Abstract
Ligniera junci, a plasmodiophorid parasite of Juncus spp. was found at three locations in Tyrol. The morphologically similar plasmodiophorid of Poaceae Polymyxa graminis was found to co-occur at two of these locations. This is the first record of these species in Austria. Colour photographs of resting spores of Ligniera junci in roots of Juncus triglumis and Polymyxa graminis in roots of Poaceae are given. A description of Ligniera junci supported by light micrographs of different stages of the life-cycle (sporosori, zoosporangia, zoospores) is presented.
Keywords: Plasmodiophorids, Ligniera junci, Polymyxa graminis
Plasmodiophorids are an enigmatic group of obligate biotrophic pathogens of higher plants. They have long been treated as a basal group of fungi (Braselton 1995), but recent molecular phylogenies point to a close affiliation with the protozoan phylum Cercozoa (Cavalier-Smith & Chao 2003). Plasmodiophorids are characterised by a multi-stage life cycle with zoosporic, plasmodial, and resting stages (Braselton 2001).
Only a small number of plasmodiophorids are quite well studied, especially plasmodiophorids which cause plant diseases (e. g., Plasmodiophora brassicae Woronin) and/or have economic significance as vectors of plant viruses (e. g. Polymyxa graminis Ledingham). Species without economic impact are reported rarely, probably because of their “hidden” life as obligate plant parasites. Sorosphaera veronicae J. Schröt., a plasmodiophorid recently reported to occur in Veronica spp. in Austria (Neuhauser & al. 2005), can be identified by the galls on the above ground plant parts of their host, but many plasmodiophorids can only be recognised by the presence of their resting spores in the roots (Karling 1968, Braselton 2001, Kirchmair & al. 2005, Neuhauser & al. 2009 a). This soil-borne and plant-associated nature of plasmodiophorids has hindered comprehensive research on the distribution of economically less important species. Some of them might be very common but reported records are rare.
During the vegetation periods in 2008 and 2009 sporosori of a plasmodiophorid parasite could be observed in root hairs of nearly every uprooted Juncus triglumis L. at the Rotmoosferner glacier foreland in the Central Alps (Ötztal, Tyrol, Austria). The species could be identified as Ligniera junci (Schwartz) Maire & A. Tison, which is the first record for this species in Austria. Moreover, the occurrence of the Poaceae-infesting plasmodiophorid Polymyxa graminis in the rhizosphere of different Poaceae was indicated by DNA-sequences. The glacier foreland of the Rotmoosferner is a well-studied region in respect to vegetation succession patterns, (Raffl & al. 2006), mycorrhiza status of different plants (e.g., Mühlmann & Peintner 2008, Fleisch 2009) or soil fungal communities (Oberkofler & Peintner 2009). Despite all attempts in studying biodiversity at the glacier foreland of the Rotmoosferner there were no hints on the occurrence of plasmodiophorids, neither by direct observations nor by molecular soil analyses.
In autumn 2008 different Juncus spp. were sampled from different habitats in Tyrol. Ligniera junci was detected at two more sites (Brandenberg, Münster). Polymyxa graminis could be found at two sites in Brandenberg, Tyrol, where the occurrence of S. veronicae a plasmodiophorid of Veronica spp. was reported earlier (Neuhauser & al. 2005).
Material and methods
Lightmicroscopic investigations
For light microscopy a Nikon Optiphot light microscope (DIC, 100 × oil) was used.
DNA extraction, PCR, sequence analyses
Root samples were rinsed with tap water and screened microscopically for the presence of plasmodiophorids. Roots with a proven infection were used for DNA extraction. DNA was extracted from roots as described previously (Neuhauser & al. 2009b). Plasmodiophorid specific primers Psvit F (ACG CGT TCC AAC TTC TTA GAG GGA) / Psvit R (CAT GCC TCT CTG AGT ATC GGT TTC) designed in our working group were used for the amplification of the 18S rDNA region (Neuhauser & al. 2010).
Material examined
Ligniera junci
Austria, Tyrol, Brandenberg (47° 29′ 20″ N, 11° 54′ 46″ E, 989 m A.S.L.), in roots of Juncus spec., 8. 9. 2008, leg. S. Neuhauser (IB 2008/0031). Austria, Tyrol, Münster (47° 24′ 54″ N, 11° 50′ 19″ E, 519 m A.S.L.), in roots of Juncus effuses, 5. 10. 2008, leg. S. Neuhauser, M. Kirchmair (IB 2008/0032); - Obergurgl, Rotmoostal (46° 50′ 46″ N, 11° 01′ 05″ E, 2252 m A.S.L.), in roots of Juncus triglumis, 16. 7. 2008, leg. S. Neuhauser & M. Kirchmair (IB 2008/0030; GenBank GQ892043).
Polymyxa graminis
Austria, Tyrol, Brandenberg (47° 29′ 20″ N, 11° 54′ 46″ E, 989 m A.S.L.), 8. 9. 2008, in roots of Poaceae, leg. S. Neuhauser & M. Kirchmair (IB 2008/33; GenBank GQ892044); - - (47° 29′ 17″ N, 11° 54′ 09″ E, 956 m A.S.L.)8. 9. 2008, in roots of Poaceae, leg. S. Neuhauser & M. Kirchmair (IB 2008/35).
Ligniera junci (Schwartz) Maire & A. Tison, 1911
C. R. Hebd. Seances. Acad. Sci. 152: 206. (Figs. 4-6, 8-12)
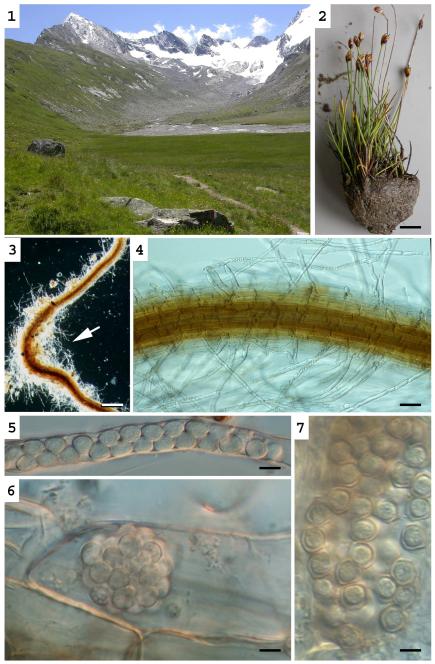
Figs. 1-6.
Liniera junci. - Fig. 1. Habitat at the Rotmoosvalley, Ötztal, Austria. - Fig. 2. Host species Juncus triglumis. - Figs. 3-4. Root of J. triglumis infected with L. junci. Resting spores can mainly be found in regions with numerous root hairs (arrows). - Figs 5-6. Resting spores in a root hair (5) and in a root cortical cell (6). - Fig. 7: Polymyxa graminis: Resting spores in a root cortical cell of a Poaceae. – Bars Bars: 1cm (Fig. 2), 200 μm (Fig. 3), 50 μm, (Fig. 4) 5 μm (Figs 6-7):
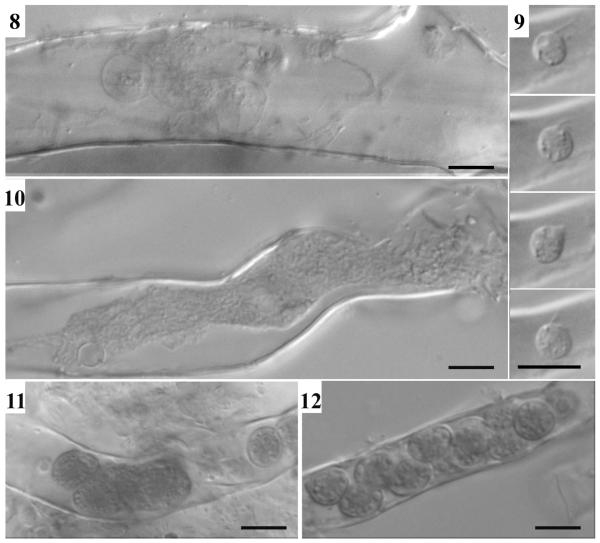
Figs. 8-12.
Liniera junci. - Fig. 8. Thin-walled, lobed zoosporangia. - Fig. 9. Primary (?) zoospore in a root hair of Juncus triglumis. - Fig.10. Plasmodium. - Figs. 11-12. Formation of resting spores. – Bars: 5μm
≡ Sorosphaera junci Schwartz 1910. Ann. Bot. London 24: 514, pl. 40.
Resting spores of L. junci were observed in root hairs and root cortical cells of Juncus triglumis L. and Juncus effuses L. The resting spores are subsphaerical to obvoid: (4.5-)5.4 ± 0.6(−6.8) × (3.3-)4.6 ± 0.5(−5.6) μm; Q = (1.0-)1.2 ± 0.1(−1.6); n = 49. The resting spores are united into sporosori which are highly variable in size and shape, depending on the shape of the host cell: Linear series of resting spores were observed especially in root hairs (Figs. 4-5, 12), while in cortical cells globose or cylindrical aggregates were observed (Fig. 6).
Zoosporangia with delicate walls are lobed, forming loose aggregates filling up parts or the entire host cell (Fig. 8). Primary (?) zoospores are biflagellate, amoeboid, 3-4 μm (Fig. 9). Primary plasmodia (Fig. 10) separate into resting spores (Fig. 11) with robust, smooth walls (Figs. 5, 6, 12).
Resting spores can mainly be found in regions with numerous root hairs, but it remains unclear if such regions are preferably infected or if an infection with L. junci induces the formation of root hairs.
Polymyxa graminis Ledingham, 1939
Canad. J. Res. C., 17: 50, figs 1-3, pls. 1-4 (Fig. 7).
Resting spores of P. graminis were observed in root cortical cells of different Poaceae. The resting spores are subsphaerical to obvoid (3.8-)5.3 ± 0.6(−7.4) × (3.2-)4.6 ± 0.6(−6.3) μm in diam.; Q = (1-)1.2 ± 0.1(−1.6); n = 59. The resting spores are united into loose sporosori which fill up the entire host cell (Fig. 7). The lobed irregular sporangia with exit tubes - which are the only morphologic character to discriminate Polymyxa from the genus from Ligniera (Karling 1968) - could not be observed. Primary and secondary zoospores were not observed. The identity of the record was confirmed by molecular analyses.
Comments
Schwartz (1910) found the resting spores of Sorosphaera junci united into spherical or ellipsoidal sporosori in root cortical cells or as loose aggregates filling the root cells of Juncus bufoninus L. No measurements are given within the original description but interpreting Schwartzs (1910) drawings a diameter of the resting spores between 4.1 and 5.5 μm can be estimated. Maire & Tison (1911) established the genus Ligniera based on L. junci and the then new described two species L. radicalis Maire & Tison in roots of Callitriche stagnalis Scop. and L. verrucosa Maire & Tison in roots of Veronica arvensis L. They specify the resting spore diameter of 5-7 μm for L. junci and 4-5 μm for L. radicalis. The same dimensions are given by Cook (1926). Karling (1968) and Dylewsky (1990) followed the opinion of Cook (1933) who concluded from infection experiments that L. radicalis and L. junci are conspecific and referred to a resting spore diameter of 4-7 μm.
Neither Schwartz (1910) nor any other, later author reporting on Ligniera junci mentioned the high abundance of sporosori in root hairs - as observed in the present study. They reported the resting spores in root cortical cells of Juncus species.
Fron & Gaillant (1925) described Ligniera pilorum in root hairs of different Poaceae. The diameter of the resting spores is given as 4-6 μm and therefore identical with those of L. junci but in contrast to the latter species all infected root hairs exhibited clear hypertrophies and became clavate.
In the years following his discovery of L. junci Schwartz described a range of Ligniera species at the type locality of L. junci: L. graminis (Schwartz 1911), L. bellidis and L. menthae (Schwartz 1914). These species were differentiated mainly by their host plants. Cook (1926) collected different plants at Schwartz’ “Ligneria site”. He found a range of plants infected with what he thought to be L. junci (Poa annua L., Plantago major L., Ranunculus aquatilis L., Mentha pulegium L., Callitriche stagnalis Scop., Veronica beccabunga L., Juncus articulatus L.). For testing the hypothesis of conspecifity Cook (1926) potted seedlings of each plant species into sterile soil and infected them with infested roots. All of them were successfully infected in different frequencies. The conspecifity of Schwartz’ (1911, 1914) later described Ligniera species and L. radicalis (parasitic in Callitriche; Maire & Tison 1911) seems convincing. But there is one drawback in the argumentation: Polymyxa graminis described by Ledingham (1939) is hardly distinguishable from Ligniera at the base of resting spore morphology. There are records of P. graminis on many different hosts (Karling 1968, Legrève & al. 2000). In the present study we could find Ligniera junci and Polymyxa graminis within a few square meters in a swamp and confirmed the species identity by molecular data. Although Cooks (1926) investigations were made very carefully, a mixing up with P. graminis in experiments seems at least imaginable as he did not use roots from the original host plant (Juncus bufoninus). Many of the older records of L. junci might be mistakenly identified as has already been pointed out by Barr (1981).
Besides the fact that we found resting spores mainly in root hairs and only rarely in root cortical cells, our collections fit perfectly with the original description of L. junci by Schwartz (1910) regarding resting spore size and form, size and form of sporosori in cortical cells, and of their host plant.
Thin-walled, lobed zoosporangia which discriminate Ligniera from Polymyxa as well as unusual flagellate zoospores within root hairs as described by Cook (1926) could be observed (Fig. 9). Moreover, sequence data confirmed the separation of the two taxa.
Acknowledgments
The authors wish to thank R. Pöder, University of Innsbruck, Austria for passing on his knowledge and expertise. We are indebted to B. Erschbamer for help with the determination of the Juncus species. Sigrid Neuhauser thanks the Austrian Science Fund for a Hertha–Firnberg research grant (FWF; project T379-B16).
Contributor Information
Sigrid Neuhauser, Institut für Mikrobiologie, Leopold-Franzens-Universität, Technikerstr. 25, 6020 Innsbruck, Österreich, sigrid.neuhauser@uibk.ac.at.
Martin Kirchmair, Institut für Mikrobiologie, Leopold-Franzens-Universität, Technikerstr. 25, 6020 Innsbruck, Österreich, martin.kirchmair@uibk.ac.at.
References
- Braselton JP. Current status of the plasmodiophorids. Crit. Rev. Microbiol. 1995;21:263–275. doi: 10.3109/10408419509113543. [DOI] [PubMed] [Google Scholar]
- Braselton JP. Plasmodiophoromycota. In: McLaughlin DJ, McLaughlin EG, Lemke PA, editors. The Mycota VII Part A. Systematics and evolution. Springer; Berlin, Heidelberg: 2001. pp. 81–91. [Google Scholar]
- Barr DJS. Polymyxa graminis. Fungi Canadenses. National Mycological Herbarium, Biosystematic Research Centre, Agriculture Canada; Ottawa, Ontario, Canada: 1981. (Fungi Canadenses No. 199.). [Google Scholar]
- Cavalier-Smith T, Chao EEY. Phylogeny and classification of phylum Cercozoa (Protozoa) Protist. 2003;154:341–358. doi: 10.1078/143446103322454112. [DOI] [PubMed] [Google Scholar]
- Cook WRI. The genus Ligniera Maire and Tison. Trans. Brit. Mycol. Soc. 1926;11:196–213. Plates 8, 9. [Google Scholar]
- Cook WRI. A monograph of the Plasmodiophorales. Arch. Protistenkunde. 1933;80:179–254. [Google Scholar]
- Dylewski DP. Phylum Plasmodiophoromycota. In: Margulis L, Corliss JO, Melkonian M, Chapman DJ, editors. Handbook of Protoctista. Jones & Barlett; London: 1990. pp. 399–416. [Google Scholar]
- Fleisch M. Mykorrhizastatus von Pionierpflanzen und viviparen Pflanzen aus Primärsukzessionsstandorten des Rotmoosferner Gletschervorfeldes. University of Innsbruck; 2009. Diploma Thesis. [Google Scholar]
- Fron M, Gaillant M. Contribution à l’étude du genre Ligniera. Bull. Soc. Myc. France. 1925;41:388–390. T. XLI, Pl. X. [Google Scholar]
- Karling JS. The Plasmodiophorales. 2nd edn. Hafner; New York, London: 1968. [Google Scholar]
- Kirchmair M, Neuhauser S, Huber L. Sorosphaera viticola sp. nov. (Plasmodiophorids), an intracellular parasite in roots of grape vine. Sydowia. 2005;57:223–232. [Google Scholar]
- Ledingham GA. Studies on Polymyxa graminis, n. gen. n. sp., a plasodiophoraceous root parasite of wheat. Canad. J. Res. C. 1939;17:38–51. figs. 1-3, pls. 1-4. [Google Scholar]
- Legrève A, Vanpee B, Delfosse P, Maraite H. Host range of tropical and sub-tropical isolates of Polymyxa graminis. Eur. J Pl. Pathol. 2000;106:379–389. [Google Scholar]
- Maire M, Tison A. Sur quelques Plasmodiophoracées non hypertrophiantes. Compt. Rend. Hebd. Seances. Acad. Sci. 1911;152:206–208. [Google Scholar]
- Mühlmann O, Peintner U. Ectomycorrhiza of Kobresia myosuroides at a primary successional glacier forefront. Mycorrhiza. 2008;18:355–362. doi: 10.1007/s00572-008-0188-z. [DOI] [PubMed] [Google Scholar]
- Neuhauser S, Huber L, Kirchmair M. Sorosphaera veronicae; neu für Österreich. Österr. Z. Pilzk. 2005;14:303–308. [Google Scholar]
- Neuhauser S, Huber L, Kirchmair M. Sorosphaera viticola a plasmodiophorid parasite of grapevine. Phytopatholog. Mediterr. 2009 a;48:136–139. [PMC free article] [PubMed] [Google Scholar]
- Neuhauser S, Huber L, Kirchmair M. A DNA based detection method for Roesleria subterranea in grapevine roots and soil samples. Phytopatholog. Mediterr. 2009 b;48:59–72. [PMC free article] [PubMed] [Google Scholar]
- Neuhauser S, Bulman S, Kirchmair M. Plasmodiophorids: The challenge to understand soil-borne, obligate biotrophs with a multiphasic life cycle. In: Gherbawy Y, Voigt K, editors. Current advances in molecular identification of fungi. Springer; Berlin: 2010. in press. [Google Scholar]
- Oberkofler I, Peintner U. Detection of soil fungal communities in an alpine primary successional habitat: Does pooling of DNA extracts affect investigations? Ann. Microbiol. 2009;58:585–595. [Google Scholar]
- Raffl C, Mallaun M, Mayer R, Erschbamer B. Vegetation succession pattern and diversity changes in a glacier valley, Central Alps, Austria. Arctic Antarctic Alpine Res. 2006;38:421–428. [Google Scholar]
- Schwartz EJ. Parasitic root diseases of the Juncaceae. Ann. Bot. London. 1910;24:511–522. pl. XL. [Google Scholar]
- Schwartz EJ. A new parasitic fungus found in the roots of grasses. Ann. Bot. London. 1911;25:591–597. pl. LXI. [Google Scholar]
- Schwartz EJ. The Plasmodiophoraceae and relationship to the Mycetozoa and Chytrideae. Ann. Bot. London. 1914;26:227–240. pl. XXII. [Google Scholar]