Google Scholar]
- Ho YJ, Pawlak CR, Guo L, Schwarting RK. Acute and long-term consequences of single MDMA administration in relation to individual anxiety levels in the rat. Behav Brain Res. 2004;149:135–144. doi: 10.1016/s0166-4328(03)00220-1. [DOI] [PubMed] [Google Scholar]
- Kalant H. The pharmacology and toxicology of "ecstasy" (MDMA) and related drugs. CMAJ. 2001;165:917–928. [PMC free article] [PubMed] [Google Scholar]
- Kehne JH, McCloskey TC, Taylor VL, Black CK, Fadayel GM, Schmidt CJ. Effects of the serotonin releasers 3,4-methylenedioxymethamphetamine (MDMA), 4-chloroamphetamine (PCA) and fenfluramine on acoustic and tactile startle reflexes in rats. J Pharmacol Exp Ther. 1992;260:78–89. [PubMed] [Google Scholar]
- Leonardi ET, Azmitia EC. MDMA (ecstasy) inhibition of MAO type A and type B: comparisons with fenfluramine and fluoxetine (Prozac) Neuropsychopharmacology. 1994;10:231–238. doi: 10.1038/npp.1994.26. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Assessing stress in zebrafish: Anxiolytic effects of nicotine. Neurotoxicology and Teratology. 2006;28:709–710. [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA ("Ecstasy") after pretreatment with the 5-HT(2) antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Lin HQ, Burden PM, Christie MJ, Johnston GA. The anxiogenic-like and anxiolytic-like effects of MDMA on mice in the elevated plus-maze: a comparison with amphetamine. Pharmacol Biochem Behav. 1999;62:403–408. doi: 10.1016/s0091-3057(98)00191-9. [DOI] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Rivera A, Maldonado E, Cavas M, de la Calle A. Anxiogenic-like activity of 3,4-methylenedioxy-methamphetamine ("Ecstasy") in the social interaction test is accompanied by an increase of c-fos expression in mice amygdala. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:249–254. doi: 10.1016/j.pnpbp.2003.10.016. [DOI] [PubMed] [Google Scholar]
- NIDA. MDMA (Ecstasy) Bethesda, MD: NIH; 2010. [Google Scholar]
- Parrott AC. The psychotherapeutic potential of MDMA (3,4-methylenedioxymethamphetamine): an evidence-based review. Psychopharmacology (Berl) 2007;191:181–193. doi: 10.1007/s00213-007-0703-5. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Rabin RA, Winter JC, Dlugos CA. d-LSD-induced c-Fos expression occurs in a population of oligodendrocytes in rat prefrontal cortex. Eur J Pharmacol. 2008;583:40–47. doi: 10.1016/j.ejphar.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Sackerman J, Donegan JJ, Cunningham CS, Nguyen NN, Lawless K, Long A, Benno RH, Gould GG. Zebrafish Behavior in Novel Environments: Effects of Acute Exposure to Anxiolytic Compounds and Choice of Danio rerio Line. Int J Comp Psychol. 2010;23:43–61. [PMC free article] [PubMed] [Google Scholar]
- Salzmann J, Marie-Claire C, Le Guen S, Roques BP, Noble F. Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br J Pharmacol. 2003;140:831–838. doi: 10.1038/sj.bjp.0705506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce-Levie K, Viswanathan SS, Hen R. Locomotor response to MDMA is attenuated in knockout mice lacking the 5-HT1B receptor. Psychopharmacology (Berl) 1999;141:154–161. doi: 10.1007/s002130050819. [DOI] [PubMed] [Google Scholar]
- Seibt KJ, Oliveira Rda L, Zimmermann FF, Capiotti KM, Bogo MR, Ghisleni G, Bonan CD. Antipsychotic drugs prevent the motor hyperactivity induced by psychotomimetic MK-801 in zebrafish (Danio rerio) Behav Brain Res. 2010;214:417–422. doi: 10.1016/j.bbr.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Stephenson CP, Hunt GE, Topple AN, McGregor IS. The distribution of 3,4-methylenedioxymethamphetamine "Ecstasy"-induced c-fos expression in rat brain. Neuroscience. 1999;92:1011–1023. doi: 10.1016/s0306-4522(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Stewart A, Kadri F, DiLeo J, Chung K, Cachat J, Goodspeed J, Suciu C, Roy S, Gaikwad S, Wong K, Elegante M, Elkhayat S, Wu N, Gilder T, Tien D, Kalueff AV. The Developing Utility of Zebrafish in Modeling Neurobehavioral Disorders. Int J Comp Psychol. 2010;23:104–121. [Google Scholar]
- Stewart A, Wong K, Cachat J, Gaikwad S, Kyzar E, Wu N, Hart P, Piet V, Utterback E, Elegante M, Tien D, Kalueff A. Zebrafish models to study drug abuse-related phenotypes. Revs in Neurosci. 2011a doi: 10.1515/RNS.2011.011. (in press) [DOI] [PubMed] [Google Scholar]
- Stewart A, Wu N, Cachat J, Hart P, Gaikwad S, Wong K, Utterback E, Gilder T, Kyzar E, Newman A, Carlos D, Chang K, Hook M, Rhymes K, Caffery M, Greenberg M, Zadina J, Kalueff A. Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011b doi: 10.1016/j.pnpbp.2010.11.035. in press. [DOI] [PubMed] [Google Scholar]
- Stove CP, De Letter EA, Piette MH, Lambert WE. Mice in ecstasy: advanced an imal models in the study of MDMA. Curr Pharm Biotechnol. 2010;11:421–433. doi: 10.2174/138920110791591508. [DOI] [PubMed] [Google Scholar]
- Sumnall HR, O'Shea E, Marsden CA, Cole JC. The effects of MDMA pretreatment on the behavioural effects of other drugs of abuse in the rat elevated plus-maze test. Pharmacol Biochem Behav. 2004;77:805–814. doi: 10.1016/j.pbb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Swain HA, Sigstad C, Scalzo FM. Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio) Neurotoxicol Teratol. 2004;26:725–729. doi: 10.1016/j.ntt.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Tang R, Dodd A, Lai D, McNabb WC, Love DR. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin (Shanghai) 2007;39:384–390. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, "Ecstasy") on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, Goodspeed J, Suciu C, Tan J, Grimes C, Chung A, Rosenberg M, Gaikwad S, Denmark A, Jackson A, Kadri F, Chung KM, Stewart A, Gilder T, Beeson E, Zapolsky I, Wu N, Cachat J, Kalueff AV. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behav Brain Res. 2010a;208:450–457. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Wong K, Stewart A, Gilder T, Wu N, Frank K, Gaikwad S, Suciu C, Dileo J, Utterback E, Chang K, Grossman L, Cachat J, Kalueff AV. Modeling seizure-related behavioral and endocrine phenotypes in adult zebrafish. Brain Res. 2010b;1348:209–215. doi: 10.1016/j.brainres.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Zakhary SM, Ayubcha D, Ansari F, Kamran K, Karim M, Leheste JR, Horowitz JM, Torres G. A behavioral and molecular analysis of ketamine in zebrafish. Synapse. 2010 doi: 10.1002/syn.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]