Abstract
The present studies determined the role of tumor necrosis factor (TNF)/tumor necrosis factor receptor (TNFR) interactions on cytolytic (CTL) activity of splenic and intrahepatic lymphocytes (IHL) isolated from mice undergoing graft versus host disease, induced by transfer of B6 T cells to major histocompatibility complex (MHC) class I disparate bm1 × B6 F1 mice. Allospecific killing of anti-H-2bm1 splenic and hepatocyte targets was assessed by 4-h 51Cr release and 16-h DNA lysis assays, respectively, utilizing spleen cells (SpC) and IHL isolated (1) from sublethally irradiated bm1 × B6 F1 who had received B6 spleen and bone marrow cells, and a control adenovirus (Adv-βgal) or a TNF inhibitor expressing adenovirus (Adv-TNFi), or (2) from bm1 × B6 F1 recipients of B6, B6.129-Tnfrsf1atm1Mak/J (TNFR1−/−), B6.129S2-Tnfrsf1btm1Mwm/J (TNFR2−/−), or B6.129S-Tnfrsf1atm1Imx Tnfrsf1btm1Imx/J (TNFR−/−) SpC and bone marrow cells, or (3) from in vitro-activated SpC. Splenic and IHL from bone marrow transplant recipients who had received Adv-TNFi at the time of transplant displayed lower allospecific CTL activity than controls. Addition of TNFR-Ig or a TNF antibody before the CTL activity assay further reduced allospecific killing against bm1 SpC blast targets. Both TNF/TNFR1 and TNF/TNFR2 interactions were critical for the development of optimal CTL activity against allospecific hepatocyte targets. Further, TNFR1- and TNFR2-deficient SpC from MHC class I disparate mixed lymphocyte cultures displayed lower CTL activity and expression of effector molecules than control B6 SpC. TNF/TNFR interactions were critical for the development of optimal CTL activity of IHL and splenic cytotoxic T cells against MHC class I disparate SpC blast and hepatocyte targets in MHC class I disparate graft versus host disease.
Introduction
Acute graft versus host disease (GVHD) is a major obstacle to a safe allogeneic hematopoietic stem cell transplantation, leading to significant morbidity and mortality. GVHD occurs when transplanted donor T lymphocytes react to foreign host alloantigens. Many murine hepatic GVHD models are CD8+ T cell mediated (El-Hayek and others 2005). This observation may be partially explained by the fact that major histocompatibility complexes (MHC) class I antigens are constitutively expressed on biliary epithelium and the vascular endothelial cells (Williams and Thiele 1994; El-Hayek and others 2005). Recently, hepatocytes have also been noted to have abundant surface MHC class I antigens, which may be induced by specific cytokines and diseases (Gouw and others 1988; Ferrara and Deeg 1991; Goes and others 1996; Dillon and others 1997).
Immunopathophysiology of hepatic GVHD likely consists of 3 steps (1) intensive conditioning regimens that cause extensive injury to host tissue and a release of inflammatory cytokines with subsequent upregulation of MHC antigens and (2) an activation of donor CD8+ T cells, which results in (3) further target host tissue damage (Ferrara and Deeg 1991). The mechanisms of cytotoxic T cells' involvement in GVHD may include a number of different pathways, direct lysis of cellular targets or via cytokines, such as interferon-gamma (IFN-γ). The lysis may be initiated via 2 pathways, the tumor necrosis factor (TNF)/tumor necrosis factor receptor (TNFR) and Fas (also known as CD95 and APO-1)/Fas ligand (CD95:FasL) pathways or the granzyme/perforin pathway (Ferrara and Deeg 1991). Pathologists have observed a number of cell types (endothelial, biliary epithelial, and hepatocytes) undergoing destruction in liver biopsy specimens. Importantly, the apoptosis of specific targets may be mediated by different pathways.
TNF/TNFR interactions appear to be important in the development of GVHD (El-Hayek and others 2005). In human studies, anti-TNFα antibody treatment has been shown to be effective in the treatment of steroid-refractory acute GVHD (Kobbe and others 2001; Couriel and others 2004; Patriarca and others 2004; Uberti and others 2005; Ferrara 2007). Patients treated with etanercept were more likely to achieve complete remission than were patients treated with steroids alone (69% versus 33%; P < 0.001). Importantly, the addition of etanercept resulted in high response rates in the skin, liver, and gastrointestinal tract, suggesting a role for TNF in all 3 organs (Levine and others 2008).
The importance of TNF in experimental models involving hepatic manifestations of acute GVHD has been controversial. However, TNF blockade has been shown to reduce overall histopathological scores of hepatic GVHD in an experimental model of MHC class I disparate GVHD induced by transfer of B6 CD8+ spleen cells (SpC) and T cell-depleted bone marrow cells (BMC) into sublethally irradiated bm1 × B6 F1 mice. Histological features of hepatitis were observed in the control livers, whereas normal liver histology was noted in bone marrow transplant (BMT) recipients who had received a TNF inhibitor (Brown and others 2005). Of importance, the TNF inhibitor was an artificial protein, consisting of the extracellular domain of the human 55 kDa receptor TNFR fused to a mouse IgG heavy chain (TNFR-Ig). The experiments reported use of an adenoviral transduction system, whereby the cDNA encoding for TNFR-Ig inserted into an adenoviral vector (Adv-TNFi) was used to block TNF/TNFR interactions in vivo. This adenoviral transduction system has previously been shown to yield high serum levels of the chimeric TNF inhibitor protein (1 mg/mL for 60 days) with a specific neutralizing activity estimated to be 100-fold higher than that mediated by known antibodies (Kolls and others 1994; Brown and others 1997).
The present studies were designed to determine the role of TNF on cytolytic (CTL) activity of responder SpC and intrahepatic lymphocytes (IHL) isolated from mice with GVHD against MHC class I disparate splenic and hepatocyte targets. TNF sensitive allospecific CTL activity of both splenic and IHL against MHC class I disparate primary splenic blasts targets was noted. Further, TNF/TNFR1 and TNF/TNFR2 interactions were critical for the development of optimal CTL activity of IHL function against allospecific primary hepatocyte targets. Of note, lack of TNF/TNFR1 interactions during the development of MHC class I disparate GVHD was associated with lower RNA levels of effector molecules, granzyme a, granzyme b, and perforin. Responder SpC that lacked TNFR1 and/or TNFR2 isolated from an MHC class I disparate mixed lymphocyte culture (MLC) had reduced CTL activity and RNA expression of effector molecules, granzyme a and perforin.
Materials and Methods
Mice
C57BL/6J (B6), B6.C-112 (bm1), B6.129-Tnfrsf1atm1Mak/J (TNFR1−/−), and B6.129S2-Tnfrsf1btm1Mwm/J (TNFR2−/−) and B6;129S-Tnfrsf1atm1Imx Tnfrsf1btm1Imx/J (TNFR−/−) were obtained from The Jackson Laboratory. B6 females and bm1 males were bred to produce an F1 strain (bm1 × B6 F1). All animals received care as outlined in the Guide for Care and Use of Laboratory Animals. Animal welfare and ethics considerations were fully enacted in compliance with national regulations.
Adenoviral vectors
The E1-deleted, replication-deficient, β-galactosidase-encoding recombinant adenovirus (Adv-βgal) was propagated in 293 culture and purified on a cesium chloride gradient, and titers of infectious virus were determined by plaque assay. Mice were injected with 109–1010 plaque-forming units. Serum was collected from mice injected with a replication-deficient adenoviral vector encoding chimeric fusion protein consisting of the extracellular domain of the human 55-kDa TNFR linked to the hinge and Fc regions of murine immunoglobulin G1 (Adv TNFi) and from the control Adv-βgal (Kolls and others 1994; Brown and others 1997; Kafrouni and others 2003).
Transplantation
Sublethally irradiated (500 cGy), bm1 × B6 F1 recipient mice were infused with 1 × 107 B6 (SpC) and 5 × 106 T cell-depleted BMC. After transplant, 1 × 109 plaque-forming units of TNF inhibitor-encoding-adenovirus (Adv-TNFi) or β-galactosidase-encoding adenovirus (Adv-βgal) was injected in the lateral vein of the transplant recipients. In some experiments, these recipient mice were maintained on acidified (pH 2), antibiotic (neomycin 100 mg/mL, and polymyxin B 10 mg/mL) water for 2 days before transplantation and 1 week after transplantation (Brown and others 1999; Kafrouni and other 2003); weights were monitored from 0 to 21 days post-transplantation.
Histological scoring of hepatic GVHD
Liver architecture was assessed in 3-μm-thick hematoxylin and eosin-stained sections of formalin fixed liver. Composite scoring assessed 3 components of hepatic GVHD: lobular hepatitis, venulitis, and bile duct inflammation (El-Hayek and others 2005).
Isolation of splenic and hepatic lymphocytes
SpC were harvested on days 5–7, by surgically removing the spleen, mincing, and disrupting the spleen over a funnel covered with nylon mesh, and washing repeatedly with Hank's balanced salt solution into a 50 mL conical tube. Flow cytometry demonstrated >70% CD3+ T cells. IHL were isolated as previously described (Watanabe and others 1992; Kafrouni and others 2001). After intraperitoneal injection with 10 U heparin and CO2 narcosis, the mouse's abdomen was entered under sterile technique. The inferior vena cava was tied above right renal vein, the portal vein was cut, and the abdominal portion of the vena cava was perfused with 20 mL phosphate-buffered saline or saline heated to 37°C. Euthanasia was performed by CO2 asphyxiation per protocol. The liver was cut, mashed, and filtered through 100 μm mesh screen. The cell suspension was centrifuged 3 mL Ficoll-Hypaque solution at 900g for 30 min and the cell pellet was used for CTL assays. Flow cytometry demonstrated >80% CD8+ T cells.
Mixed lymphocyte culture
Triplicate wells of control B6, TNFR1−/−, TNFR2−/−, or TNFR−/− responder SpC (3 × 105) were cultured with MHC class I disparate bm1 stimulator cells (3 × 105) or B6, TNFR1−/−, TNFR2−/−, or TNFR−/− syngeneic stimulator cells (3 × 105), controls for each allogeneic culture, respectively, for 5 days before harvest and used in real-time (RT) polymerase chain reaction (PCR) assays. The stimulator cells were SpC that had been T cell depleted by exposing SpC to anti CD4+ antibody (GK1.5) and anti CD8+ T cell antibody (YTS169) followed by incubation with complement. In other MLCs, B6 responder SpC were cultured with irradiated MHC class I and II disparate DBA/2J stimulator cells (Brown and Thiele 2000).
Hepatocyte isolation
Primary mouse hepatocytes were isolated as previously described (Kafrouni and others 2001). The liver was perfused with 30 mL of the liver perfusion medium at 3 cc/min and 60 mL of the liver digestion medium containing collagenase I at 3 cc/min. After the capsule was removed and the hepatocytes released, the cells were collected and centrifuged at 500 rpm for 5 min and resuspended in 50% Percoll and centrifuged. Live cells (5 × 103/well) were cultured in 96-well plate coated with collagen type 1, and the hepatocyte medium containing Williams E Media, dexamethasone (1 μM), insulin (5 μg/mL), transferrin (5 μg/mL), selenous acid (5 ng/mL), penicillin (100 units/mL), streptomycin (100 mg/mL), epidermal growth factor (1 ng/mL), hepatocyte growth factor (25 ng/mL), and 10% fetal bovine serum (GemiBio) was added followed by 10 μci/mL of 3H-Thymidine. Cells were incubated for 24 h and then used as targets in the after CTL assays.
CTL assays: chromium release and JAM assays
For in vitro assays, MHC class I disparate, anti-H-2bm1-stimulated B6 SpC were harvested and washed. The targets, bm1 blasts, whole bm1 SpC, stimulated with Con A (2.5 mg/mL) for 48 h, were labeled with 100 to 200 μCi Na2CrO4 at 37°C and washed before incubation with varying numbers of B6 SpC in triplicate cultures with and without TNFR-Ig protein. After 4 h at 37°C, 100 μL of supernatant was harvested from experimental and control wells, and the percentage of specific lysis was calculated as previously described, % specific lysis = experimental release (cpm) − spontaneous release (cpm)/maximal release (cpm) − spontaneous release (cpm) × 100 (Kafrouni and others 2001).
For ex vivo CTL assays, SpC and IHL were harvested at 5–7 and 12–21 days, respectively, from sublethally irradiated bm1 × B6 F1 who received MHC class I disparate, B6 donor BMC, and SpC and either Adv-TNFi or Adv-βgal. The bm1 blast targets were prepared as previously described and incubated with varying numbers of anti-H-2bm1 SpC and IHL in triplicate.
To determine the effect of TNFR-Ig on the final effector function of SpC and IHL, TNFR-Ig or control Ig (0.5 mg/mL) was added at the beginning of the 4-h CTL assay as previously described. In other experiments, the anti-mTNFα (TNSF 1A/MP6-XT22) and purified rat IgG (0.5 mg/mL; R&D Systems) were used in the 4-h CTL assay, performed in triplicate. All results were reported as mean ± standard error of the mean.
To assess the cytolysis of the primary allospecific hepatocytes, the JAM assay was performed. After incubation, the medium was removed, cells were washed with Hank's balanced salt solution, and intrahepatic lymphocyte effector cells were incubated with hepatocyte targets at effector to target ratio of 10:1 and 100:1. After 18 h, the cell medium was removed and 50 μL of 0.25% trypsin added for 3 min and then harvested with Micro96 Harvester. Cells were suctioned to fiberglass filters. Unlysed DNA was collected on filter and counted by liquid scintillation, as a measure of the DNA retained by living cells rather than the cellular components lost by dying cells (Kafrouni and others 2001).
Real-time polymerase chain reaction
Live responder SpC were harvested, washed, and centrifuged to remove nonviable cells (including the irradiated stimulator cells) and counted on a Multisizer 3 Coulter Counter (Beckman), 5 days after culture with irradiated bm1 stimulator cells. RNA was then extracted from these SpC from in vivo-stimulated anti-H-2bm1 IHL, separated as described above, 21 days after BMT, using the Qiagen extraction kit under an RNase/DNase free field. Sixty microliters of the extracted total RNA was then reverse transcribed (Invitrogen Superscript II). The cDNA samples were then diluted to 25 ng/μL and placed on RT plate to assay for mRNA levels of 18S, IFN-γ, granzyme A, and perforin with Sybr green enzyme. Quantitative assay plate (ABI 384-well optical reaction plate) was prepared with the primers 18S, granzyme A, and perforin (25 nM scale). Primers included granzyme A (Mm00439191_m1), granzyme b (Mm00439191_m1), and perforin (Mm00812512_m1) TaqMan gene expression assay, (Applied Biosystems). Data were analyzed in SDS2 (Applied Biosystems). Quantitative RT plates were analyzed using the comparative cycle time method, in which each sample is normalized to the reference gene (18S) and to a calibrator (syngeneic control).
Statistical analysis
GVHD-induced weight loss and the cellular experiments were analyzed by Student's t-test, and P < 0.05 was considered significant.
Results
TNF blockade decreased anti-H-2bm1 CTL activity of SpC isolated from sublethally irradiated MHC class I disparate bm1 × B6 F1 recipients of B6 BMC and SpC
To assess whether TNF/TNFR interactions played a role in activation of splenic cytotoxic T cells, experiments were performed in which allospecific splenic cytotoxic T cells were generated in vivo by transferring whole SpC and T cell-depleted B6 BMC into sublethally irradiated bm1 × B6 recipients. TNF blockade was induced by the injection of an adenoviral vector expressing TNFR-Ig (Adv-TNFi) directly after BMT. Splenic cytotoxic T cells were isolated and included in the analysis only from recipient mice that expressed evidence of histopathological features in the liver as described in Materials and Methods and/or expression of the TNFR-Ig protein in the serum. Splenic cytotoxic T cells were isolated and mixed with chromium-labeled MHC class I disparate bm1 blasts, at varying ratios. The splenic cytotoxic T cells isolated from control BMT recipients who received the control adenovirus revealed a high allospecific lysis of 75% ± 1% to 82% ± 2% at 20:1 and 50:1 effector:target ratio, whereas the splenic cytotoxic T cells from BMT recipients who had received Adv-TNFi exhibited allospecific lysis of 19% ± 1.3% to 20% ± 2.1% at the same effector:target ratios. With the addition of TNFR-Ig before the 4-h CTL assay, a reduction of allospecific lysis was noted at the same effector:target ratios in the cytotoxic T lymphocyte isolated from the recipients of the control Adv 49% ± 1% to 55% ± 2% and the recipients of Adv-TNFi (0–0% ± 0.3%) (Fig. 1a). These experiments suggested that TNF/TNFR interactions were critical for the development of allospecific optimal splenic cyotoxic T cell function and may play a role in the effector functions of this same splenic cytotoxic T cell.
FIG. 1.
TNF blockade decreased anti-H-2bm1 CTL activity of splenic cytotoxic T cells isolated from sublethally irradiated bm1 × B6 F1 recipients of B6 bone marrow cells and spleen cells. Splenic cytotoxic T cells were isolated 7 days after BMT from sublethally irradiated bm1 × B6 F1 transplant recipients of B6 spleen cells and bone marrow cells, who had received either the Adv-TNFi or the control βgal-Adv. TNFR-Ig (0.5 μg/μL) was added in some experiments before 4-h CTL assay (a). In vivo-activated splenic cytotoxic T cells were isolated from sublethally irradiated bm1 × B6 F1 recipients of B6 spleen cells and bone marrow cells 5 days after transplantation. A purified monoclonal TNF Ab or control antibody (R&D Systems) was added at the initiation of the CTL assay (b). BMT, bone marrow transplant; CTL, cytolytic; TNF, tumor necrosis factor; TNFR, tumor necrosis factor receptor.
To determine whether other TNF antibodies could reduce the allospecific lysis, SpC were isolated 5 days after BMT from sublethally irradiated bm1 × B6 F1 recipients of B6 BMC and SpC. A purified monoclonal TNF Ab or control Ab (R&D Systems) was added at the initiation of the CTL assay. Decreased anti-H-2bm1 CTL activity of B6 splenic cytotoxic T cell was noted with the addition of TNF Ab (Fig. 1b) (experiment 1: 36% ± 1.2% to 15.4% ± 0.9%, P = 0.003, and experiment 2: 24.6% ± 0.5% to 10% ± 1.5%, P = 0.01). These experiments confirmed that TNF/TNFR interactions were critical for the lysis of the in vivo-activated SpC against an allospecific MHC class I disparate bm1 blasts in a 4-h CTL assay. Further, the decrease in the allospecific lysis was applicable to both a TNFR-Ig inhibitor protein and a purified monoclonal TNF antibody.
TNF blockade decreased allospecific CTL activity of IHL isolated from recipients of an MHC class disparate BMT
In previous experiments, intrahepatic CD8+ lymphocytes were shown to have high levels of IFN-γ 21 days post-transplant (El-Hayek and others 2005), an indication of activated cytotoxic T cell. To assess the CTL effector functions of intrhepatic lymphocytes (IHL) against MHC class I disparate bm1 splenic blasts, in vivo-activated IHL were isolated from livers of sublethally irradiated bm1 × B6 F1 recipients of B6 BMC and SpC 12 days after transplantation. BMT recipients had received either Adv-TNFi or control Adv-βgal. Viable cells were harvested on day 12 and CTL activity was assessed against anti-H-2bm1 bm1 blast target cells. The allospecific CTL activity of IHL isolated from the bm1 × B6 F1 recipients of the B6 BMC and SpC (17% ± 10% to 69% ± 12% at 50:1 to 100:1) was higher than the recipients of the BMT recipients who had received the Adv-TNFi (0% ± 1% to 4% ± 0.2% at 50:1 and 100:1) (Fig. 2a).
FIG. 2.
TNF blockade decreased anti-H-2bm1 CTL activity of IHL isolated from sublethally irradiated bm1 × B6 F1 recipients of B6 spleen cells and bone marrow cells. In vivo-activated IHL were isolated from livers of sublethally irradiated bm1 × B6 F1 recipients of B6 spleen cells and bone marrow cells 12 days after transplantation. BMT recipients had received either Adv-TNFi or control Adv-βgal. Viable cells were harvested on day 12 and CTL activity was assessed against anti H-2 bm1 blast target cells (a). In other experiments, a purified monoclonal TNF Ab or control antibody (R&D Systems) was added at the initiation of a 4-h 51Cr release assay of IHL mixed with MHC class I disparate bm1 targets (b). MHC, major histocompatibility complex; IHL, intrahepatic lymphocytes.
To assess whether this finding was specific only for the development phase of the IHL, other experiments were performed to assess whether TNF/TNFR interactions were critical during a 4-h CTL assay. Specifically, to assess the role of TNF in the CTL effector functions of IHL against bm1 splenic blasts, in vivo-activated IHL isolated from livers of bm1 × B6 F1 recipients of B6 SpC and BMC and SpC 12 days after transplantation were used in CTL assays. A control antibody or a TNF antibody was added at the initiation of a 4-h assay. Decreased anti-H-2bm1 CTL activity of IHL was noted at effector target ratios of 2.5 and 5:1 with the addition of TNF antibody [11.5% ± 7.5% to 34% ± 3.5% (control) versus 4.6% ± 6% to 25% ± 3% (TNF antibody)] (Fig. 2b). These experiments suggested that TNF/TNFR interactions were critical for the development of allospecific optimal IHL function and may play a minor role in the effector functions of IHL isolated from an MHC class I disparate GVHD model against allospecific splenic blast targets.
TNF/TNFR1 and TNF/TNFR2 interactions were critical for the development of optimal CTL activity of IHL against primary hepatocyte targets
Experiments were performed to assess the role of TNF in the development of an activated IHL with the ability to lyse primary MHC class I disparate hepatocyte targets. To determine the role of the lack of specific TNF/TNFR interactions, IHL were isolated from livers of sublethally irradiated bm1 × B6 F1 recipients of B6, TNFR1−/−, TNFR2−/−, and TNFR−/− BMC, and SpC and primary mouse hepatocytes were isolated from MHC class I disparate bm1 mice (Kafrouni and others 2001; Kafrouni and others 2003). Briefly, live hepatocytes were cultured in 96-well plated with collagen type I and hepatocyte medium and 3H-Thymidine for 24 h. Activated anti-H-2bm1 IHL effectors were incubated with the allospecific hepatocyte targets at varying effector to target ratio. After 18 h, unlysed DNA was collected on filter and counted. Of interest, the allospecific CTL activity of IHL increased from 45% to 60% in recipients of control B6 SpC. A marked decrease of CTL activity against MHC class I disparate hepatocytes was noted in recipients of SpC that lacked TNFR1 (0%–7%), TNFR2 (11%–12%), and TNFR1 and 2 (0%–1%). Hence, interactions between TNF and either TNFR1 or TNFR2 on donor SpC during the initiation of GVHD were critical for the development of optimal CTL activity of IHL against MHC class I hepatocyte targets (Fig. 3).
FIG. 3.
TNF/TNFR1 and TNF/TNFR2 interactions were critical for the development of optimal allospecific CTL effection function of IHL against primary hepatocyte targets. Eighteen-hour cultures were performed with freshly isolated IHL from bm1 × B6 recipients of B6, TNFR1, TNFR2, or TNFR-deficient spleen cells and bone marrow cells and 3H-Thymidine-labeled allospecific bm1 hepatocytes at effector to target ratios of 10:1 and 40:1. After cells were suctioned to fiberglass filter, cytolysis was assessed by the amount of unlysed DNA detected.
Cytotoxic T cell effector molecules were lower in IHL isolated from MHC class I disparate BMT recipients who received the TNF inhibitor and in recipients of TNFR1−/− BMC and SpC
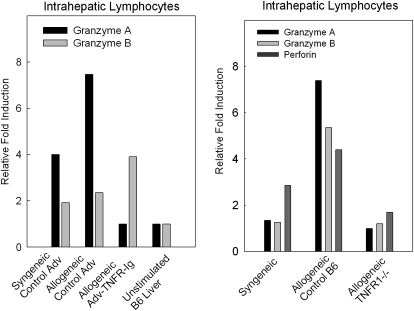
RT PCR was performed on RNA extracted of IHL isolated from sublethally irradiated bm1 × B6 F1 recipients of B6 and either Adv-TNFi or control Adv-βgal to quantify the relative expression levels of granzyme A and granzyme B mRNA. Briefly, RNA was isolated from liver from the control and experimental BMT recipients. Granzyme A RNA levels were reduced from 8-fold to nearly 2-fold after TNF blockade, whereas granzyme B showed no changes in the levels (Fig. 4).
FIG. 4.
Effector molecules were lower in IHL isolated from recipients of MHC class I disparate spleen cells and bone marrow cells who received the TNF inhibitor and in recipients of TNFR1−/− bone marrow cells and spleen cells. RNA was extracted from in vivo-stimulated anti-H-2bm1 IHL 21 days after receipt of (1) B6 bone marrow cells and spleen cells and either Adv-TNFi or Adv-βgal or (2) of B6 or TNFR1−/− spleen cells and bone marrow cells. Real-time polymerase chain reaction was performed as previously described in the Materials and Methods section.
To assess the role of the lack of specific receptors on donor T cells, we performed RT PCR assays of IHL isolated from bm1 × B6 F1 recipients of either control B6 or TNFR1 donor SpC and BMC 21 days after transplantation. In a representative experiment illustrated in Fig. 4, levels of granzyme a, granzyme b, and perforin were lower in IHL isolated from recipients of TNFR1−/− donor SpC and BMC than from control B6 donor SpC and BMC. These experiments confirm that cytotoxic T cell effector molecules are dependent on an intact TNF/TNFR1 pathway during the development of responder IHL in an MHC class I disparate GVHD.
CTL activity against MHC class I disparate splenic blasts and cytotoxic T cell effector molecules' expression were lower in SpC that lacked TNFR1−/−, TNFR2−/− or TNFR−/− than control B6 responder SpC isolated after a 5-day MHC class I disparate MLC
The previous experiments confirmed that TNF/TNFR interactions were important in the development of in vivo-activated GVHD SpC and IHL and may play a minor role in the lysis of splenic blast targets. The role of each the TNFRs in CTL activity against MHC class I disparate targets was assessed by using SpC from control B6, TNFR1−/−, and TNFR2−/− mice in MHC class I disparate MLC. Allospecific lysis of bm1 blasts increased from 53% to 99% with control B6 responder SpC (20:1 to 50:1 E:T ratios). The allospecific lysis of bm1 blasts was lower with TNFR2−/− SpC (35% to 63% at 20:1 to 50:1 E:T ratios, respectively) and further reduced with the absence of TNFR1 (0% to 16% at 20:1 to 50:1 E:T ratios) (Fig. 5a). TNFR1 and TNFR2 were critical for the development of optimal CTL activity against MHC class I disparate splenic blasts.
FIG. 5.
CTL activity and granzyme A, and perforin mRNA expression was lower in spleen cells that lacked TNFR1−/−, TNFR2−/−, or TNFR−/− than control B6 responder spleen cells isolated from an MHC class I disparate mixed lymphocyte culture. In vitro-activated responder spleen cells were harvested on day 5 from a mixed lymphocyte culture utilizing B6, TNFR1−/−, TNFR2−/−, or TNFR−/− responder spleen cells with irradiated bm1 stimulator cells. CTL activity of MHC class I disparate-stimulated spleen cells was determined as indicated in the Materials and Methods section (a). Real-time polymerase chain reaction was performed on RNA extracted from the spleen cells of control and experimental groups to quantify the relative expression levels effector molecules, granzyme A (b) and perforin (c).
Other effector mechanisms also seemed to play a role in the lysis of targets that were sensitive to TNF/TNFR interactions during the development of the cytotoxic T cells. To assess whether the development of specific cytotoxic T cell effector molecules were dependent on an intact TNF/TNFR pathway, in vitro-activated responder SpC were harvested from MLC utilizing B6, TNFR1−/−, TNFR2−/−, or TNFR−/− responder SpC with irradiated bm1 stimulator cells. RT-PCR was performed on RNA extracted from the SpC of control and experimental groups to quantify the relative expression levels of granzyme A and perforin mRNA. Granzyme A RNA levels were reduced from 20-fold to the control level with the lack of any TNFR (Fig. 5b), whereas perforin was reduced from 8-fold to the control level with the lack of both TNFR1 and 2 (Fig. 5c). These experiments confirm that granzyme A and perforin (effector molecules) are dependent on an intact TNF/TNFR pathway during the development of responder SpC in an MHC class I disparate MLC.
Discussion
GVHD, a principal cause of morbidity after an allogeneic hematopoietic cell transplantation, has no standard therapy available for patients with steroid refractory disease. In previous experiments, our laboratory had demonstrated the critical role of TNF/TNFR interactions in the development of histopathological lesions and the optimal activation of CD8+ T cells in an MHC class I disparate hepatic GVHD (El-Hayek and others 2005). In this article, the experimental results support that TNF/TNFR interactions are critical for the development of allospecific optimal splenic and intrahepatic CD8+ T cell function against both splenic blasts and primary hepatocyte targets. The mechanism of the decrease in CTL activity against allospecific splenic blasts may be linked to the decrease in specific effector molecules, granzymes and perforin, with TNF playing a minor direct role in the effector functions since CTL activity is reduced when a purified TNF antibody is present during the 4-h short-term CTL assay.
Of importance, these experiments are the first to report a decrease in CTL activity of IHL from livers of recipients of SpC and BMC that lacked TNFR1 or TNFR2, against allospecific primary hepatocyte targets in 18-h CTL assays. Further, effector molecules, likely markers of activation, are also lower in these in vivo-activated IHL isolated from recipients of donor SpC and BMC, which lacked TNF/TNFR interactions. Of interest, the lysis of these primary hepatocyte targets with cytolytically active IHL isolated from livers of mice undergoing GVHD was only observed at 18 h, suggesting a slow mechanism for CTL activity. Other investigators have noted that hepatocytes may lose expression of specific surface receptors during in vitro culture (Chen and others 2005), which may affect the predominance of a pathway utilized in lysis.
These experiments differ from our previous work because they used MHC class I disparate blast splenic cells and primary hepatocyte targets and an MHC class I only allospecific antigenic stimulus, whereas previous work utilized MHC class I and II disparate allospecific differences as well as P815 mastocytoma tumor cells as targets (Kafrouni and others 2003). These experiments differ from Hattori's work with the utilization of MHC class I disparate stimulus and the use of different targets (Hattori and others 1998). Our data may differ from other investigators secondary to the targets, con A-stimulated splenic blasts and primary hepatocyte targets, utilized in the experiments. Cultured cell line targets may have lower amount of surface TNFRs, which may affect the pathway of lysis. Through the use of splenic blasts, the role of TNF/TNFR interactions during the effector phase of a very sensitive target may have been observed. Although other targets may not be killed by this effector mechanism, this target does illustrate the role of TNF/TNFR interactions that may be possible in specific environments or disease states. Hence, the TNF sensitivity of splenic blasts may suggest the optimal role that TNF may play in the CTL effector function and that in specific instances, TNF may play such a role in cytolysis in other targets of GVHD.
Other mechanisms involved in the hepatic CTL activity of IHL against splenic blasts may be associated with TNF/TNFR interactions affect on other effector molecules. Specifically, granzymes and perforin mRNA levels were lower in IHL isolated from bm1 × B6 recipients of MHC class I disparate TNFR1−/− BMC and SpC than from control B6 as well as from in vitro-activated anti-H2bm1 TNFR1−/− and TNFR2−/− SpC than from control B6 SpC. Further, low levels of granzyme A and perforin were noted in syngeneic-stimulated B6, TNFR1−/−, TNFR2−/−, and TNFR−/− SpC (data not shown) with no statistical difference noted between the strains of mice, suggesting that the difference is not associated with the genetic manipulation.
Our findings may differ from previous studies because of the use of specific TNF inhibitors that inhibit the interactions of membrane bound TNF (Hattori and others 1998). Some investigators have reported that IHL induce an acute GVHD through a FAS/FAS ligand-mediated pathway (Hattori and others 1998). Our studies, would favor the multiplicity of pathways of hepatic GVHD with some contribution by TNF/TNFR interactions
Previously we have shown that IFN-γ is upregulated in the liver immune cells and is dependent on TNF/TNFR interactions (Brown and others 2005). IFN-γ has been shown to be important in the induction of MHC class I and II antigens in arterial endothelium (Goes and others 1996), which may be important during hepatic GVHD. Early induction showing enhanced MHC Class I expression on hepatocytes, bile duct epithelium, and sinusoidal endothelium, and Class II antigen on Kupffer cells and sinusoidal endothelium occurs in a number of disease processes (Chen and others 2005). MHC class I is constitutively expressed in the liver and it can be induced during disease processes, and this may be the reason that cyotoxic T cells play a major role in the pathogenesis of GVHD manifestations in the liver (Chen and others 2005).
The interest of TNF blockade in GVHD dates back to Herve and Holler, where antibodies against TNF were utilized in human trials as a preconditioning protocol before BMT, which resulted in a minimal reduction in grade of intestinal GVHD (Herve and others 1992; Holler and others 1995). Further analysis of the role of TNF, a cytokine that plays a prominent role in both innate and adaptive immune response, has been performed in human trials. The mechanisms of its actions include (1) induction of apoptosis, (2) activation of macrophages, neutrophils, eosinophils, T and B cells, (3) production of a cascade of other inflammatory cytokines, (4) increased expression of human leukocyte antigen molecules, and (5) facilitation of cytotoxic T cell lysis (Couriel and others 2004). Investigators have noted the importance of TNF/TNFR interactions in murine models of GVHD. Ferrara found significantly reduced mortality and morbidity in his experimental GVHD model, although in an MHC class I and II disparate model with lethal irradiation in recipients deprived of TNF/TNFR interactions via use of TNFR-deficient donors (Hill and others 2000). Of note, TNFR1 was critical for optimal allospecific stimulation in his model.
In therapy for human GVHD, both infliximab (humanized mouse TNF monoclonal antibody) and etanercept (the human 75 kDa receptor TNFR fused to a mouse IgG heavy chain; 75 kDa TNFR-Ig) have recently become available (Kobbe and others 2001; Couriel and others 2004; Patriarca and others 2004; Uberti and others 2005; Ferrara 2007; Levine and others 2008). Investigators have recently treated patients with new-onset GVHD with steroids plus etanercept and found that patients treated with etanercept were more likely to achieve complete remission (Levine and others 2008). Mixed results have been reported, with some showing improvement with acute skin and gut disease as high as 86% and 77% and less with liver involvement (30%) (Courier and others 2004). Similar to our studies, the latest human trials have demonstrated that the addition of etanercept to steroids resulted in high response rates in all 3 target organs, Further, since TNFα inhibition increases response rates but does not completely eliminate GVHD, there may be both TNFα-dependent and TNFα-independent pathways of disease (Levine and others 2008).
In summary, TNF-TNFR interactions are necessary for the development of the cytolytically active IHL and splenic cytotoxic T cells in MHC class I disparate GVHD. In addition TNF-TNFR interactions are necessary for the optimal effector CTL function of both IHL and splenic cyototoxic T cells from mice recipients of MHC class I disparate SpC and BMC against splenic blasts. This article is the first to describe the importance of TNF/TNFR interactions in the development of cytolytically active IHL against primary hepatocyte targets. This murine model suggests a mechanism of the observation of improved survival after TNF blockade in human GVHD, that is, optimal CTL activity through the TNF/TNFR interactions. This set of experiments suggests that specific targets may be more susceptible to the killing by TNF/TNFR interactions.
Acknowledgments
We give special thanks to Jake Kreck and Guadalupe Jasso for their excellent technical assistance. This work was supported by Veterans Affairs Merit Review Grant and National Institute of Health Grant NIH/NHLBI R01 HL69006.
Author Disclosure Statement
The authors have no conflict of interest.
References
- Brown GR. Lee E. El-Hayek J. Kintner K. Luck C. IL-12-independent LIGHT signaling enhances MHC class II disparate CD4+ T cell alloproliferation, IFN-gamma responses, and intestinal graft-versus-host disease. J Immunol. 2005;174:4688–4695. doi: 10.4049/jimmunol.174.8.4688. [DOI] [PubMed] [Google Scholar]
- Brown GR. Lindberg G. Meddings J. Silva M. Beutler B. Thiele D. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology. 1999;116(3):593–601. doi: 10.1016/s0016-5085(99)70181-2. [DOI] [PubMed] [Google Scholar]
- Brown GR. Thiele DL. Enhancement of MHC class I-stimulated alloresponses by TNF/TNF receptor (TNFR)1 interactions and of MHC class II-stimulated alloresponses by TNF/TNFR2 interactions. Eur J Immunol. 2000;30:2900–2907. doi: 10.1002/1521-4141(200010)30:10<2900::AID-IMMU2900>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Brown GR. Thiele DL. Silva M. Beutler B. Adenoviral vectors given intravenously to immunocompromised mice yield stable transduction of the colonic epithelium. Gastroenterology. 1997;112:1586–1594. doi: 10.1016/s0016-5085(97)70040-4. [DOI] [PubMed] [Google Scholar]
- Chen M. Tabaczewski P. Truscott SM. Van Kaer L. Stroynowski I. Hepatocytes express abundant surface class I MHC and efficiently use transporter associated with antigen processing, tapasin, and low molecular weight polypeptide proteasome subunit components of antigen processing and presentation pathway. J Immunol. 2005;175:1047–1055. doi: 10.4049/jimmunol.175.2.1047. [DOI] [PubMed] [Google Scholar]
- Couriel D. Saliba R. Hicks K. Ippoliti C. de Lima M. Hosing C. Khouri I. Andersson B. Gajewski J. Donato M. Anderlini P. Kontoyiannis DP. Cohen A. Martin T. Giralt S. Champlin R. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood. 2004;104:649–654. doi: 10.1182/blood-2003-12-4241. [DOI] [PubMed] [Google Scholar]
- Dillon PW. Belchis D. Minnick K. Tracy T. Differential expression of the major histocompatibility antigens and ICAM-1 on bile duct epithelial cells in biliary atresia. Tohoku J Exp Med. 1997;18:33–40. doi: 10.1620/tjem.181.33. [DOI] [PubMed] [Google Scholar]
- El-Hayek JM. Rogers TE. Brown GR. The role of TNF in hepatic histopathological manifestations and hepatic CD8+ T cell alloresponses in murine MHC class I disparate GVHD. J Leukoc Biol. 2005;78:1001–1007. doi: 10.1189/jlb.1204730. [DOI] [PubMed] [Google Scholar]
- Ferrara JL. Novel strategies for the treatment and diagnosis of graft-versus-host-disease. Best Pract Res Clin Haematol. 2007;20:91–97. doi: 10.1016/j.beha.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Ferrara JL. Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- Goes N. Urmson J. Hobart M. Halloran PF. The unique role of interferon-gamma in the regulation of MHC expression on arterial endothelium. Transplantation. 1996;6:1889–1894. doi: 10.1097/00007890-199612270-00036. [DOI] [PubMed] [Google Scholar]
- Gouw AS. Huitema S. Grond J. Slooff MJ. Klompmaker IJ. Gips CH. Poppema S. Early induction of MHC antigens in human liver grafts. An immunohistologic study. Am J Pathol. 1988;133:82–94. [PMC free article] [PubMed] [Google Scholar]
- Hattori K. Hirano T. Miyajima H. Yamakawa N. Tateno M. Oshimi K. Kayagaki N. Yagita H. Okumura K. Differential effects of anti-Fas ligand and anti-tumor necrosis factor alpha antibodies on acute graft-versus-host disease pathologies. Blood. 1998;91:4051–4055. [PubMed] [Google Scholar]
- Herve P. Flesch M. Tiberghien P. Wijdenes J. Racadot E. Bordigoni P. Plouvier E. Stephan JL. Bourdeau H. Holler E. Phase I-II trial of a monoclonal anti-tumor necrosis factor alpha antibody for the treatment of refractory severe acute graft-versus-host disease. Blood. 1992;79:3362–3368. [PubMed] [Google Scholar]
- Hill GR. Teshima T. Rebel VI. Krijanovski OI. Cooke K. Brinson YS. Ferrara JL. The p55 TNF-alpha receptor plays a critical role in T cell alloreactivity. J Immunol. 2000;164:656–663. doi: 10.4049/jimmunol.164.2.656. [DOI] [PubMed] [Google Scholar]
- Holler E. Kolb HJ. Mittermuller J. Kaul M. Ledderose G. Duell T. Seebe B. Schleuning M. Hintermeier-Knabe R. Ert B. Modulation of acute graft-versus-host-disease after allogeneic bone marrow transplantation by tumor necrosis factor alpha (TNF alpha) release in the course of pretransplant conditioning: role of conditioning regimens and prophylactic application of a monoclonal antibody neutralizing human TNF alpha (MAK 195F) Blood. 1995;86:890–899. [PubMed] [Google Scholar]
- Kafrouni MI. Brown GR. Thiele DL. Virally infected hepatocytes are resistant to perforin-dependent CTL effector mechanisms. J Immunol. 2001;167:1566–1574. doi: 10.4049/jimmunol.167.3.1566. [DOI] [PubMed] [Google Scholar]
- Kafrouni MI. Brown GR. Thiele DL. The role of TNF-TNFR2 interactions in generation of CTL responses and clearance of hepatic adenovirus infection. J Leukoc Biol. 2003;74:564–571. doi: 10.1189/jlb.0103035. [DOI] [PubMed] [Google Scholar]
- Kobbe G. Schneider P. Rohr U. Fenk R. Neumann F. Aivado M. Dietze L. Kronenwett R. Hunerliturkoglu A. Haas R. Treatment of severe steroid refractory acute graft-versus-host disease with infliximab, a chimeric human/mouse antiTNFalpha antibody. Bone Marrow Transplant. 2001;28:47–49. doi: 10.1038/sj.bmt.1703094. [DOI] [PubMed] [Google Scholar]
- Kolls J. Peppel K. Silva M. Beutler B. Prolonged and effective blockade of tumor necrosis factor activity through adenovirus-mediated gene transfer. Proc Natl Acad Sci USA. 1994;91:215–219. doi: 10.1073/pnas.91.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JE. Paczesny S. Mineishi S. Braun T. Choi SW. Hutchinson RJ. Jones D. Khaled Y. Kitko CL. Bickley D. Krijanovski O. Reddy P. Yanik G. Ferrara JL. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca F. Sperotto A. Damiani D. Morreale G. Bonifazi F. Olivieri A. Ciceri F. Milone G. Cesaro S. Bandini G. Dini G. Corradini P. Fanin R. Infliximab treatment for steroid-refractory acute graft-versus-host disease. Haematologica. 2004;89:1352–1359. [PubMed] [Google Scholar]
- Uberti P. Ayash L. Ratanatharathorn V. Silver S. Reynolds C. Becker M. Reddy P. Cooke KR. Yanik G. Whitfield J. Jones D. Hutchinson R. Braun T. Ferrara JL. Levine JE. Pilot trial on the use of etanercept and methylprednisolone as primary treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:680–687. doi: 10.1016/j.bbmt.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Watanabe H. Ohtsuka K. Kimura M. Ikarashi Y. Ohmori K. Kusumi A. Ohteki T. Seki S. Abo T. Details of an isolation method for hepatic lymphocytes in mice. J Immunol Methods. 1992;146:145–154. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]
- Williams FH. Thiele DL. The role of major histocompatibility complex and non-major histocompatibility complex encoded antigens in generation of bile duct lesions during hepatic graft-vs.-host responses mediated by helper or cytotoxic T cells. Hepatology. 1994;19:980–988. [PubMed] [Google Scholar]