Abstract
A novel chitin-degrading aerobe, Chitinibacter tainanensis, was isolated from a soil sample from southern Taiwan, and was proved to produce N-acetyl glucosamine (NAG). Chitin degrading factors (CDFs) were proposed to be the critical factors to degrade chitin in this work. When C. tainanensis was incubated with chitin, CDFs were induced and chitin was converted to NAG. CDFs were found to be located on the surface of C. tainanensis. N-Acetylglucosaminidase (NAGase) and endochitinase activities were found in the debris, and the activity of NAGase was much higher than that of endochitinase. The optimum pH of the enzymatic activity was about 7.0, while that of NAG production by the debris was 5.3. These results suggested that some factors in the debris, in addition to NAGase and endochitinase, were crucial for chitin degradation.
Keywords: CDFs, Chitinibacter tainanensis, chitin, NAG, fermentation
1. Introduction
Chitin is the most abundant polysaccharide on the earth except cellulose, and it is a major component of most fungal cell walls, insect exoskeletons and the shells of crustaceans [1]. The chemical structure of chitin is a polymer of N-acetyl-d-glucosamine (NAG) linked by beta-glycosidic bond. Derivatives of chitin, including polysaccharides, oligosaccharides and monosaccharides, have performed several therapeutic activities such as immunomodulation [2,3], antitumor [4] osteoarthritis treatment [5], and so on [6–9]. Also, in the recent decade, NAG, the end hydrolytic product of chitin, has become an attractive biomaterial as food supplements and cosmetics [10–13].
Production of NAG by acid hydrolysis of chitin is expensive owing to low yield. The product manufactured by N-acetylation of glucosamine is not approval as a natural material due to the chemical process [14]. Several natural methods have been used to produce NAG. Using these methods, NAG is obtained by chitin hydrolysis using chitin degrading enzymes, containing endochitinases and N-acetylglucosaminidase (NAGase). The enzymes were usually isolated from microorganisms having strong chitinolytic activities [15,16]. Sashiwa et al. performed selective and efficient methods to produce NAG using a mixed enzyme preparation with high NAGase/endochitinase ratio [14,17,18].
In our laboratory, a novel chitin-degrading aerobe, Chitinibacter tainanensis, was isolated from a soil sample of southern Taiwan [19], and it was proven to produce NAG by degrading chitin. The yield was 0.75 g/g with α-chitin as substrate and was 0.98 g/g with β-chitin as substrate. A needle type crystal was produced after concentration and crystallization, and the purity was measured to be higher than 99%. The product was natural and was proven to be feasible for edible or cosmetic usage [9,12,20].
In this study, the mechanism by which C. tainanensis transforms chitin into NAG was elucidated by fermentation. A novel prospect for NAG obtained from chitin digestion by chitin degradation factors (CDFs) was also proposed.
2. Results and Discussion
2.1. Degradation of Chitin by “Chitin Degrading Factors”
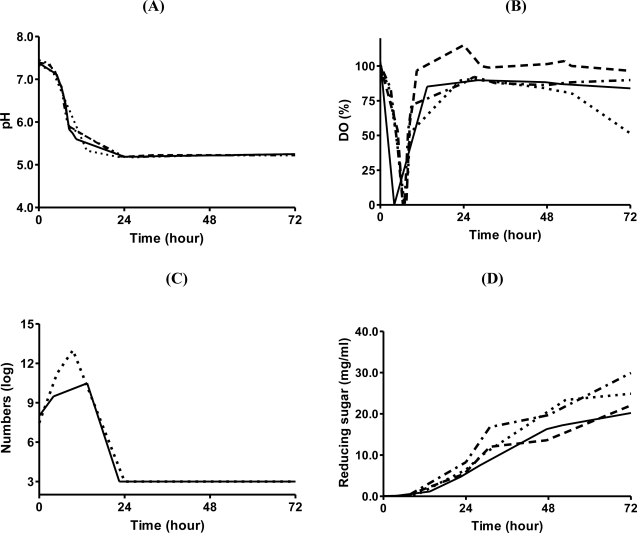
In order to understand the mechanism of chitin degradation by C. tainanensis, chitin degradation studies were performed in a 5 L fermenter. C. tainanensis was incubated in B-H broth containing 4% α-chitin powder. Dissolved oxygen (DO), pH, total aerobe count and reducing sugar were measured continuously for a period of 72 hours. The pH value of the broth decreased gradually from 7.4 to 5.3 in 14 hours (Figure 1A). DO decreased for 4 hours, after that, it increased up to 100% (Figure 1B). This implied that C. tainanensis proliferated rapidly for 14 hours, and some acidic metabolites were released and accumulated in the broth. After incubation, C. tainanensis was dead owing to the acidic broth, confirmed by counting of total aerobe number (Figure 1C).
Figure 1.
Parameters in fermentation. Each factor was evaluated more than once (indicated in brackets). (A) pH (triplicate); (B) DO (quadruplicate); (C) total aerobe number (duplicate); (D) reducing sugar (quadruplicate).
It was interesting to notice that the concentration of reducing sugar in the broth was low within the first 14 hours; however, when C. tainanensis was incubated for longer than 14 hours, the level of reducing sugar increased over the incubation time of the tested period of 72 hours (Figure 1D). This suggested that some factors that were able to degrade chitin completely were produced simultaneously with C. tainanensis proliferation. The hydrolytic product was used as a carbon source for bacterial proliferation, and acidic metabolites were produced simultaneously. C. tainanensis was dead at pH 5.3; however, the assumed factors still existed in the broth. NAG, the final hydrolytic product of chitin degradation, was not consumed, but accumulated in the broth instead. The assumed factors were nominated “CDFs”.
2.2. Chitin Degrading Activity of Bacterial Debris
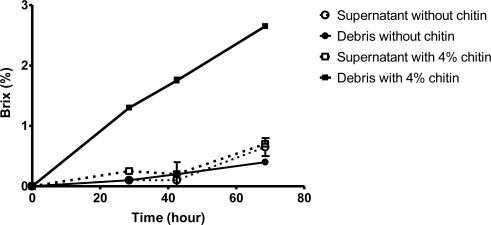
The bacterial debris was obtained by centrifugation, and it was suspended in 0.1 M NaOAc, pH 5.0. After 4% β-chitin was added, the brix increased dramatically in the reaction containing bacterial debris, while that in the reaction containing supernatant increased moderately. In another experiments, β-chitin was hardly degraded by the bacterial debris obtained from the incubation broth using glucose as a carbon source (Figure 2). β-chitin was more susceptible than α-chitin, and higher reducing sugar was produced [21]. NAG concentration was directly measured using a hand refractometer due to its high concentration in the broth. These results reflected that CDFs were located on the bacterial debris, and were induced by chitin powder.
Figure 2.
Chitin degrading effects of supernatant and bacterial debris isolated from the broth with or without 4% beta-chitin.
In order to understand how CDFs hydrolyzed chitin, endochitinase and NAGase activities were measured. Both enzyme activities were detected in the bacterial debris. The specific activity of NAGase was much higher than that of endochitinase (data not shown). These results were in agreement with previous reports [14,17,18].
2.3. Chitin Degrading Effects of Fractions Isolated from the Bacterial Debris
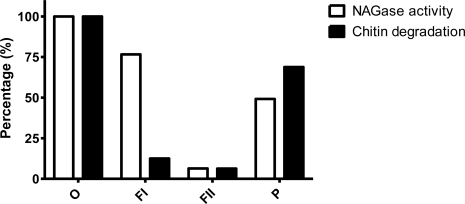
Since assumed CDFs were located on the debris of C. tainanensis, it was reasonable to identify the structural information of CDFs. Bacterial debris (O), dramatically shaken fraction (FI), surfactant extracted fraction (FII) and residue (P) were obtained as described in the Experimental section. Chitin degrading effects and NAGase activities of the four fractions were compared, and those of bacterial debris were set as 100% (Figure 3). Chitin was not efficiently degraded in fraction FI which possessed high NAGase activity. Soluble surface components were almost extracted by shaking treatment due to low chitin degrading effect in fraction FII. In the residual fraction (P), chitin was efficiently degraded although lower NAGase activity existed in this fraction. These results suggested that CDFs strongly interacted with the bacterial surface, and were similar to the cellulosome concept in cellulose degradation [22]. In addition, NAGase activity did not play major roles in chitin degradation of C. tainanensis.
Figure 3.
NAGase activity (□) and chitin degrading activity (▪) of different fractions isolated from the bacterial debris of C. tainanensis. Bacterial debris (O); dramatically shaken fraction (FI); surfactant extracted fraction (FII); residue (P).
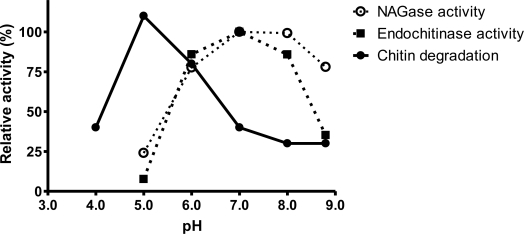
Chitin degrading effects and enzymatic activities of the bacterial debris were also performed in buffers with different pHs. It was surprising that the optimum pH value of the chitin degrading effect was about 5.3, while those of endochitinase and NAGase isolated from the bacterial debris were about 7.0 (Figure 4). These results suggested that the mechanism of chitin degradation of C. tainanensis was novel and different from that of the enzymatic method published before. Some unknown factors not yet explored played important roles in chitin degradation.
Figure 4.
Relative activities of chitin degrading effect, NAGase and endochitinase in buffers of various pH.
3. Experimental Section
3.1. Materials
Powdered α-chitin and β-chitin used in this study were supplied from Charming and Beauty company (Taipei, Taiwan). Media for microorganism incubation were purchased from Difco Laboratories (Dentroit, MI). Colloidal chitin was prepared from α-chitin using the method of Jeniaux [23,24], and other chemicals used in this study were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
3.2. Fermentation of C. tainanensis
The agar containing B-H broth, Difco Bushnell-Haas broth, and 0.2% colloidal chitin was used as a selective medium. The colonies with surrounding clear zones were obtained and grown in 400 mL Luria-Bertani medium at 30 °C overnight. A fresh overnight culture was transferred to a 5 L fermenter containing 4 L B-H broth supplemented with 4% α-chitin powder as a sole carbon source. The agitation rate and air feeding were set at 200 rpm and 4 L/min. All fermentations were performed at 30 °C. pH and DO were detected by an appropriate electrode, and the reducing sugar was analyzed with a spectrophotometer using potassium ferricyanide as substrate [25]. Numbers of bacteria in the fermenter were counted after spraying the diluted broth on nutrient agar following 48 hours incubation.
3.3. Preparation of Bacterial Debris and Its Components
C. tainanensis was incubated in B-H broth containing 4% β-chitin powder for 48 hours. Bacterial debris was collected by centrifugation, and was suspended in an equal volume of 0.1 M NaOAc, pH 5.0. Bacterial debris (O) was shaken dramatically to obtain the surface component in supernatant (FI) after centrifugation, and the pellet was further treated with 0.1% N-lauroylsarcosine to isolate the other component on the surface of bacterial debris (FII). After centrifugation, the residues were suspended in equal volume of 0.1 M NaOAc, pH 5.0 for test.
3.4. Analysis of Chitin Degrading Effect and Enzymatic Activity
Chitin was used as substrate to analyze the chitin degrading effect. Briefly, in the sterilized test tube, chitin was added to the bacterial debris or its components described above at a final concentration of 4%, and 10 μg/mL of tetracycline was added subsequently to prevent microorganism contamination. NAG released in the reaction was detected using a hand refractometer after centrifugation, and the result was expressed as brix (%). The yield was calculated from the equation: Yield (%) = NAG (g)/Chitin (g) added [14].
Endochitinase and NAGase activities were analyzed using 0.18 mM PNP-(GlcNAc)3 and 0.18 mM PNP-GlcNAc as substrate, respectively [7,20,24,26,27]. After 30 min incubation, NaOH was added to a final concentration of 0.1 M to terminate the reaction. The absorbance at 405 nm was measured using a spectrophotometer. When enzyme activity of insoluble component was analyzed, the insoluble component was removed by centrifugation before colorimetric measurement.
4. Conclusion
CDFs, chitin degrading factors, were proposed to describe the chitin degrading effect of C. tainanensis. When C. tainanensis was incubated with chitin, CDFs were induced and chitin was converted to NAG. After consumption of NAG, the medium became sour simultaneously, and the bacterium was dead, then NAG was accumulated in the medium. CDFs were found to be located on the surface of C. tainanensis. Chitin degradation and enzymatic activity measurement showed that chitin degradation of C. tainanensis was different from that of enzymatic method, and some unknown factors not yet explored played important roles in chitin degradation.
Acknowledgments
This work was financially supported in part by the Chang Gung Memorial Hospital Grant CMRPD150381 and BMRP440, National Science Council grant 98-2320-B-182-014-MY3 to C. R. Shen. It also did by National Science Council grant 96-2221-E-131-008 and 100-2914-I-131-001-A1 as well as Ministry of Education Industry-Academy Cooperation grant 98B-38-006 as well as 99B-38-050 to C. L. Liu.
References
- 1.Flash J, Pilet PE, Jolles P. What’s new in chitin research? Experientia. 1992;48:701–716. doi: 10.1007/BF02124285. [DOI] [PubMed] [Google Scholar]
- 2.Shibata Y, Foster LA, Metzger WJ, Myrvik QN. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Infect. Immun. 1997;65:1734–1741. doi: 10.1128/iai.65.5.1734-1741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibata Y, Honda I, Justice JP, van Scott MR, Nakamura RM, Myrvik QN. Th1 adjuvant N-acetyl-d-glucosamine polymer up-regulates Th1 immunity but down-regulates Th2 immunity against a mycobacterial protein (MPB-59) in interleukin-10-knockout and wild-type mice. Infect. Immun. 2001;69:6123–6130. doi: 10.1128/IAI.69.10.6123-6130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki K, Mikami T, Okawa Y, Tokoro A, Suzuki S, Suzuki M. Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose. Carbohydr. Res. 1986;151:403–408. doi: 10.1016/s0008-6215(00)90359-8. [DOI] [PubMed] [Google Scholar]
- 5.Creamer P. Osteoarthritis pain and its treatment. Curr Opin Rheumatol. 2000;12:450–455. doi: 10.1097/00002281-200009000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Aam BB, Heggset EB, Norberg AL, Sorlie M, Varum KM, Eijsink VG. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs. 2010;8:1482–1517. doi: 10.3390/md8051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang CJ, Liu YK, Liu CL, Shen CN, Kuo ML, Su CC, Tseng CP, Yen TC, Shen CR. Inhibition of acidic mammalian chitinase by RNA interference suppresses ovalbumin-sensitized allergic asthma. Hum. Gene Ther. 2009;20:1597–1606. doi: 10.1089/hum.2008.092. [DOI] [PubMed] [Google Scholar]
- 8.Shen CR, Juang JH, Tsai ZT, Wu ST, Tsai FY, Wang JJ, Liu CL, Yen TC. Preparation, characterization and application of superparamagnetic iron oxide encapsulated with N-[(2-hydroxy-3-trimethylammonium) propyl] chitosan chloride. Carbohydr. Polym. 2011;84:781–784. [Google Scholar]
- 9.Chen JK, Shen CR, Liu CL. N-acetylglucosamine: production and applications. Mar. Drugs. 2010;8:2493–2516. doi: 10.3390/md8092493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talent JM, Gracy RW. Pilot study of oral polymeric N-acetyl-d-glucosamine as a potential treatment for patients with osteoarthritis. Clin. Ther. 1996;18:1184–1190. doi: 10.1016/s0149-2918(96)80073-7. [DOI] [PubMed] [Google Scholar]
- 11.Salvatore S, Heuschkel R, Tomlin S, Davies SE, Edwards S, Walker-Smith JA, French I, Murch SH. A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment Pharmacol. Ther. 2000;14:1567–1579. doi: 10.1046/j.1365-2036.2000.00883.x. [DOI] [PubMed] [Google Scholar]
- 12.Bissett DL, Robinson LR, Raleigh PS, Miyamoto K, Hakozaki T, Li J, Kelm GR. Reduction in the appearance of facial hyperpigmentation by topical N-acetyl glucosamine. J. Cosmet. Dermatol. 2007;6:20–26. doi: 10.1111/j.1473-2165.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- 13.Kimball AB, Kaczvinsky JR, Li J, Robinson LR, Matts PJ, Berge CA, Miyamoto K, Bissett DL. Reduction in the appearance of facial hyperpigmentation after use of moisturizers with a combination of topical niacinamide and N-acetyl glucosamine: results of a randomized, double-blind, vehicle-controlled trial. Br. J. Dermatol. 2010;162:435–441. doi: 10.1111/j.1365-2133.2009.09477.x. [DOI] [PubMed] [Google Scholar]
- 14.Sashiwa H, Fujishima S, Yamano N, Kawasaki N, Nakayama A, Muraki E, Sukwattanasinitt M, Pichyangkura R, Aiba SI. Enzymatic production of N-acetyl-d-glucosamine from chitin. Degradation study of N-acetylchitooligosaccharide and the effect of mixing of crude enzymes. Carbohydr. Polym. 2003;51:391–395. [Google Scholar]
- 15.Kuk JH, Jung WJ, Hyun JG, Ahn JS, Kim KY, Park RD. Selective preparation of N-acetyl-D-glucosamine and N,N′-diacetylchitobiose from chitin using a crude enzyme preparation from Aeromonas sp. Biotechnol. Lett. 2005;27:7–11. doi: 10.1007/s10529-004-6300-3. [DOI] [PubMed] [Google Scholar]
- 16.Li YL, Wu ST, Yu ST, Too JR. Screening of a microbe to degrade chitin. Taiwan. J. Agric. Chem. Food Sci. 2005;43:410–418. [Google Scholar]
- 17.Sashiwa H, Fujishima S, Yamano N, Kawasaki N, Nakayama A, Muraki E, Aiba SI. Production of N-acetyl-d-glucosamine from beta-chitin by enzymatic hydrolysis. Chem Lett. 2001:308–309. [Google Scholar]
- 18.Sashiwa H, Fujishima S, Yamano N, Kawasaki N, Nakayama A, Muraki E, Hiraga K, Oda K, Aiba S. Production of N-acetyl-d-glucosamine from alpha-chitin by crude enzymes from Aeromonas hydrophila H-2330. Carbohydr. Res. 2002;337:761–763. doi: 10.1016/s0008-6215(02)00034-4. [DOI] [PubMed] [Google Scholar]
- 19.Chern LL, Stackebrandt E, Lee SF, Lee FL, Chen JK, Fu HM. Chitinibacter tainanensis gen. nov., sp. nov., a chitin-degrading aerobe from soil in Taiwan. Int. J. Syst. Evol. Microbiol. 2004;54:1387–1391. doi: 10.1099/ijs.0.02834-0. [DOI] [PubMed] [Google Scholar]
- 20.Liu CL, Shen CR, Hsu FF, Chen JK, Wu PT, Guo SH, Lee WC, Yu FW, Mackey ZB, Turk J, Gross ML. Isolation and identification of two novel SDS-resistant secreted chitinases from Aeromonas schubertii. Biotechnol. Prog. 2009;25:124–131. doi: 10.1002/btpr.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurita K, Kaji Y, Mori T, Nishiyama Y. Enzymatic degradation of beta-chitin: Susceptibility and the influence of deacetylation. Carbohydr. Polym. 2000;42:19–21. [Google Scholar]
- 22.Shoham Y, Lamed R, Bayer EA. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol. 1999;7:275–281. doi: 10.1016/s0966-842x(99)01533-4. [DOI] [PubMed] [Google Scholar]
- 23.Hsu SC, Lockwood JL. Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl. Microbiol. 1975;29:422–426. doi: 10.1128/am.29.3.422-426.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen CR, Chen YS, Yang CJ, Chen JK, Liu CL. Colloid chitin azure is a dispersible, low-cost substrate for chitinase measurements in a sensitive, fast, reproducible assay. J. Biomol. Screen. 2010;15:213–217. doi: 10.1177/1087057109355057. [DOI] [PubMed] [Google Scholar]
- 25.Imoto T, Yagishita K. A simple activity measurement of lysozyme. Agri. Biol. Chem. 1971;35:1154–1156. [Google Scholar]
- 26.Wang S, Moyne A, Thottappilly G, Wu S, Locy RD, Singh NK. Purification and characterization of a Bacillus cereus exochitinase. Enzyme Microb. Technol. 2001;28:492–498. doi: 10.1016/s0141-0229(00)00362-8. [DOI] [PubMed] [Google Scholar]
- 27.Guo SH, Chen JK, Lee WC. Purification and characterization of extracellular chitinase from Aeromonas schubertii. Enzyme Microb. Technol. 2004;35:550–556. [Google Scholar]