Abstract
To expand the potential of pseudopterosins and seco-pseudopterosins isolated from the octocoral Pseudopterogorgia elisabethae of San Andrés and Providencia islands (southwest Caribbean Sea), we report the anti-microbial profile against four pathogenic microorganisms (Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa and Candida albicans) and report a more complete cytotoxic profile against five human cells lines (HeLa, PC-3, HCT116, MCF-7 and BJ) for the compounds PsG, PsP, PsQ, PsS, PsT, PsU, 3-O-acetyl-PsU, seco-PsJ, seco-PsK and IMNGD. For the cytotoxic profiles, all compounds evaluated showed moderate and non-selective activity against both tumor and normal cell lines, where PsQ and PsG were the most active compounds (GI50 values between 5.8 μM to 12.0 μM). With respect to their anti-microbial activity the compounds showed good and selective activity against the Gram-positive bacteria, while they did not show activity against the Gram-negative bacterium or yeast. PsU, PsQ, PsS, seco-PsK and PsG were the most active compounds (IC50 2.9–4.5 μM) against S. aureus and PsG, PsU and seco-PsK showed good activity (IC50 3.1–3.8 μM) against E. faecalis, comparable to the reference drug vancomycin (4.2 μM).
Keywords: marine natural products, pseudopterosins, seco-pseudopterosins, Pseudopterogorgia elisabethae, cytotoxic activity, antimicrobial activity
1. Introduction
The discovery of selective and potent therapeutic activity of pseudopterosins and seco-pseudopterosins isolated from the octocoral Pseudopterogorgia elisabethae [1–14] and the high degree of chemical variation among specimens collected at different locations throughout the Caribbean region [1,2], have motivated several authors to pursue this field of study. Thus far, 30 pseudopterosins (PsA-Y, iso-PsE, 2-O-Ac-PsQ, 3-O-Ac-PsQ, 2-O-Ac-PsU and 2-O-Ac-PsQ) [3–11] and 11 seco-pseudopterosins (seco-PsA-K) [8–13] have been isolated from specimens collected in the Bahamas, Bermuda, the Florida Keys and the Colombian islands of San Andrés and Providencia.
The pseudopterosins (PsA-D, PsE, iso-PsE, PsM-O, PsX and PsY) and seco-pseudopterosins (seco-PsA-G) isolated from specimens collected in the North Caribbean Sea (the Bahamas, Bermuda and the Florida Keys) have been evaluated as anti-inflammatory, analgesic and antimicrobial agents [2–6,8,12]. Among them, PsN, PsA, iso-PsE and PsE were found to be the most potent compounds in mouse ear anti-inflammatory assays [3,4,6,8]. Furthermore PsA and PsE, appeared to prevent eicosanoid biosynthesis by inhibition of PLA2, 5-LO and COX, degranulation of leukocytes and the consequent liberation of lysosomal enzymes [15,16]. PsA-E, PsK, PsX, PsY and seco-PsA-D showed excellent and selective activity against the Gram-positive bacteria Streptococcus pyogenes, Staphylococcus aureus, and Enterococcus faecalis [5,12]. Pseudopterosins are also used in several skin care products [1,17]. Methopterosin is a simple derivative of PsA and has completed Phase I and II clinical trials as a wound healing agent [17–20].
Specimens of P. elisabethae collected in the southwest Caribbean Sea, specifically at the Islands of San Andres and Providencia (Colombia), have been shown to contain new pseudopterosins (PsP-V, 2-O-Ac-PsQ, 3-O-Ac-PsQ, 2-O-Ac-PsU and 2-O-Ac-PsQ, PsG and PsK), seco-pseudopterosins (seco-PsH-K), and an inter-converting mixture of non-glycosylated diterpenes (10-acetoxy-9-hydroxy- and 9-acetoxy-10-hydroxy-amphilecta-8,10,12,14-tetraenes (IMNGD)) [7,9–11]. However, only a limited number of these compounds has been evaluated for anti-inflammatory [21], anti-tuberculosis, anti-viral, anti-malarial and anti-cancer [11] activity.
In regards to their anti-inflammatory activity, our experiments showed that fractions enriched with pseudopterosins (PsG, PsK, PsP, PsQ, PsS, PsT and PsU) and seco-pseudopterosins (seco-PsJ and seco-PsK) were able to inhibit the inflammation in a 12-O-tetradecanoyl-phorbol-acetate (TPA)-induced edema assay, with results comparable to those shown by indomethacin used as the control standard [21]. Additionally, in in vitro experiments, PsQ, PsS, PsT, PsU and IMNGD showed good activity for the inhibition of MPO release; PsP, PsT and IMNGD inhibit NO release [21] and PsR inhibited thromboxane B2 (TXB2) and the superoxide anion (O2−) [11].
In other experiments, PsP was evaluated for anti-viral activity (against HSV-1, HSV-2, HVMV and VZV), and PsQ, PsR, PsU, PsV, seco-PsH and seco-PsI evaluated as anti-malaria agents (against Plasmodium falciparum) and as anti-tuberculosis agents (against Mycobacterium tuberculosis H37Rv) [11]. There is only one report discussing the cytotoxic activity of PsQ, PsR, PsU, PsV, and 3-O-acetyl-PsU against MCF-7 (breast cancer), NCI-H460 (non-small-cell lung cancer), and SF-268 (CNS) cells [11]. These data suggest that some of these pseudopterosins have some cytotoxicity but lack potency.
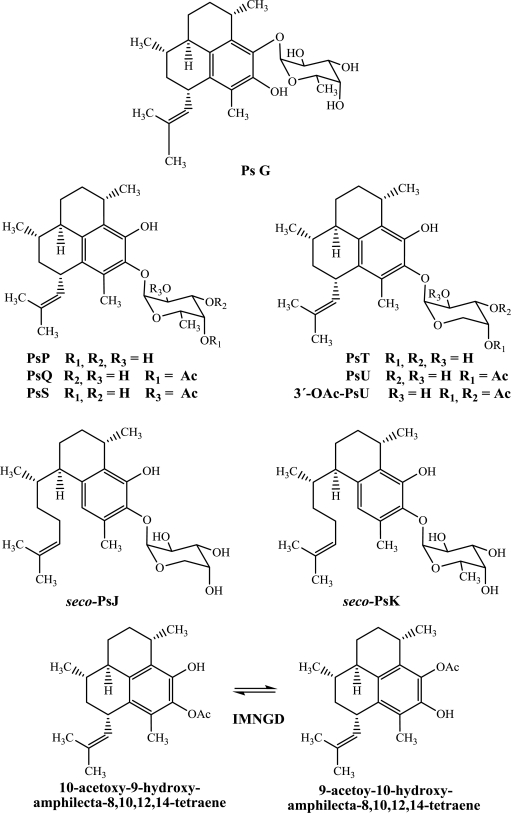
While there has been considerable effort towards characterizing the biological activity of pseudopterosins isolated from octocoral samples collected in the northern Caribbean, much is unknown about the bioactivity of such compounds isolated from octocoral collected from the Islands of San Andrés and Providencia. For this reason, and with a goal of expanding the potential of these compounds, here we report for the first time the antimicrobial profile against four pathogen microorganisms (S. aureus, E. faecalis, Pseudomonas aeruginosa and Candida albicans) and report a more complete cytotoxic profile against five human cells lines (HeLa, PC-3, HCT116, MCF-7 and BJ) for the compounds: PsG, PsP, PsQ, PsS, PsT, PsU, 3-O-acetyl-PsU, seco-PsJ, seco-PsK and IMNGD (Figure 1).
Figure 1.
Chemical structures of pseudopterosins, seco-pseudopterosins and IMNGD isolated from P. elisabethae (SW Caribbean Sea).
2. Results and Discussion
In this study, PsG, PsP, PsQ, PsS, PsT, PsU, 3-O-acetyl-PsU, seco-PsJ, seco-PsK and IMNGD were isolated (Figure 1) by flash chromatography and HPLC and identified by spectroscopic means. The structures of all compounds were previously reported by us [7,10], and preliminary assessment of their cytotoxic (Table 1) and antimicrobial (Table 2) properties is presented here.
Table 1.
Cytotoxic effect of diterpenes isolated from P. elisabethae (SW Caribbean) in human cancer cell lines (HeLa, PC-3, HCT116, MCF-7) and normal BJ cells.
| Compounds |
GI50 ± S.E (μM) |
||||
|---|---|---|---|---|---|
| HeLa | PC-3 | HCT116 | MCF7 | BJ | |
| PsG | 9.22 ± 0.45 | 8.83 ± 0.54 | 12.04 ± 0.36 | 9.42 ± 0.43 | 7.62 ± 0.38 |
| PsP | 10.31 ± 0.49 | 13.77 ± 0.58 | 17.89 ± 0.45 | 12.58 ± 0.45 | 10.40 ± 0.42 |
| PsQ | 5.82 ± 0.33 | 7.81 ± 0.35 | 7.66 ± 0.27 | 8.44 ± 0.41 | 4.47 ± 0.31 |
| PsS | 13.79 ± 0.39 | 52.05 ± 0.31 | 33.50 ± 0.33 | 26.25 ± 0.45 | 29.14 ± 0.45 |
| PsT | 14.58 ± 0.35 | 21.99 ± 0.46 | 24.24 ± 0.42 | 14.72 ± 0.46 | 12.94 ± 0.37 |
| PsU | 15.63 ± 0.32 | 24.81 ± 0.34 | 23.44 ± 0.40 | 26.46 ± 0.46 | 9.35 ± 0.34 |
| 3-O-Ac-PsU | 44.61 ± 0.35 | 26.45 ± 0.31 | 20.48 ± 0.48 | 83.93 ± 0.39 | 62.03 ± 0.45 |
| seco-PsJ | 21.08 ± 0.37 | 37.21 ± 0.41 | 31.68 ± 0.32 | 28.02 ± 0.51 | 15.00 ± 0.35 |
| seco-PsK | 15.83 ± 0.18 | 13.57 ± 0.38 | 13.28 ± 0.27 | 11.45 ± 0.36 | 8.28 ± 0.29 |
| IMNGD* | 11.20 ± 0.15 | 12.11 ± 0.20 | 19.90 ± 0.19 | 9.67 ± 0.15 | 7.91 ± 0.17 |
| Staurosporine** | 105.6 ± 0.41 | 61.82 ± 0.38 | 45.56 ± 0.45 | 176.6 ± 0.38 | 13.56 ± 0.35 |
GI50 (μg/mL),
GI50 (nM)
Table 2.
Antibacterial activity of diterpenes isolated from P. elisabethae (SW Caribbean) in two Gram-positive bacteria.
| Compounds |
IC50 ± S.E (μM) * |
|
|---|---|---|
| S. aureus | E. faecalis | |
| PsG | 4.48 ± 0.18 | 3.14 ± 0.22 |
| PsP | 14.91 ± 0.20 | 37.35 ± 0.29 |
| PsQ | 3.30 ± 0.20 | 7.38 ± 0.16 |
| PsS | 3.89 ± 0.23 | 20.20 ± 0.25 |
| PsT | 5.39 ± 0.25 | 4.38 ± 0.16 |
| PsU | 2.97 ± 0.17 | 3.19 ± 0.25 |
| 3-O-Ac-PsU | 20.23 ± 0.19 | 7.64 ± 0.16 |
| seco-PsJ | 6.52 ± 0.12 | 4.08 ± 0.19 |
| seco-PsK | 4.20 ± 0.16 | 3.82 ± 0.21 |
| IMNGD** | 2.33 ± 0.09 | 3.47 ± 0.15 |
| Penicillin G | 1.61 ± 0.08 | - |
| Vancomycin | - | 4.21 ± 0.11 |
All compounds evaluated were inactive against the Gram-negative bacterium P. aeruginosa and the yeast C. albicans.
IC50 (μg/mL).
2.1. Cytotoxic Activity
The cytotoxic activity of the pseudopterosins (PsG, PsP, PsQ, PsS, PsT, PsU and 3-O-acetyl-PsU), seco-pseudopterosins (seco-PsJ and seco-PsK) and IMNGD against five cell lines: HeLa (cervical cancer), PC-3 (prostate cancer), HCT116 (colorectal cancer), MCF-7 (breast cancer) and BJ (fibroblasts) was investigated using the MTT reduction colorimetric assay [22]. Although no compound showed significant activity, according to the GI50 cut off value of 10 nM for pure compounds suggested by Bugelski et al. [23], all compounds evaluated showed moderate to weak (GI50 5.8–83.9 μM) and non-selective activity against both tumor and normal cell lines as shown in Table 1.
While none of these compound showed comparable activity to the reference drug staurosporine (GI50 13.6–105.6 nM) against the four tumor cell lines, PsQ and PsG were the most active compounds (GI50 values between 5.8 μM to 12.0 μM) and IMNGD showed a moderate activity with GI50 values of 9.7–19.9 μg/mL. These results are comparable with those previously reported by Rodriguez et al. [11], who determined the GI50 values for PsQ, PsU and PsV in the NCI-H460-cell line. Results for PsQ showed a GI50 between 1.7–5.8 μM. In the same screen, PsU and PsV were generally much less toxic (GI50 20–100 μM). For the normal cell line BJ (Table 1), PsS, 3-O-acetyl-PsU, PsP, PsT and seco-PsJ did not show a considerable cytotoxic effect (GI50 > 10 μM) while PsQ, PsG, seco-PsK and PsU showed a moderate cytotoxic activity (GI50 4.5–9.3 μM). The IMNGD showed a moderate activity with GI50 of 8.3 μg/mL.
The selectivity of the cytotoxic activity of the compounds, measured as the differential effect on growth of different types of cell lines, was made by comparing the effect of the compounds on inhibiting cell growth in both normal and tumor cells. The results showed no selectivity of compounds between the lines used, and all compounds induced reduction in cell survival to a similar magnitude in all lines.
Preliminary conclusions regarding structure-activity relationships can be drawn from an examination of the cytotoxic activity. The position of glycosylation on the terpene skeleton appears to affect the inhibitory activity profile as, for example, PsG (glycosylated in C-9 with fucopyranose) is more active than PsP (glycosylated in C-10 with fucopyranose). Further, the type of sugar moiety also influences the activity as, for example, PsP which is glycosylated with fucopyranose is more active than PsT which is glycosylated with arabinopyranose. Likewise, PsQ (C-4’ mono-acetylated fucose as sugar moiety) is more active than PsU (C-4’ mono-acetylated arabinose as sugar moiety) and seco-PsK (non-acetylated fucose as sugar moiety) is more active than seco-PsJ (mono-acetylated arabinose as sugar moiety).
2.2. Antimicrobial Activity
The antimicrobial activity of the pseudopterosins, seco-pseudopterosins and IMNGD was investigated against one Gram-negative bacterium (P. aeruginosa), two Gram-positive bacteria (S. aureus and E. faecalis) and one yeast (C. albicans), using a microdilution method [24]. The majority of compounds evaluated showed a good IC50 (2.9–7.64 μM) and all showed a selective activity against both Gram-positive bacteria tested (Table 2), while they did not show activity against the Gram-negative bacterium or the yeast.
PsU, PsQ, PsS, seco-PsK, PsG were the most active compounds (IC50 2.3–4.5 μM) against S. aureus. The IMNGD showed good activity with IC50 of 2.3 μg/mL. For E. faecalis, PsG, PsU and seco-PsK showed better activity (Table 2) compared to the reference drug vancomycin (4.2 μM) while, seco-PsJ and PsT exhibited similar activity. The IMNGD showed good activity, with an IC50 of 3.5 μg/mL. These results are comparable with the activity previously reported for PsA-E, PsK, PsX and PsY isolated from specimens collected in the north Caribbean Sea [5], which were reported to have minimum inhibitory concentration (MIC) values between 4.2 and 8.8 μM.
The selectivity observed in our study is comparable to that reported by Ata et al., [5] who reported that other pseudopterosins (PsA-E, PsK, PsX and PsY) inhibit the growth of Gram-positive bacteria (S. pyogenes, S. aureus,and E. faecalis), while they were inactive against Gram-negative bacteria (E. coli and P. aeruginosa). This finding can be related to the fact that many Gram-negative bacteria are resistant to toxic agents in the environment, due to the barrier of lipopolysaccharides on their outer membrane [25].
Examination of the data in Table 2 suggests the following structure–activity relationships. Firstly, PsG (glycosylated in C-9 with fucopyranose) is more active than PsP (glycosylated in C-10 with fucopyranose). Secondly, PsT glycosylated with arabinopyranose is more active than PsP, which is glycosylated with fucopyranose. Likewise, PsU (mono-acetylated arabinose as sugar moiety) is more active than PsQ and PsS (mono-acetylated fucose as sugar moiety). However, this behavior initially observed in pseudopterosins (amphilectane skeleton) is not retained when the results are compared with seco-psedopterosins (serrulatane skeleton), where, the seco-PsK (glycosylated with fucoyranose) showed more activity than seco-PsJ (glycosylated with arabinopyranose). Clearly, additional work is required to fully understand the structure–activity relationships for this family of terpenes.
From the above discussion, one can note that the pseudopterosins, seco-pseudopterosins and IMNGD isolated from P. elisabethae collected at the Islands of San Andrés and Providencia (southwest Caribbean Sea) have a similar drug-like potential as the related compounds isolated from specimens collected in the north Caribbean Sea (the Bahamas, Bermuda and the Florida Keys). Further, due to their wide applicability in commercial additives, in the cosmetic industry, it might be important to consider P. elisabethae collected in Colombian waters as an alternative source of such compounds. As has been shown, the anti-inflammatory, anti-microbial and cytotoxic activity of this family of compounds isolated from P. elisabethae collected at different locations at the Caribbean Sea are quite similar, regardless of a small number of differences in their structure (type of sugar moiety, position of glycosylation and stereochemistry).
3. Experimental Section
3.1. Chemicals and Reagents
The following substances were purchased from Sigma-Aldrich (St Louis, USA): Dulbecco’s modified Eagle’s medium (DMEM), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), staurosporine, vancomycin, penicillin G, gentamicin, nystatin, Luria-Bertani Broth (LB) and Sabouraud Dextrose broth (SD). Fetal bovine serum (FBS), salts for phosphate buffered saline (PBS) solution and organic solvents, were purchased from WRW (PA, USA).
3.2. Octocoral Collection
Fragments of individual colonies of P. elisabethae were collected by SCUBA (ca. 20–30 m depth) at Providencia and San Andrés islands (SW Caribbean) and identified by Dr. Monica Puyana. Voucher specimens coded as INV CNI 1612–1616, were deposited at the invertebrate collection of Museo de Historia Natural Marina Colombiana (MHNMC) at Instituto de Investigaciones Marinas de Punta Betín (INVEMAR).
3.3. Isolation and Structure Elucidation of Compounds from P. elisabethae
The dried colony fragments (30 g) from each location were extracted separately with a dichloromethane-methanol (1:1) mixture. The resulting extracts were filtered and concentrated by rotary evaporation to obtain a dark green oil. The isolation of each compound was carried out according to our previously described procedures [7,10] with some modifications. Each extract was subjected separately to flash C18 column chromatography and eluted with 500 mL of each solvent mixture of decreasing polarity (methanol-water 1:9, fraction F1; methanol-water 1:1, F2; methanol-water 4:1, F3; methanol 100% F4; ethanol 100% 5, F5; acetone 100%, F6; dichloromethane-methanol 5:5, F7; and dichloromethane 100%, F8). Checking by LC-MS and TLC, the fractions F4 and F5 from the Providencia extract contained a mixture of pseudopterosins and seco-pseudopterosins and F5 from the San Andrés extract contained the interconverting mixture of non-glycosylated diterpenes (IMNGD). Fraction F4 and F5 from Providencia were mixed and subsequently separated by flash chromatography using a diol column (150 g) yielding PsG (19.1 mg), PsP (9.6 mg), PsQ (58.1 mg), PsS (2.3 mg), PsT (14.5 mg), PsU (46.6 mg), 3-O-Ac-PsU (3.5 mg), seco-PsJ (16.2 mg) and seco-PsK (1.2 mg). Final purification of all compounds was performed on HPLC, using a column Gemini C-18 (5 μm, 10 × 250 mm) and MeOH-water (9:1) as the mobile phase with a 3.0 mL/min flow rate. The isolated compounds were carefully identified by comparison of their spectral data with that shown by our previously isolated compounds [7,8]. Purity was confirmed by HPLC and NMR.
3.4. Cell Lines
The cell lines HeLa (cervical cancer), PC-3 (prostate cancer), HCT116 (colorectal cancer), MCF-7 (breast cancer) and normal human cell line BJ (skin fibroblasts) were used and maintained in DMEM with 5% of FBS and gentamicin 50 μg/mL. Cultures were held in 75 cm2 culture flasks at 37 °C, 5% CO2 and 100% relative humidity, changing media at least twice a week.
3.5. Cytotoxic Assay
Cells were harvested, counted, and transferred into 96 well plates and incubated for 24 h prior to the addition of the test compounds. Test sample solutions (10 μL) at the desired dilutions were added to the wells containing the cells and incubated for 48 h. Microtitration colorimetric method of MTT reduction was used to determine surviving cells at the end of the treatment period [22].
A solution of MTT (0.5 mg/mL) was prepared in PBS and 100 μL of this solution was added to each well after the removal of 100 μL of treatment solution. The plates were incubated at 37 °C for 4 h. The solution in each well, containing media, unmetabolized MTT, and dead cells, was removed by suction and 100 μL of DMSO was added to each well. The DMSO was mixed by pipetting and optical density was recorded using a BioTek Synergy HT microplate reader to measure the absorbance at 570 nm. Percentages of cell survival relative to vehicle control wells (wells containing only cells and DMSO) were calculated.
Stock solutions (5 mg/mL) were prepared by dissolving pure compounds in DMSO and storing at 4 °C. Serial dilutions with culture media were prepared just prior to addition to test plates. The concentration of DMSO in wells was consistent across dilutions at 0.38% v/v. Staurosporine was used as the positive control and vehicle (DMSO + media) as blank. Control and test compounds (PsG, PsP, PsQ, PsS, PsT, PsU, 3-O-Ac-PsU, seco-PsJ, seco-PsK and IMNGD) were assayed in duplicate for each concentration and replicated for each cell line at concentrations of 5000, 1000, 500, 100, 50, 10, 5 and 1 nM for staurosporine and 0.1, 0.5, 1.0, 2.5, 5.0, 7.5, 10.0, and 20.0 μg/mL for compounds.
3.6. Microbial Strains and Culture Media
Compounds isolated from P. elisabethae were tested against a panel of microorganisms including the bacteria Staphylococcus aureus ATCC 375, Enterococcus faecalis ATCC 10741, Pseudomonas aeruginosa ATCC 14210 and the yeast Candida albicans ATCC 14035. Bacterial strains were cultured in Luria-Bertani Broth (LB) and C. albicans was cultured in Sabouraud dextrose broth (SD), for 18 h at 37 °C and 220 rpm in a humidified incubator.
3.7. Antimicrobial Assay
The antimicrobial activity was determined by a microdilution method (IC50) in 96-well microtiter plates using LB media for bacteria and SD media for the yeast seeded liquid media [24]. In all cases, a pre-inoculated dilution was made with fresh broth. A volume of 180 μL of each microorganism suspension was inoculated in each well (105 cells/mL) and mixed with 20 μL of treatment solutions. Stock solutions were prepared by dissolving pure compounds and controls in 20% DMSO and storing at 4 °C. Penicillin G (S. aureus), vancomycin (E. faecalis), gentamicin (P. aeruginosa) and nystatin (C. albicans) were used as positive controls and vehicle (DMSO + media) as blank. The final DMSO concentration used for dissolving the extracts was less than 20%. Controls and test compounds (PsG, PsP, PsQ, PsS, PsT, PsU, 3-O-Ac-PsU, seco-PsJ, seco-PsK and IMNGD) were assayed in duplicate for each of the following concentrations: 64.0, 32.0, 16.0, 4.0, 1.0, 0.25 and 0.06 μg/mL.
Microplates were incubated for 24 h at 37 °C and optical density was recorded using a BioTek Synergy HT microplate reader to measure the absorbance at 600 nm. For each experiment, correction for background absorbance was made by subtracting the value for OD600 after the compounds were added (time 0 h). Percentages of microorganism survival relative to vehicle control wells (wells containing only microorganism and DMSO) were calculated. The IC50 of the controls were also determined in parallel experiments in order to control for the sensitivity of the standard test organisms.
3.8. Statistical Analysis
Data were normalized and analyzed using non-linear regression to elucidate IC50 values from sigmoidal dose-response curves. Comparison between cell lines and microorganisms used extra sum-of-squares F test to evaluate IC50 between data sets. P < 0.05 was considered as indicative of significance using GraphPad Software, Prism V. 5.0.
4. Conclusions
All results presented here contribute to the demonstration that the compounds isolated from P. elisabethae (SW Caribbean) are promising molecules with potentially useful antimicrobial activity profiles. This confirms that this marine organism has great value as a source of lead compounds with pharmaceutical applications. Specifically, PsU, PsQ, PsS, seco-PsK and PsG are the most promising anti-bacterial agents. For this reason, it will be interesting to continue with molecular targeted high-throughput screens in order to understand their mechanism of action.
Acknowledgments
This work was partially financed by grants from Colciencias and Universidad Nacional de Colombia. The Ministerio de Ambiente, Vivienda y Desarrollo Territorial granted permission (permission No. 4 of 10/02/2010) for collecting samples and performing the research on P. elisabethae at the archipelago of San Andrés and Providencia, Colombian Caribbean. R. Kerr gratefully acknowledges financial support from the Natural Sciences and Engineering Council of Canada (NSERC), the Canada Research Chair Program, the University of Prince Edward Island, the Atlantic Innovation Fund, and the Jeanne and Jean-Louis Lévesque Foundation.
Footnotes
Samples Availability: Available from the authors.
References
- 1.Marrero J, Rodriguez II, Rodriguez AD. The natural products chemistry of the gorgonian genus Pseudopterogorgia (Octocorallia:Gorgoniidae) In: Mander L, Liu H-W, editors. Comprehensive Natural Products II: Chemistry and Biology. 1st ed. Vol. 2. Elsevier; Oxford, UK: 2010. pp. 363–428. [Google Scholar]
- 2.Heckrodt TJ, Mulzer J. Marine Natural Products from Pseudopterogorgia elisabethae: structures, biosynthesis, pharmacology, and total synthesis. In: Mulzer J, editor. Natural Products Synthesis II. 1st ed. Vol. 244. Springer Berlin Heidelberg; New York, NY, USA: 2005. pp. 1–41. [Google Scholar]
- 3.Look SA, Fenical W, Jacobs RS, Clardy J. The pseudopterosins: anti-inflammatory and analgesic natural products from the sea whip Pseudopterogorgia elisabethae. Proc Natl Acad Sci USA. 1986;83:6238–6240. doi: 10.1073/pnas.83.17.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roussis V, Wu Z, Fenical W, Strobel SA, van-Duyne D, Clardy J. New antiinflammatory pseudopterosins from the marine octocoral Pseudopterogorgia elisabethae. J Org Chem. 1990;55:4916–4922. [Google Scholar]
- 5.Ata A, Win HY, Holt D, Holloway P, Segstro EP, Jayatilake GS. New antibacterial diterpenes from Pseudopterogorgia elisabethae. Helv Chim Acta. 2004;87:1090–1098. [Google Scholar]
- 6.Hoarau C, Day D, Moya C, Wu G, Hackim A, Jacobs RS, Little RD. iso-PsE, a new pseudopterosin. Tetrahedron Lett. 2008;49:4604–4606. [Google Scholar]
- 7.Duque C, Puyana M, Narvaez G, Paz A, Osorno O, Hara N, Fujimoto Y. Pseudopterosins P-V.New compounds from the gorgonian octocoral Pseudopterogorgia elisabethae from Providencia Island, Colombian Caribbean. Tetrahedron. 2004;60:10627–10635. [Google Scholar]
- 8.Ata A, Kerr RG, Moya CE, Jacobs RS. Identification of anti-inflammatory diterpenes from the marine gorgonian Pseudopterogorgia elisabethae. Tetrahedron. 2003;59:4215–4222. [Google Scholar]
- 9.Puyana M, Narvaez G, Paz A, Osorno O, Duque C. Pseudopterosin content variability of the purple sea whip Pseudopterogorgia elisabethae at the Islands of San Andrés and Providencia (SW Caribbean) J Chem Ecol. 2004;30:1183–1201. doi: 10.1023/b:joec.0000030271.73629.26. [DOI] [PubMed] [Google Scholar]
- 10.Duque C, Puyana M, Castellanos L, Arias A, Correa H, Osorno O, Asai T, Hara N, Fujimoto Y. Further studies on the constituents of gorgonian octocoral Pseudopterogorgia elisabethae collected in San Andrés and Providencia islands, Colombian Caribbean: isolation of a putative biosynthetic intermediate leading to erogorgiane. Tetrahedron. 2006;62:4205–4213. [Google Scholar]
- 11.Rodríguez II, Shi Y-P, García OJ, Rodríguez AD, Mayer AMS, Sánchez JA, Ortega E, González J. New pseudopterosin and seco-pseudopterosin diterpene glycosides from two Colombian isolates of Pseudopterogorgia elisabethae and their diverse biological activities. J Nat Prod. 2004;67:1672–1680. doi: 10.1021/np049802o. [DOI] [PubMed] [Google Scholar]
- 12.Look SA, Fenical W. The seco-pseudopterosins: new anti-inflammatory diterpene-glycosides from a Caribbean gorgonian octocoral of the genus Pseudopterogorgia. Tetrahedron. 1987;43:3363–3370. [Google Scholar]
- 13.Ferns TA, Kerr RG. Identification of amphilectosins as key intermediates in pseudopterosin biosynthesis. J Org Chem. 2005;70:6152–6152. doi: 10.1021/jo050282r. [DOI] [PubMed] [Google Scholar]
- 14.Duque C. Pseudopterogorgia elisabethae de San Andrés y Providencia, una pluma de mar con excelente potencial como fuente de productos naturales con aplicación industrial. Rev Acad Colomb Cienc. 2010;34:89–103. [Google Scholar]
- 15.Potts BC, Faulkner DJ. Phospholipase A2 inhibitors from marine organisms. J Nat Prod. 1992;55:1707–1717. doi: 10.1021/np50090a001. [DOI] [PubMed] [Google Scholar]
- 16.Mayer AMS, Jacobson PB, Fenical W, Jacobs RS, Glaser KB. Pharmacological characterization of the pseudopterosins: novel anti-inflammatory natural products isolated from the Caribbean soft coral Pseudopterogorgia elisabethae. Life Sci. 1998;62:PL 401–407. doi: 10.1016/s0024-3205(98)00229-x. [DOI] [PubMed] [Google Scholar]
- 17.Kijoa A, Sawanwong P. Drugs and cosmetics from the sea. Mar Drugs. 2004;2:72–82. [Google Scholar]
- 18.Gross H, König GM. Terpenoids from marine organisms: unique structures and their pharmacological potential. Phytochem Rev. 2006;5:115–141. [Google Scholar]
- 19.Haefner B. Drugs from the deep: marine natural products as drug candidates. Drug Discov Today. 2003;8:536–544. doi: 10.1016/s1359-6446(03)02713-2. [DOI] [PubMed] [Google Scholar]
- 20.Mayer AMS, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, McIntosh JM, Newman DJ, Potts B, Shuster DE. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci. 2010;31:255–265. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Correa H, Valenzuela AL, Ospina LF, Duque C. Anti-inflammatory effects of the gorgonian Pseudopterogorgia elisabethae collected at the Islands of Providencia and San Andrés (SW Caribbean) J Inflamm. 2009;6 doi: 10.1186/1476-9255-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosmann TJ. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 23.Bugelski PJ, Atif U, Molton S, Toeg I, Lord PG, Morgan G. A strategy for primary high throughput cytotoxicity screening in pharmaceutical toxicology. Pharm Res. 2000;17:1265–1272. doi: 10.1023/a:1026495503939. [DOI] [PubMed] [Google Scholar]
- 24.Vasková Z, Stachová P, Krupkováa L, Hudecováa D, Valigura D. Bis(nitrobenzoato)copper(II) complexes with nicotinamide, preparation, structure and properties. Acta Chim Slov. 2009;2:77–87. [Google Scholar]
- 25.Morton LHG, Greenway DLA, Gaylarde CC, Surman SB. Consideration of some implications of the resistance of biofilms to biocides. Int Biodeter Biodegr. 1998;41:247–259. [Google Scholar]