Abstract
Marine natural products have been the focus of discovery for new products of chemical and pharmacological interest. The aim of this study was to evaluate the antinociceptive activity of the methanolic (ME), acetate (AE), hexanic (HE) and chloroform (CE) extracts obtained from Caulerpa mexicana, and ME, CE and HE obtained from Caulerpa sertularioides. These marine algae are found all over the world, mainly in tropical regions. Models such as the writhing test, the hot plate test and formalin-induced nociception test were used to evaluate antinociceptive activity in laboratory mice. In the writhing test, all the extracts were administered orally at a concentration of 100 mg/kg, and induced high peripheral antinociceptive activity, with a reduction in the nociception induced by acetic acid above 65%. In the hot plate test, treatment with extracts from C. sertularioides (100 mg/kg, p.o.) did not significantly increase the latency of response, although the ME, AE and HE from C. mexicana showed activity in this model. This result suggests that these extracts exhibit antinociceptive activity. In the formalin test, it was observed that ME, AE and HE obtained from C. mexicana reduced the effects of formalin in both phases. On the other hand only CE from C. sertularioides induced significant inhibition of the nociceptive response in the first phase. To better assess the potential anti-inflammatory activity of the extracts, the carrageenan-induced peritonitis test was used to test Caulerpa spp. extracts on cell migration into the peritoneal cavity. In this assay, all extracts evaluated were able to significantly inhibit leukocyte migration into the peritoneal cavity in comparison with carrageenan. These data demonstrate that extracts from Caulerpa species elicit pronounced antinociceptive and anti-inflamatory activity against several nociception models. However, pharmacological and chemical studies are continuing in order to characterize the mechanism(s) responsible for the antinociceptive action and also to identify the active principles present in the Caulerpa species.
Keywords: antinociceptive, anti-inflammatory, Caulerpa mexicana, Caulerpa sertularioide, marine algae
1. Introduction
The search for pharmacological properties from natural products has led to the discovery of pharmacologically active substances, with important applications both in the experimental field and identification of active principles with therapeutic interest [1–5]. Currently, about 25–30% of all active principles used in treatments are extracted from natural products [6]. The plant kingdom is responsible for the largest share of chemical diversity recorded in the literature to date and has contributed quite significantly to the research and discovery of new drugs of natural origin, as well as the supply of substances useful for treating diseases that affect living beings [7–11]. However, it should be noted that marine natural products have also been the focus of discovery of new products of chemical and pharmacological interest [12–18].
Nonetheless, only about 20% of natural products from around the world have had their extracts submitted to pharmacological or biological tests [19]. To make matters worse, extinction of many species has become more frequent, with an estimated thousand species becoming extinct each year on the planet. Many of these species have not yet even been described, cataloged or studied [20].
Marine organisms are sources of numerous new compounds with multiple pharmacological properties [21]. The variety and complexity of small molecules which are the secondary metabolites of plants and marine organisms is difficult to be obtained by chemical synthesis by laboratory methods, these being the direct result of millions of years of evolution, reaching highly refined forms for protection against the weather and resistance to climate, pollution and predators [22].
In general, algae synthesize secondary metabolites such as terpenoids [23], alkaloids [24], flavonoids [25], tannins [26] and acetogenins [27]. Polar polyphenols may also occur in high concentrations [28,29]. In the literature, different pharmacological activities of macroalgae have been reported, including: antibacterial [30,31], antitumoral [32,33], anti-angiogenic [34,35], antiviral [36,37], antileishmania [38] and antioxidant [39] activity. However, in Brazil, this research field has not been well explored, despite the wealth of our marine flora.
Recently, the hypothesis that Caulerpa species (Chlorophyta, order Caulerpales, family Caulerpaceae) produce secondary metabolites with possible antinociceptive actions was investigated. In our preliminary investigation of the crude methanolic extract and phases from another specie from algae, Caulerpa racemosa, our group showed that this specie also had antinociceptive activity in the same models described in this work [40]. Although marine algae are an important source for biologically active natural products, few studies, specifically in Brazil, have been conducted with the the purpose of evaluating antinociceptive and antiinflamatory activity in animal models. As such, this study intended to evaluate the antinociceptive activity of extracts of macroscopic green algae Caulerpa mexican and Caulerpa sertularioides in murine models.
2. Results and Discussion
The antinociceptive potential of extracts from algae of the genus Caulerpa was evaluated using three well-accepted murine pain models, namely acetic acid-induced writhing, hot plate and formalin-induced nociception tests. The acetic acid-induced abdominal writhing and hot plate test have been reported to be useful to investigate peripheral and central activity, respectively, while the formalin-induced nociception test is valuable in detecting both effects.
Pre-treatment for all Caulerpa species resulted in significant inhibition of the acetic acid-induced writhing response. All extracts were evaluated at a dose of 100 mg/kg. The methanolic (ME), acetate (AE), hexanic (HE) and chloroform (CE) extracts from C. mexicana induced high peripheral antinociceptive activity with an inhibition of 78.4%, 73.2%, 83.1% and 77.0%, respectively. The pharmacological evaluation of extracts of C. sertularioides in the writhing test showed that the HE, CE, AE and ME induced inhibition of 66.5%, 67.0%, 60.7% and 67.0%, respectively. Moreover, these protective effects in the writhing test were also observed for dypirone (86.5%), as expected, used as the reference peripheral analgesic drug (Table 1). These data suggest that there is a possible antinociceptive action for extracts of C. Mexican and C. sertularioides. These data also corroborate a previous study on antinociceptive activity of Caulerpa racemosa [40].
Table 1.
The antinociceptive effects of extracts from algae C. mexicana (100 mg/kg, p.o.), C. sertularioides (100 mg/kg, p.o.) and dipyrone (40 mg/kg, i.p.) in the acetic acid-induced writhing model in mice.
| C. mexicana | C. sertularioides | |||
|---|---|---|---|---|
| Number of writhing | ||||
| Treatment | Mean ± S.E.M. a | I (%) b | Mean ± S.E.M. a | I (%) b |
| Vehicle | 35.5 ± 1.6 | – | 34.9 ± 1.9 | – |
| Dypirone | 8.0 ± 2.3 | 86.5 ** | 5.9 ± 1.7 | 83.2 ** |
| HE | 6.0 ± 1.4 | 83.1 ** | 11.7 ± 2.0 | 66.5 ** |
| CE | 8.2 ± 2.3 | 77.0 ** | 11.5 ± 0.9 | 67.0 ** |
| AE | 9.5 ± 1.4 | 73.2 ** | 13.7 ± 2.8 | 60.7 ** |
| ME | 7.7 ± 0.7 | 78.4 ** | 11.5 ± 1.8 | 67.0 ** |
Represents the Mean ± S.E.M. of 6 animals. Statistical differences between the treated and the control groups were evaluated by ANOVA and Dunnett tests and the asterisks denote the significance levels in comparison with control groups;
P < 0.01;
Represents percentage inhibition. HE, hexanic; CE, chloroform; AE, acetate; ME, methanolic.
The writhing test is commonly used for screening peripherally active analgesic. Algogenic agents, such as acetic acid, provoke a stereotypical behavior in mice characterized by abdominal contractions, movements of the body as a whole, twisting of dorso abdominal muscles and a reduction in motor activity and in motor coordination [41]. This model involves different nociceptive mechanisms, such as release of biogenic amines (e.g., histamine and serotonin), cyclooxygenases and their metabolites (e.g., PGE2 and PGF2α) [42] and opioid mechanisms [43]. Furthermore, it is well established that the nociceptive response caused by acetic acid is also dependent on the release of some cytokines, such as TNF-α, interleukin 1β and interleukin 8 via modulation of macrophages and mast cells localized in the peritoneal cavity [44]. In spite of compounds that act peripherally presenting action in this model, such as local anesthetics, muscle relaxants, ansiolitic, tranquilizers, among others, this remains being a good model to investigate the central analgesic action of substances. Because of this, the hot plate test was carried out with the aim of evaluating whether the Caulerpa species could demonstrate an antinociceptive effect.
In the hot plate test, the extracts of C. sertularioides did not significantly increase the latency of response, indicating that they do not show central activity (data not shown). On the order hand, treatment with ME, AE, CE and HE from C. mexicana (Table 2) caused a marked increase in the latency time of the animals at the times of 90 and 150 (5.4 ± 0.6 s and 5.4 ± 0.4 s), 90 and 150 (5.3 ± 0.9 s and 5.9 ± 1.3 s), 90 (5.7 ± 0.7 s) and 90–120 (5.8 ± 0.9 s; 5.9 ± 1.00 s) minutes respectively. These results suggest the action of analgesic activity for C. mexicana may be mediated through inhibition of pain receptors or inhibition of mediators like ciclooxigenase (i.e., COX-3). The treatment with morphine (4.3 mg/kg, s.c.), the opioid receptor agonist, induced a significant increase in latency time in the hot plate test, as expected, which persisted for at least 150 min. The hot plate test is considered to be selective for centrally acting analgesic compounds, like morphine, while peripheral analgesics are known to be inactive on this kind of painful stimulus [45]. Although an effect in the hot plate test was observed, we cannot say that the effect of this extract is mediated centrally.
Table 2.
Time-course for response with treatment of C. mexicana extracts (100 mg/kg, p.o.) and morphine (4.3 mg/kg, s.c.) on thermal nociception (hot plate test).
| Post-treatment (min) a | ||||||
|---|---|---|---|---|---|---|
| Animal Group | 0 min | 30 min | 60 min | 90 min | 120 min | 150 min |
| Time latency (s) | ||||||
| Control | 1.4 ± 0.3 | 2.2 ± 0.6 | 1.8 ± 0.2 | 3.2 ± 0.3 | 2.8 ± 0.3 | 2.6 ± 0.5 |
| Morphine | 6.9 ± 0.4 | 5.8 ± 0.3 | 12.8 ± 0.4 ** | 10.3 ± 0.8 ** | 9.7 ± 0.7 ** | 9.7 ± 0.9 ** |
| ME | 3.5 ± 0.5 | 3.0 ± 0.4 | 4.5 ± 0.3 | 5.4 ± 0.6 * | 5.1 ± 0.8 | 5.4 ± 0.4 * |
| AE | 1.7 ± 0.4 | 3.4 ± 0.3 | 3.1 ± 0.5 | 5.3 ± 0.9 ** | 4.1 ± 0.5 | 5.9 ± 1.3 ** |
| CE | 2.7 ± 0.2 | 3.7 ± 0.5 | 4.4 ± 1.0 | 5.7 ± 0.7 * | 3.6 ± 0.6 | 3.9 ± 1.0 |
| HE | 2. 7 ± 0.3 | 2.8 ± 0.6 | 4.6 ± 0.7 | 5.8 ± 0.9 * | 5.9 ± 1.0 * | 5.1 ± 0.8 |
Results represents time latency in second (s). Represents the mean ± S.E.M. of 6 animals;
P < 0.05;
P < 0.01 (ANOVA and Dunnett tests were used to evaluate the significance levels in comparison to time-zero).
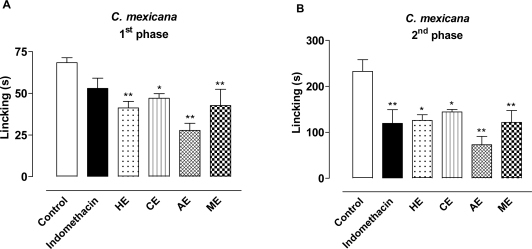
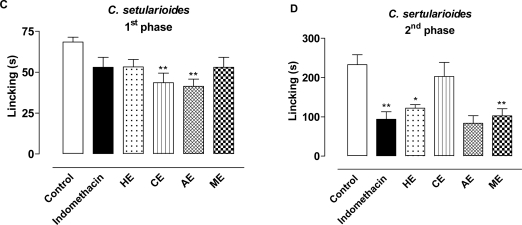
Neurogenic and inflammatory pain were evaluated using the formalin test. The first phase corresponds to acute neurogenic pain, while the second phase corresponds to inflammatory pain. The first and second phases are generally believed to reflect excitation of peripheral afferent nociceptors and central sensitization, respectively [46,47]. Substance P and bradykinin participate in the first phase, while serotonin, histamine, bradykinin, nitric oxide and prostaglandins are involved in the second phase [48]. Different mechanisms have been shown to be involved in first and second phase nociceptive behaviors, based on the differential pharmacology associated with these behaviors. For example, while second phase behaviors are selectively attenuated by cyclooxygenase inhibitors, first and second phase behaviors are attenuated by opioids [47]. Treatment with HE, CE, AE and ME from C. mexicana induced an inhibition of 39.7%, 31.1%, 60.2% and 50.2%, respectively, in the first phase (Figure 1A). Furthemore, all extracts of C. mexicana induced significant inhibition in the second phase, with an inhibition of 47.7% (ME), 68.7% (AE) 45.8% (HE) and 37.9% (CE) (Figure 1B). The pharmacological evaluation of extracts of C. sertularioides in the formalin test showed that the CE and AE could reduce the duration (36.2% and 40.0% inhibition) of paw licking in the first phase (Figure 1C). On the other hand, in the second phase CE was not active, while ME, AE and HE, were able to decrease the time that animals spent licking the injected paw (55.8%, 64% and 47.5% inhibition, respectively) (Figure 1D). Treatment with indomethacin, the NSAID significantly inhibited formalin induced nociception in the second phase (48.7% inhibition), but not the first phase. Thus, the results shown in the first phase of the model corroborate with those obtained in the writhing test, confirming the antinociceptive activity of the extracts.
Figure 1.
Effect of extracts of algae C. mexicana, C. sertularioides and indomethacin administered orally at a dose of 100 mg/kg in the formalin test (6 animals). (A) and (C) represents 1st phase, (B) and (D) represents 2nd phase. Statistical differences between the treated and the control groups were evaluated using ANOVA and Dunnett tests and the asterisks denote the significance levels in comparison with control groups, * P < 0.05, ** P < 0.01.
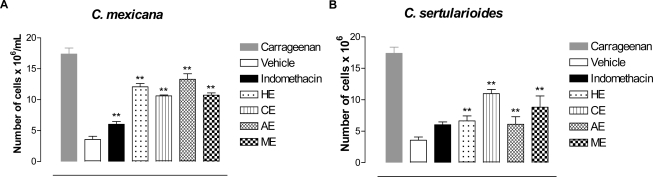
Since reduction of the second phase in the formalin test implied a possible anti-inflammatory mechanism, and considering previous studies from our group [40] that have demonstrated that another specie of Caulerpa has anti-inflammatory activity, we decided to evaluate the activity of these species in models of cell migration. To determine the effects of extracts from Caulerpa spp. on peritoneal inflammation induced by carrageenan, the mice were treated with test samples. Using carrageenan as a stimulus, it was possible to produce an acute inflammatory response after 4 h in the peritoneal cavity of mice, with a large number of leukocytes in the exudates. With the aim of evaluating a possible inhibitory effect of Caulerpa spp. extracts on cell migration into the peritoneal cavity, the carrageenan-induced peritonitis test was used. In this assay, all extracts evaluated were able to significantly inhibit leukocyte migration into the peritoneal cavity in comparison with carrageenan. The HE, CE, AE and ME from C. mexicana inhibited 30.5%, 38.9%, 23.5%, 38.26%, respectively (Figure 2A). While the extracts from C. sertularioides induced inhibition of 61.8% (HE), 36.9%, (CE), 71.7% (AE) and 49.25% (ME) (Figure 2B). The treatment with indomethacin inhibited 65.4% leukocyte migration.
Figure 2.
The effect of Caulerpa spp. extracts on cell migration. Caulerpa spp. extracts (100 mg/kg, p.o.) and indomethacin (35.7 mg/kg, p.o.) were evaluated using the carrageenan-induced peritoneal inflammation test. Each point represents the mean ± S.E.M. of six animals. Statistical differences between the treated and the control groups were evaluated by ANOVA and Dunnett tests, and the asterisks denote the significance levels in comparison with control groups, * P < 0.05, ** P < 0.01.
3. Experimental Section
3.1. Extraction and Isolation
The algae C. mexicana and C. sertularioide were collected from the coastal region of Bessa (7°03′52″S/34°49′51″W), João Pessoa, Paraíba State, Brazil in April 2008. The specimens were identified by Dr. George Emmanuel Cavalcanti de Miranda. Voucher specimens of C. mexicana (JPB 13985), C. sertularioides (JPB 13983) have been deposited in the Lauro Pires Xavier Herbarium at the Federal University of Paraíba (Universidade Federal da Paraíba), Brazil. The fresh algae were lyophilized and exhaustively extracted with hexane, chloroform, ethyl acetate, methanol and water in a Soxhlet apparatus, to obtain the respective extracts.
3.2. Biological Activity Tests
3.2.1. Drugs and Reagents
The following drugs and reagents were used: acetic acid (Merck), dipyrone (Sigma Chemical), morphine sulfate (Dimorf-Cristalia-BR), indomethacin (Merck), arabic gum (Sigma Chemical) and Tween 20 (Sigma). A solution of formalin 2.5% was prepared with formaldehyde (Merck) in saline (NaCl 0.9%). The botanical material was used as suspensions in Tween 80 (s.q.f.) and arabic gum (vehicle) in all the experiments and were administered by oral route at a dose of 100 mg/kg. Dipyrone, morphine and indomethacin were used as reference drugs. Dipyrone and indomethacin were administered by oral route and morphine by subcutaneous route. The control group was composed of the vehicle (arabic gum).
3.2.2. Animals
All experiments were performed with male and female Swiss mice (20–25 g). Animals were maintained in a room at a controlled temperature of 22 ± 2 °C for 12-h light/dark cycle with free access to food and water. Eight hours before each experiment animals received only water, in order to avoid food interference with substance absorption. Animal care and research protocols were in accordance with the principles and guidelines for the care of laboratory animals and the ethical guidelines for investigations of experimental pain in conscious animals [49]. The experiments were performed with the approval of the protocol by the local Institutional Ethics Committee-UFAL (No. 006443/2005-78).
3.2.3. Acetic Acid-Induced Writhing Test
The writhing test was carried out as described by Koster et al. [50]. Groups of mice (n = 6) were treated with the methanolic (ME), ethyl acetate (AE), hexanic (HE) and chloroform (CE) extracts from Caulerpa species (100 mg/kg, p.o.), dipyrone (40 mg/kg, p.o.) and the vehicle (p.o.). The writhings were induced by intraperitoneal injection with a 0.6% acetic acid solution (0.1 mL/10 g) 40 min after treatment. The number of writhings were counted starting at 5 min after injection of the stimulus for 20 min. Antinociceptive activity was expressed as percent inhibition of the usual number of writhings observed in control animals.
3.2.4. Hot Plate Test
The hot plate test was performed following the method of Eddy and Leimbach [51]. Different groups of animals (n = 6) received ME, AE, HE and CE from Caulerpa species (100 mg/kg, p.o.), morphine (4.3 mg/kg, s.c.) and the vehicle (0.5 mL, p.o.) Then, mice were placed on the equipment, which was maintained at 55 ± 1 °C, and the reaction time was noted by observing either the licking of the fore and hind paws or jumping at 30, 60, 90, 120 and 150 min after administration of the extracts. The baseline was considered as the mean reaction time obtained at 30 min and 60 min before administration of the phases, compounds or morphine and was defined as the normal reaction of the animal to temperature. The cut-off time used to prevent skin damage was 15 s.
3.2.5. Formalin-Induced Nociception
The formalin test was carried out as described by Hunskaar, Hole and Tjølsen et al. [52,53]. Animals received a dose of 20 μL of a 2.5% formalin solution (0.92% formaldehyde, in saline) on the ventral surface of the right hind paw. Animals were observed from 0 to 5 min (neurogenic phase) and from 15 to 30 min (inflammatory phase) and the time that they spent licking the injected paw was recorded and considered as indicative of nociception. Animals received ME, AE, HE and CE from Caulerpa species (100 mg/kg, p.o.) or the standard drug (indomethacin, 35.7 mg/kg, p.o.) 40 min before formalin injection. Control animals received only the vehicle (arabic gum).
3.2.6. Carrageenan-Induced Peritonitis in Mice
For this series of experiments, the method described by Ferrándiz and Alcaraz [54] was used. Carrageenan (Sigma Aldrich) was freshly prepared (10 mg/mL) in sterile 0.9% w/v saline, and 250 μL was injected i.p. After 4 h, the animals were killed by cervical dislocation. The peritoneal cavity was washed with 1.5 mL cold PBS, and after gentle manual massage, the exudate was retrieved and its volume measured. The number of recruit leukocytes to the peritoneum was counted in a Neubauer chamber and results were expressed as cells × 106/mL. The exudate was collected and used freshly for cell counts and cytospin preparations. The Caulerpa extracts (100 mg/kg, p.o.), the carrageenan group (arabic gum, p.o.) and the reference drug (indomethacin, 35.7 mg/kg, p.o.) were administered 30 min before the carrageenan injection. In the negative control group, animals just received the same dose of a vehicle (arabic gum, p.o.) 30 min before the saline injection by intraperitoneal route.
3.3. Statistical Analysis
Data are reported as mean ± S.E.M. and were analyzed statistically using analysis of variance (ANOVA) followed by Dunnett’s test. Results with P < 0.05 were considered significant (* P < 0.05, ** P < 0.01).
4. Conclusions
In conclusion, this study has shown that extracts from Caulerpa species have significant antinociceptive and anti-inflammatory effects in laboratory animals at the dose and route investigated. It can be argued that the extracts have antinociceptive activity and may possibly act via inhibition of inflammatory mediators. However, pharmacological and chemical studies are needed in order to characterize the mechanism(s) responsible for the antinociceptive and anti-inflammatory action and also to identify other active agents present in this plant. Moreover, the results obtained in this work contribute significantly to the pharmacological studies from marine products.
Acknowledgments
The authors are grateful to the CAPES, CNPq (Edital 10/2006), MCT, FAPEAL, INCT-INOFAR/CNPq and the Ministry of Environment (License MMA/CGEN 18/2007) for the joint funding of this research project. The authors wish to thank several of their colleagues working at the Federal University of Alagoas and Federal University of Paraíba for their constructive criticism and assistance in carrying out this project.
References
- 1.Konig GM, Wright AD. Marine natural products research: current directions and future potential. Planta Med. 1995;62:193–211. doi: 10.1055/s-2006-957861. [DOI] [PubMed] [Google Scholar]
- 2.Sousa FCF, Melo CTV, Citó MCO, Félix FHC, Vasconcelos SMM, Fonteles MMF, Barbosa Filho JM, Viana GSB. Medicinal plants and their bioactive constituents: A scientific review of bioactivity and potential benefits in the anxiety disorders in animal models. Braz J Pharmacogn. 2008;18:642–654. [Google Scholar]
- 3.Corrêa MFP, Melo GO, Costa SS. Natural products from plant origin potentially useful in the asthma therapy. Braz J Pharmacogn. 2008;18:785–797. [Google Scholar]
- 4.Barbosa-Filho JM, Alencar AA, Nunes XP, Tomaz ACA, Sena-Filho JG, Athayde-Filho PF, Silva MS, Souza MFV, Cunha EVL. Sources of alpha-, beta-, gamma-, delta- and epsilon-carotenes: a twentieth century review. Braz J Pharmacogn. 2008;18:135–154. [Google Scholar]
- 5.Rocha L, Almeida JRGS, Macêdo RO. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12:514–535. doi: 10.1016/j.phymed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Silva JSE, Moura MD, Oliveira RAG, Diniz MFFM, Barbosa-Filho JM. Natural products inhibitors of ovarian neoplasia. Phytomedicine. 2003;10:221–232. doi: 10.1078/094471103321659988. [DOI] [PubMed] [Google Scholar]
- 7.Vuorella P, Leinonenb M, Saikkuc P, Tammelaa P, Rauhad JP, Wenneberge T, Vuorella H. Natural products in the process of finding new drug candidates. Curr Med Chem. 2004;11:1375–1389. doi: 10.2174/0929867043365116. [DOI] [PubMed] [Google Scholar]
- 8.Agra MF, Silva KN, Basílio IJLD, França PF, Barbosa-Filho JM. Survey of medicinal plants used in the region Northeast of Brazil. Braz J Pharmacogn. 2008;18:472–508. [Google Scholar]
- 9.Almeida RN, Navarro DS, Barbosa-Filho JM. Plants with central analgesic activity. Phytomedicine. 2001;8:310–322. doi: 10.1078/0944-7113-00050. [DOI] [PubMed] [Google Scholar]
- 10.Truti MCT, Ferreira ICP, Zamuner MLM, Nakamura CV, Sarragiotto MH, Souza MC. Antiprotozoal and moluscicidal actives of five Brasilians plants. Braz J Med Biol Res. 2005;38:1873–1878. doi: 10.1590/s0100-879x2005001200016. [DOI] [PubMed] [Google Scholar]
- 11.Quintans-Júnior LJ, Almeida JRGS, Lima JT, Nunes XP, Siqueira JS, Oliveira LEG, Almeida RN, Athayde-Filho PF, Barbosa-Filho JM. Plants with anticonvulsant properties—a review. Braz J Pharmacogn. 2008;18:798–819. [Google Scholar]
- 12.Mariath IR, Falcão HS, Barbosa-Filho JM, Sousa LCF, Tomaz ACA, Batista LM, Diniz MFFM, Athayde-Filho PF, Tavares JF, Silva MS, Cunha EVL. Plants of the American continent with antimalarial activity. Braz J Pharmacogn. 2009;19:158–192. [Google Scholar]
- 13.Iannitti T, Palmieri B. An update on the therapeutic role of alkylglycerols. Mar Drugs. 2010;8:2267–2300. doi: 10.3390/md8082267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrita MT, Vale C, Rauter AP. Halogenated compounds from marine algae. Mar Drugs. 2010;8:2301–2317. doi: 10.3390/md8082301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JL, Xia WS, Liu P, Cheng QY, Tahirou T, Gu WX, Li B. Chitosan modification and pharmaceutical/biomedical applications. Mar Drugs. 2010;8:1962–1987. doi: 10.3390/md8071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Sullivan L, Murphy B, McLoughlin P, Duggan P, Lawlor PG, Hughes H, Gardiner GE. Prebiotics from marine macroalgae for human and animal health applications. Mar Drugs. 2010;8:2038–2064. doi: 10.3390/md8072038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aratake S, Trianto A, Hanif N, de Voogd NJ, Tanaka J. A new polyunsaturated brominated fatty acid from a Haliclona sponge. Mar Drugs. 2009;7:523–527. doi: 10.3390/md7040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebouvier N, Jullian V, Desvignes I, Maurel S, Parenty A, Dorin-Semblat D, Doerig C, Sauvain M, Laurent D. Antiplasmodial activities of homogentisic acid derivative protein kinase inhibitors isolated from a Vanuatu marine sponge Pseudoceratina sp. Mar Drugs. 2009;7:640–653. doi: 10.3390/md7040640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truti MCT, Ferreira ICP, Zamuner MLM, Nakamura CV, Sarragiotto MH, Souza MC. Antiprotozoal and moluscicidal actives of five Brasilians plants. Braz J Med Biol Res. 2005;38:1873–1878. doi: 10.1590/s0100-879x2005001200016. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros JD. A biotecnologia e a extinção de espécies, crises da modernidade. Rev Biotecnol Cienc Desenvolv. 2003;30:109–113. [Google Scholar]
- 21.Arif JM, Al-Hazzani AA, Kunhi M, Al-Khodairy F. Marine compounds: Anticancer or genotoxic. J Biomed Biotechnol. 2004;2:93–98. doi: 10.1155/S1110724304307060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viegas C, Jr, Bolzani VS, Barreiro EJ. Os produtos naturais e a química medicinal moderna. Quim Nova. 2006;29:326–337. [Google Scholar]
- 23.Cen-Pacheco F, Nordstrom L, Souto ML, Martin MN, Fernandez JJ, Daranas AH. Studies on polyethers produced by red algae. Mar Drugs. 2010;8:1178–1188. doi: 10.3390/md8041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guven KC, Percot A, Sezik E. Alkaloids in marine algae. Mar Drugs. 2010;8:269–284. doi: 10.3390/md8020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stafford HA. Flavonoid evolution—an enzymatic approach. Plant Physiol. 1991;96:680–685. doi: 10.1104/pp.96.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano J, Puupponen-Pimia R, Dauer A, Aura AM, Saura-Calixto F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009;53:S310–S329. doi: 10.1002/mnfr.200900039. [DOI] [PubMed] [Google Scholar]
- 27.Narkowicz CK, Blackman AJ. Further acetogenins from Tasmanian collections of Caulocystis cephalornithos demonstrating chemical variability. Biochem Syst Ecol. 2006;34:635–641. [Google Scholar]
- 28.Teixeira VL, Kelecom A, Gottlieb OR. Produtos naturais de algas marinhas. Quim Nova. 1991;14:83–90. [Google Scholar]
- 29.Pereira SMB, Oliveira-Carvalho MF, Angeiras JAP, Oliveira NMB, Torres J, Gestinari LM, Badeira-Pedrosa ME, Cocentino ALM, Santos MD, Nascimento PRF, Cavalcanti DR. Algas bentônicas do Estado de Pernambuco. In: Tabarelli M, Silva JMC, editors. Diagnóstico da Biodiversidade de Pernambuco. Massagana e SECTMA; Recife, Brazil: 2002. pp. 97–124. [Google Scholar]
- 30.Lima-Filho JVM, Carvalho AFFU, Freitas SM, Melo VMM. Antibacterial activity of extracts of six macroalgae from the Northeastern Brazilian coast. Braz J Microbiol. 2002;33:311–313. [Google Scholar]
- 31.Li ZY. Advances in marine microbial symbionts in the China sea and related pharmaceutical metabolites. Mar Drugs. 2009;7:113–129. doi: 10.3390/md7020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machado FLD, Kaiser CR, Costa SS, Gestinari LM, Soares AR. Braz J Pharmacogn. 2010;20:441–452. [Google Scholar]
- 33.Taskin E, Caki Z, Ozturk M, Taskin E. Assessment of in vitro antitumoral and antimicrobial activities of marine algae harvested from the Eastern Mediterranean sea. Afr J Biotechnol. 2010;9:4272–4277. [Google Scholar]
- 34.Dias PF, Siqueira JM, Vendruscolo LF, Neiva TD, Gagliardi AR, Maraschin M, Ribeiro-Do-Valle RM. Antiangiogenic and antitumoral properties of a polysaccharide isolated from the seaweed Sargassum stenophyllum. Cancer Chemother Pharmacol. 2005;56:436–446. doi: 10.1007/s00280-004-0995-7. [DOI] [PubMed] [Google Scholar]
- 35.Matsubara K, Xue C, Zhao X, Mori M, Sugawara T, Hirata T. Effects of middle molecular weight fucoidans on in vitro and ex vivo angiogenesis of endothelial cells. Int J Mol Med. 2005;15:695–699. [PubMed] [Google Scholar]
- 36.Romanos MTV, Andrada-Serpa MJ, Santos MGM, Ribeiro ACF, Yoneshiguevalentin Y, Costa SS, Wigg MD. Inhibitory effect of extracts of Brazilian marine algae on human T-cell lymphotropic virus type 1 (HTLV-1) induced syncytium formation in vitro. Cancer Investig. 2002;20:46–54. doi: 10.1081/cnv-120000365. [DOI] [PubMed] [Google Scholar]
- 37.Mayer AMS, Rodriguez AD, Berlinck RGS, Hamann MT. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim Biophys Acta Gen Subj. 2009;1790:283–308. doi: 10.1016/j.bbagen.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genovese G, Tedone L, Hamann MT, Morabito M. The mediterranean red alga Asparagopsis: A source of compounds against. Leishmania Mar Drugs. 2009;7:361–366. doi: 10.3390/md7030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocha FD, Pereira RC, Kaplan MAC, Teixeira VL. Natural products from marine seaweeds and their antioxidant potential. Braz J Pharmacogn. 2007;17:631–639. [Google Scholar]
- 40.Souza ET, Queiroz AC, Miranda GEC, Lorenzo VP, Silva EF, Freire-Dias TLM, Cupertino-Silva YK, Melo GMA, Chaves MCO, Barbosa-Filho JM, Athayde-Filho PF, Santos BVO, Alexandre-Moreira MS. Antinociceptive activities of crude methanolic extract and phases, n-butanolic, chloroformic and ethyl acetate from Caulerpa racemosa (Caulerpaceae) Braz J Pharmacogn. 2009;19:115–120. [Google Scholar]
- 41.Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 42.Duarte IDG, Nakamura M, Ferreira SH. Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res. 1988;21:341–343. [PubMed] [Google Scholar]
- 43.Collier HOJ, Dinneen JC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato AB, Poole S, Ferreira SH, Cunha FQ. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol. 2000;387:111–118. doi: 10.1016/s0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- 45.Chu C, Huang Y, Chen YF, Wu JH, Rahman K, Zheng HC, Qin LP. Anti-nociceptive activity of aqueous fraction from the MeOH extracts of Paederia scandens in mice. J Ethnopharmacol. 2008;118:177–180. doi: 10.1016/j.jep.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Dickenson AH, Sullivan AF. Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurons. Neurosci Lett. 1987;83:207–211. doi: 10.1016/0304-3940(87)90242-4. [DOI] [PubMed] [Google Scholar]
- 47.Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol. 2001;90:2386–2402. doi: 10.1152/jappl.2001.90.6.2386. [DOI] [PubMed] [Google Scholar]
- 48.García MD, Fernandez MA, Alvarez A, Saenz MT. Anti-nociceptive and anti-inflammatory effect of the aqueous extract from leaves of Pimenta racemosa var. ozua (Mirtaceae) J Ethnopharmacol. 2004;91:69–73. doi: 10.1016/j.jep.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Zimmermann M. Ethical guidelines for investigation of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 50.Koster R, Anderson M, De-Beer EJ. Acetic acid analgesic screen. Fed Proc. 1959;18:418–420. [Google Scholar]
- 51.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyland dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- 52.Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 53.Tjolsen A, Hole K. Animal models of analgesia. In: Dickenson A, Besson J, editors. The Pharmacology of Pain. Vol. 130. Springer Verlag; Berlin, Germany: 1997. pp. 1–20. [Google Scholar]
- 54.Ferrándiz ML, Alcaraz MJ. Antiinflammatory activity and inhibition of arachidonic acidmetabolism by flavonoids. Inflamm Res. 1991;32:283–288. doi: 10.1007/BF01980887. [DOI] [PubMed] [Google Scholar]