Abstract
Accumulating evidence indicates that interleukin (IL)-27, a member of the IL-12 family of cytokines, antagonizes pathological Th17 effector cell responses. Relatively little is known about the cytokines that regulate human Th17 cells. In this study, we investigated the effect of IL-27 on the differentiation of human Th17 cells and on committed memory Th17 cells. We demonstrate that IL-27 suppresses the development of human Th17 cells by downregulating retinoid orphan nuclear receptor C expression and that this inhibition is associated with the induction of the intracellular signaling factors STAT1 and induction of the suppressor of cytokine signaling protein 1. The IL-27-mediated inhibition of IL-17 is independent of IL-10. We show that IL-27 inhibits differentiation of naïve T cells into IL-17+ T cells under different Th17 polarizing conditions. IL-27 suppresses other Th17 subset cytokines such as IL-22 and IL-21 but not tumor necrosis factor-α. Moreover, we also show that IL-27 inhibits IL-17 production by committed Th17 memory cells, which is independent of IL-10. These studies show that IL-27 negatively regulates both the developing and committed human Th17 responses and therefore may be a promising therapeutic approach in the treatment of Th17-mediated diseases.
Introduction
Th17 cells represent a novel CD4+ T cell lineage that appear to be essential in the pathogenesis of numerous inflammatory and autoimmune diseases. In mice, differentiation of naïve T cells into interleukin (IL)-17-secreting T cells is promoted by IL-6 and transforming growth factor-β (TGF-β) and is mediated by the transcription factors, retinoid orphan nuclear receptor γt (RORγt), and STAT3 (Chen and others 2006; Ivanov and others 2006; Mangan and others 2006; Veldhoen and others 2006). In humans, IL-1β with either IL-6 or IL-23 were identified as the critical factors promoting Th17 differentiation and expression of RORC (Acosta-Rodriguez and others 2007; Wilson and others 2007). TGF-β was found to be dispensable for the development of human IL-17+ T cells. Th17 cells were shown to differentiate from a CD4+CD45RA+CD161+ precursor cell in the presence of IL-1β and IL-23 (Cosmi and others 2008). Additional studies reported that TGF-β is indeed essential for human Th17 differentiation. Under serum-free conditions, naïve T cells required TGF-β, IL-1β, IL-6, and IL-23 to differentiate into IL-17-secreting cells (Manel and others 2008; Volpe and others 2008). In both the murine and human system, Th17 differentiation is inhibited by interferon (IFN)-γ and IL-4 (Acosta-Rodriguez and others 2007; Harrington and others 2005; Park and others 2005).
IL-27, a member of the IL-12 cytokine family, is a heterodimeric cytokine comprising the Epstein-Barr-virus-induced gene 3 (EBi3) and p28 subunits (Pflanz and others 2002). EBi3 and p28 are structurally homologous to IL-12p40 and IL-12p35, respectively. IL-27 mediates its effects by signaling through a receptor complex composed of a unique subunit, WSX-1 (IL-27Rα) and gp130, a common receptor chain shared by several cytokines (Pflanz and others 2004). The IL-27R is expressed on T and B cells, NK cells, mast cells, macrophages, dendritic cells, and endothelial cells. IL-27 activates the STAT family of signal transducers and transcription factors, specifically, STAT1, STAT3, and, to a lesser extent, STAT4 (Hibbert and others 2003).
IL-27 has a proinflammatory role since it promotes Th1 differentiation by inducing expression of T-bet and the IL-12Rβ2 in a STAT1-dependent manner (Hibbert and others 2003; Lucas and others 2003; Takeda and others 2003). IL-27 inhibits Th2 differentiation by downregulating GATA-3 with a concomitant upregulation of T-bet (Yoshimoto and others 2007). IL-27 suppresses the development of TGF-β-induced Foxp3+ T regulatory cells (Tregs) (Neufert and others 2007). In contrast, subsequent studies have described IL-27 as having an anti-inflammatory function. IL-27R-deficient mice infected with Toxoplasma gondii develop severe neuroinflammation with increased production of IFN-γ and tumor necrosis factor (TNF)-α during the acute disease (Villarino and others 2003). During the chronic phase of the disease, an increased frequency of IL-17+ T cells was found in the central nervous system (CNS) (Stumhofer and others 2006). Increases in IL-17+ T cells in the brain were reported in IL-27R-deficient mice with experimental autoimmune encephalomyelitis (EAE) induced by immunization with myelin oligodendrocyte glycoprotein peptide (Batten and others 2006). In vitro, the IL-27-mediated inhibition of Th17 development was dependent on STAT1 but was independent of IL-6 signaling through suppressor of cytokine signaling protein 3 (SOCS3) or IFN-γ production (Batten and others 2006; Stumhofer and others 2006). IL-27 ameliorates EAE with suppression of both Th1 and Th17 responses in the CNS and in vitro IL-27 treatment of effector cells inhibits IL-17 encephalitogenic responses (Fitzgerald and others 2007).
The mechanisms by which IL-27 suppresses Th17 responses remain unresolved and may involve IL-10. IL-27 potently enhanced IL-10 production in naïve T cells activated under Th1 or Th2 but not Th17 polarizing conditions (Stumhofer and others 2007). T cells from IL-27R-deficient mice chronically infected with T. gondii displayed a reduced capacity to produce IL-10. However, IL-27 inhibition of IL-17 responses was seen in IL-10-deficit animals. IL-27 induced the differentiation of IL-10+IFN-γ+ T-bet+ CD4+ effector T cells, and IL-10 mediated the suppressive effect of IL-27 on encephalitogenic Th17 cells EAE (Fitzgerald and others 2007). Optimal induction of IL-10 by IL-27 required accessory non-T cells to be present. IL-27 induction of IL-10 in IFN-γ+Foxp3–Th1 cells was dependent on STAT1 (Batten and others 2008).
The role of IL-27 in human Th17 differentiation has not been thoroughly investigated. In an article demonstrating the role of IL-27 in an in vivo mouse model, it was reported that IL-27 inhibits the development of human Th17 cells (Diveu and others 2009). IL-27 was reported to promote the differentiation of IL-10-secreting Tr1 cells and to inhibit Th17 development (Murugaiyan and others 2009). In this study, we investigate the role of IL-27 in the development of human Th17 and in memory IL-17-secreting cells. We demonstrate that IL-27 regulates both the generation of human Th17 cells and already differentiated memory Th17 cells. The IL-27-induced inhibition of IL-17 results in downregulation of the Th17-specific transcription factor, RORC, is independent of IL-10, and is associated with the induction of the intracellular signaling factors STAT1 and induction of the suppressor protein SOCS1.
Materials and Methods
Media, cytokines, and Abs
RPMI 1640 was supplemented with 45 μg/mL penicillin and streptomycin, 2 mM L-glutamine (all from Gibco), and 10% heat-inactivated fetal calf serum (FCS) (Hyclone Laboratories). X-VIVO 20 serum-free medium was purchased from Lonza. Unconjugated monoclonal antibodies (mAbs) specific for CD3, CD28, and IFN-γ as well as the conjugated Abs to CD4, CD45RA, CD45RO, IFN-γ, TNF-α, and IL-10 were obtained from BD Pharmingen. IL-17 and IL-22 mAbs and polyclonal anti-IL-4 were obtained from R&D Systems. Antibodies to phosphorylated tyrosine residues of STAT1, STAT3, and STAT4 were purchased from BD Pharmingen. Recombinant IL-23, IL-27, and TGF-β were purchased from R&D Systems, rIL-6 from BioSource International, and rIL-1β was a gift from Hoffmann-LaRoche. IFN-α and IFN-β were gifts from Drs. Fitzgerald-Bocarsly and Pachner, respectively, and IFN-γ was obtained from BD Pharmingen. Polyclonal antibodies to SOCS1 (SC-9021), SOCS3 (SC-9023), and the secondary Ab (SC-2004) were purchased from Santa Cruz Biotechnology.
Isolation of CD4+ naïve and memory T cells
Human peripheral blood mononuclear cells (PBMC) studies were approved by the Institutional Review Board of New Jersey Medical School in accordance with regulations mandated by the Department of Health and Human Services. Informed consent was obtained from each subject. PBMC were isolated from heparinized venous blood obtained from healthy individuals by Ficoll-Hypaque gradient centrifugation (Sigma-Aldrich). Naïve and memory CD4+ T cells were purified using magnetic beads and reagents from Miltenyi Biotec. CD4+ T cells were isolated from PBMC by immunomagnetic depletion of non-T helper cells (CD4+ T Cell Isolation kit) according to the manufacturer's instructions. Naïve CD45RA+ T cells were purified from the CD4+ T cells by negative selection using CD45RO beads, and the purity was >95% in all experiments as assessed by immunofluorescence flow cytometry. The positive fraction was highly enriched for CD4+CD45RO+ memory T cells.
Activation of naïve and memory T cells
Naïve CD4+CD45RA+ T cells (1 × 105/well) were cultured in 10% heat-inactivated FCS/RPMI 1640 containing CD28 Ab (2 μg/mL) in 96-well U-bottom plates coated with anti-CD3 (5 μg/mL) for 6 days. Cultures were generated in the presence of rIL-1β (10 ng/mL), rIL-23 (50 ng/mL), anti-IFN-γ (5 μg/mL), and a polyclonal anti-IL-4 (0.1 μg/mL) with and without human rIL-27 (100 ng/mL). For some experiments, naïve T cells were resuspended in X-VIVO 20 serum-free medium and activated with immobilized anti-CD3 and soluble anti-CD28 in the presence of rIL-1β (10 ng/mL), rIL-6 (40 ng/mL), rIL-23 (50 ng/mL), and rTGF-β (5 ng/mL) with and without rIL-27 (100 ng/mL). Anti-IFN-γ and anti-IL-4 were added to the cultures. Cell-free culture supernatants were collected on day 6 for ELISA and stored at −80°C until testing.
Memory CD4+CD45RO+ T cells (5 × 105/mL) were cultured in 10% heat-inactivated FCS/RPMI 1640 containing CD28 in 96-well U-bottom plates coated with anti-CD3 in the presence and absence of rIL-1β, rIL-6, rIL-23, rTGF-β, and rIL-27. Cell-free culture supernatants were collected on day 6 for ELISA and stored at −80°C until testing.
Cytokine analysis
IL-17 levels were measured by ELISA as described (Liu and Rohowsky-Kochan 2008) using a plate-bound capture mAb and a biotinylated polyclonal detection Ab (R&D Systems). IL-21 and IL-22 levels were measured by ELISA (eBioscience) as per the manufacturer's instructions. IFN-γ and IL-10 were quantitated using the OptEIA human IFN-γ kit and IL-10 kit (BD Pharmingen) according to the manufacturer's instructions.
Intracellular cytokine staining
For intracellular cytokine staining, cultured cells were restimulated with phorbol-12-myristate-13-acetate (PMA) (20 ng/mL) and ionomycin (100 ng/mL) in the presence of brefeldin A (all from Sigma Aldrich) for 5 h. Surface staining was performed with fluorochrome-conjugated anti-CD4 and anti-CD45RO. After fixation and permeabilization with 0.5% saponin, cells were stained with a biotinylated anti-IL-17 and streptavidin PECY7 (BD Pharmingen) or with anti-IFNγ-PE, anti-IL-22-PE, and anti-TNFα-FITC. Data were acquired on a FACS Calibur cytoflorometer and analyzed using Cellquest software (BD).
For analysis of phosphorylated STAT proteins, PBMC were incubated with rIL-27, rIL-6, rIFN-α, β, or γ for 5, 30, 60, and 180 min. Cells were fixed, permeabilized with 100% cold methanol, and stained with antibodies CD4, CD45RO, and to phosphorylated STAT1, STAT3, and STAT4. Data were acquired and analyzed as described above.
Real-time quantitative polymerase chain reaction
Total RNA was extract from cells using RNeasy Mini Kit (Qiagen) and cDNA synthesis was performed for each RNA sample using Superscript First-Strand Synthesis system (Invitrogen). TaqMan real-time polymerase chain reaction (PCR) was performed utilizing primers and probes from Applied Biosystems as described (Liu and Rohowsky-Kochan 2008). Gene expression is shown as expression fold value 2-ΔΔCt (Liu and Rohowsky-Kochan 2008).
Western blotting
Activated cells (4 × 106) were homogenized in lysis buffer and 50 μg of total protein were loaded on a 12% NuPAGE BisTris gel (Invitrogen) and electrophoresed for 50 min at 200 V in 3-[N-morpholino] propanesulfonic acid (MOPS) sodium dodecyl sulfate running buffer. Proteins were transferred onto a polyvinylidene difluoride membrane for 60 min at 30 V using Invitrogen semi-dry transfer procedure. Immunodetection was performed using either an anti-SOCS1 or anti-SOCS3 at a 1:400 dilution followed by a horseradish peroxidase-coupled secondary antibody (1:5,000). The reaction was observed using Pierce ECL Western Blotting Substrate kit (Thermo Scientific) and exposure of blots to HyBlot CL autoradiography film (Denville Scientific, Inc.) for 30 s–20 min and quantified using Un-Scan-It software (Silk Scientific). Data were normalized to signals obtained with housekeeping proteins alpha-tubulin or GAPDH antibodies.
Statistical analysis
The 2-tailed paired Student's t-test was used for data analysis of 2 groups and the 1-way analysis of variance (Kruskal–Wallis test) was used for analysis of >2 groups. P < 0.05 was considered statistically significant. To test for associations, Spearman's correlation coefficients were calculated.
Results
IL-27 inhibits Th17 differentiation
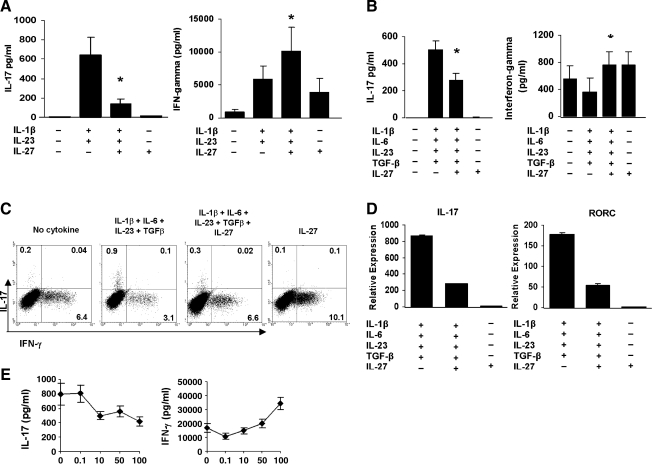
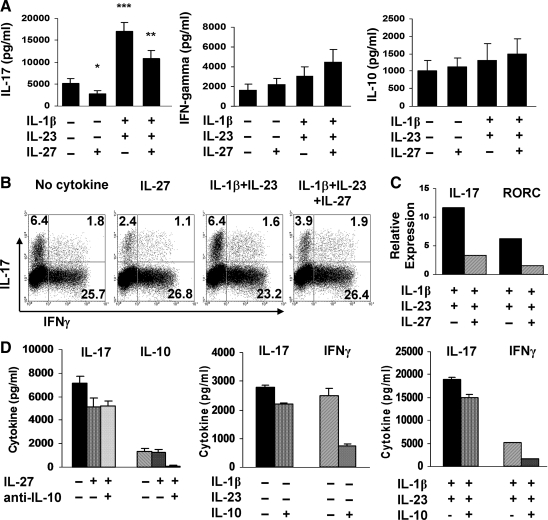
We investigated whether IL-27 was able to inhibit Th17 differentiation of human naïve T cells under different culture conditions. Naïve CD4+CD45RA+ T cells purified from the PBMC of healthy individuals were activated for 6 days with plate-bound anti-CD3 and soluble anti-CD28 either in 10% FCS/RPMI in the presence of IL-1β and IL-23 with and without IL-27 or in serum-free media with IL-1β, IL-6, IL-23, and TGF-β with and without IL-27. In agreement with published studies, CD3/CD28 activation in serum-containing media in the presence IL-1β and IL-23 induced IL-17 production (Fig. 1A), whereas activation of naïve T cells in serum-free media with IL-1β, IL-6, IL-23, and TGF-β triggered IL-17 (Fig. 1B) (Acosta-Rodriguez and others 2007; Wilson and others 2007; Cosmi and others 2008; Manel and others 2008; Volpe and others 2008). Addition of IL-27 significantly diminished the IL-1β/IL-23-induced IL-17 secretion (P < 0.008) (Fig. 1A) and the IL-1β, IL-6, IL-23, and TGF-β-induced IL-17 production (P < 0.0001) (Fig. 1B). IL-27 alone had no effect on IL-17 levels regardless of the media. In contrast, IL-27 significantly enhanced IL-1β and IL-23-induced IFN-γ secretion (P < 0.02) (Fig. 1A) and IFN-γ secretion in the presence of IL-1β, IL-6, IL-23, and TGF-β (P < 0.0003) (Fig. 1B). IL-27 alone increased IFN-γ levels, though not reaching significance in serum-containing conditions. Intracellular cytokine analysis of day 6 cultured cells showed that IL-27 inhibited the percent IL-17+ T cells and increased IFN-γ+ T cells, confirming the protein data by ELISA (Fig. 1C). IL-27-induced inhibition of IL-17 was associated with a marked reduction in IL-17 and RORC mRNA expression (Fig. 1D). The results from the intracellular cytokine staining and mRNA analysis under serum-containing conditions (data not shown) were similar to the serum-free conditions. The IL-27 effect on IL-17 and IFN-γ secretion was dose dependent and optimum at 100 ng/mL (Fig. 1E). These results indicate that IL-27 suppresses the differentiation of human Th17 cells under different polarizing conditions.
FIG. 1.
IL-27 inhibits human Th17 differentiation. Naïve CD4+CD45RA+ T cells were activated with plate-bound anti-CD3 and soluble CD28 in the presence of IL-1β and IL-23 with and without IL-27 for 6 days in 10% FCS/RPMI. (A) ELISA of IL-17 and IFN-γ in cell-free culture supernatants. Data are expressed as the mean ± SEM of 16 different healthy individuals. *P = 0.007 for IL-17 and P = 0.01 for IFN-γ. (B) Naïve T cells were activated as in (A) with IL-1β, IL-6, IL-23, and TGF-β with and without IL-27 under serum-free conditions. Data are expressed as the mean ± SEM of 8 different healthy individuals. *P < 0.0001 for IL-17 and P < 0.0002 for IFN-γ. (C) Intracellular staining of IL-17 and IFN-γ in cells from one individual activated in (B). Numbers in quadrants indicate percent cells in the CD4+CD45RO+ gate. One of 4 representative donors is shown. (D) Real-time polymerase chain reaction of IL-17 and RORC mRNA expressed as 2-ΔΔCt normalized to HPRT-1. Data from one of 2 donors are depicted as the mean ± SD of triplicate wells. (E) Dose–response curve for IL-27-mediated inhibition of IL-17 secretion. Results from one of 2 donors are shown and represent the mean ± SD of triplicate wells. RORC, retinoid orphan nuclear receptor C; IL, interleukin; TGF, transforming growth factor; FCS, fetal calf serum; IFN, interferon; SEM, standard error of the mean; SD, standard deviation.
IL-27 inhibits other Th17 subset cytokines
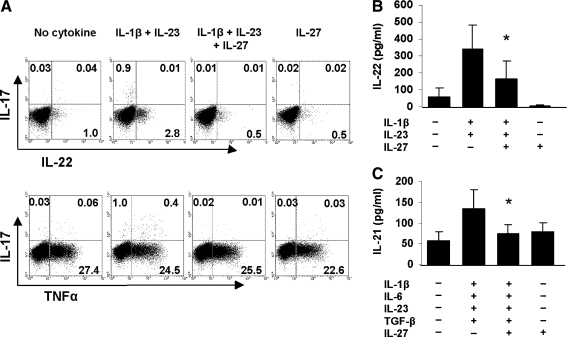
We tested whether IL-27 inhibits other members of the Th17 cytokine family. Naïve T cells were activated with CD3/CD28 under Th17 polarizing conditions with and without IL-27, and the frequency of IL-22- and TNF-α secreting T cells was ascertained. The percent IL-22+ T cells decreased from 2.8% to 0.5% in the presence of IL-27, but no effect was observed on the percent TNF-α+ T cells (Fig. 2A). ELISA results on IL-22 levels in culture supernatants corroborated the intracellular flow cytometry data (Fig. 2B). The effect of IL-27 on IL-21 production was determined by measuring levels of the cytokine in supernatants from activated naïve T cells. IL-27 significantly inhibited (P = 0.04) IL-21 levels in the presence of IL-1 β, IL-6, IL-23, and TGF-β but not when added alone (Fig. 2C). Thus, IL-27 negatively regulates certain but not all Th17 subset cytokines.
FIG. 2.
IL-27 inhibits other cytokines of the Th17 subset. Naïve T cells activated as described in Fig. 1A were restimulated with phorbol-12-myristate-13-acetate (PMA)/ionomycin for 5 h in the presence of brefeldin A and intracellular cytokine staining for IL-22 and TNF-α was performed (A). Numbers in quadrants indicate percent IL-22+ and TNF-α+ cells of gated CD4+CD45RO+ T cells. One of 5 representative donors is illustrated. ELISA of IL-22 levels in culture supernatants (B). Data are expressed as the mean ± SD of 7 different healthy donors. *P < 0.01. ELISA of IL-21 levels in the culture supernatants (C). Data are expressed as the mean ± SD of 6 different healthy donors. *P < 0.04. TNF, tumor necrosis factor.
IL-27 activates STAT1 and STAT3
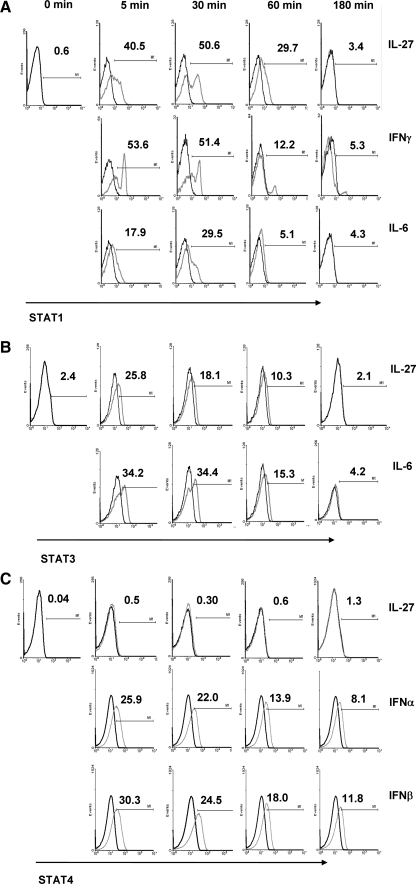
In mice, IL-27 inhibition of IL-17 production was reported to be STAT1 dependent (Stumhofer and others 2006). We analyzed IL-27-induced activation of STAT phosphorylation and the kinetics of the response after ligation of the IL-27 receptor on human PBMC. In these experiments, IL-27 was compared with IFN-γ and IL-6, IL-6, or IFN-α and IFN-β for activation of STAT1, STAT3, and STAT4, respectively. We stimulated PBMC with each cytokine for 0, 5, 30, 60, and 180 min and monitored the phosphorylation of STAT1, STAT3, and STAT4 by intracellular flow cytometry gating on naïve T cells. Addition of IL-27 resulted in a rapid induction of STAT1 occurring within 5 min and peaking at 30 min (Fig. 3A). IFN-γ induced a rapid phosphorylation of STAT1 with ∼50% of the T cells staining positive for STAT1 by 5 min, which remained unchanged after 30 min and then declined, whereas IL-6 induced a weaker STAT1 response. The IL-27-induced STAT1 phosphorylation was more prolonged as ∼30% of the T cells stained positive for STAT1 phosporylation at 60 min compared with 12% and 5% for IFN-γ and IL-6, respectively. Activation of phosphorylated STAT3 was similar in magnitude for IL-27 and IL-6 with IL-27 inducing slightly lesser amounts of STAT3 after 5 min (Fig. 3B). The IL-27-induced STAT3 activation declined by 30 min, whereas the IL-6-induced STAT3 phosphorylation remained unchanged and declined by 60 min. Comparison of STAT1 and STAT3 phosphorylation by IL-27 shows a 3-fold increase in the frequency of STAT1-expressing cells compared to STAT3+ T cells at 30 and 60 min, respectively. IL-27 did not trigger the phosphorylation of STAT4 over the 3 h period, although phosphorylation of STAT4 was observed for both IFN-α and IFN-β (Fig. 3C). These results indicate that IL-27 phosphorylates STAT1 and STAT3 and that the IL-27-induced activation of STAT1 appears to be dominant effect.
FIG. 3.
IL-27 induces STAT1 and STAT3 expression. Peripheral blood mononuclear cells were incubated with rIL-27 for 0 to 180 min and analyzed for expression of phosphorylated STAT1, STAT3, and STAT4 in T cells by intracytoplasmic staining. Histogram depicts phosphorylation of STAT1 (A), STAT3 (B), and STAT4 (C) on gated CD4+CD45RA+ T cells with cytokine (grey line) or without cytokine (black line) stimulation. Data are representative of one of 3 individuals tested.
IL-27 upregulates SOCS1 expression
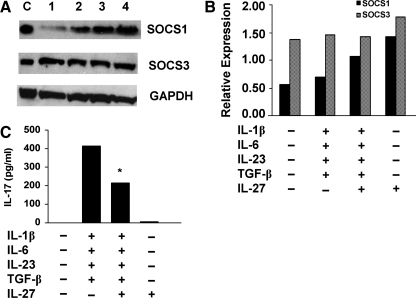
To determine which target genes are activated downstream of STAT1 and STAT3, we tested the effects of IL-27 on inducing expression of SOCS1 and SOCS3. Naïve T cells were activated by crosslinking CD3 and CD28 for 6 days in the presence of the Th17 polarizing cytokines with and without IL-27, and expression of SOCS proteins was assessed by Western blotting. Activation with CD3/CD28 induced both SOCS1 and SOCS3 expression with higher levels of SOCS3 (Fig. 4A, B). Addition of Th17 polarizing cytokines, IL-1β, IL-6, IL-23, and TGF-β resulted in slight increases in SOCS1 and SOCS3. Addition of IL-27 markedly upregulated SOCS1 protein both in the presence and absence of the Th17 polarizing cytokines. IL-27 augmented SOCS3 protein expression in the cultures activated without the Th17 polarizing cytokines. Resting, nonactivated naïve T cells did not express either SOCS1 or SOCS3, and their expression was not detected after 6 or 20 h of activation (data not shown). Measurement of secreted IL-17 levels in supernatants of cells activated in the presence of IL-27 revealed significantly decreased IL-17 levels (P < 0.03; Fig. 4C). We then asked whether there was an association between the IL-27-induced SOCS1 expression and the decreased IL-17 levels in the same cultures. An inverse correlation was observed between IL-17 levels and SOCS1 expression (correlation coefficient r = −0.84, P = 0.006). These data suggest that the IL-27-mediated inhibition of IL-17 may be mediated by SOCS1.
FIG. 4.
IL-27 promotes expression of SOCS1. Cell lysates were prepared from T cells activated with CD3/CD28 in the presence of IL-1β, IL-6, IL-23, and TGF-β with and without IL-27 for 6 days in serum-free conditions. (A) Western blot analysis using SOCS1, SOCS3, and GAPDH polyclonal Abs and 50 μg protein from cells activated with CD3/CD28 and no cytokine (Lane 1); Th17 polarizing cytokines (Lane 2); Th17 polarizing cytokines plus IL-27 (Lane 3) and IL-27 (Lane 4). Lane C = SOCS1 and SOCS3 positive control. One of 3 representative experiments is shown. (B) Graphic representation of Western results shown in (A) after normalization of band densities to GAPDH (P = 0.02). Median levels of 3 different donors from 3 independent experiments are shown. (C) ELISA of IL-17 in culture supernatants from which cell lysates were isolated. Data are expressed as the median of 3 different donors. SOCS1, suppressor of cytokine signaling protein 1.
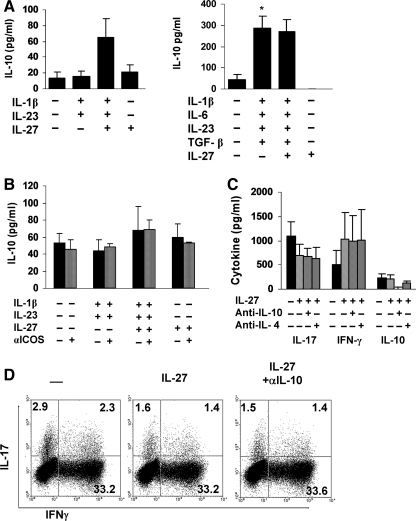
IL-27 does not induce IL-10 production in naïve T cells
We investigated whether IL-27 induced IL-10 production in human naïve T cells cultured under Th17 polarizing conditions in serum-containing and serum-free medium. Very low levels of IL-10 were detected in the supernatants of naïve T cells activated with CD3/CD28 in 10% FCS/RPMI media with 10 out of 14 individuals not secreting any detectable amount of IL-10 (Fig. 5A). No difference in IL-10 levels was observed in the presence of IL-1β and IL-23. Addition of IL-27 resulted in a modest but not significant increase in IL-10, with 8 out of 14 individuals producing IL-10 in amounts ranging from 9 to 324 pg/mL and the remaining individuals not producing any detectable amount of IL-10. IL-10 levels in cultures with IL-27 alone were similar to levels detected with CD3/CD28 crosslinking with or without IL-1β and IL-23. In serum-free media, activation with CD3/CD28 did not induce IL-10 with or without IL-27 (Fig. 5A). Addition of IL-1β, IL-6, IL-23, and TGF-β significantly increased (P = 0.03) IL-10 levels, and similar levels were detected when IL-27 was added. We then examined whether crosslinking inducible T-cell co-stimulator (ICOS) on naïve T cells during activation would lead to IL-10 secretion. Naïve T cell cultures were activated in 10% FCS/RPMI media in the absence and presence of an ICOS antibody. Ligation of ICOS did not alter the amount of IL-10 secreted regardless of whether IL-27 and/or IL-1β and IL-23 were added (Fig. 5B). These results demonstrate that IL-27 does not trigger IL-10 production in human naïve T cells.
FIG. 5.
IL-10 does not mediate IL-27-induced IL-17 inhibition. Naïve CD4+ T cells were activated with CD3/CD28 in the absence and presence of Th17 polarizing cytokines with and without IL-27 in either serum-containing medium (left graph) or serum-free medium (right graph) and IL-10 levels were measured in supernatants after 6 days by ELISA (A). Data are expressed as the mean ± SEM of 14 different healthy individuals (left graph) and 8 different healthy donors (right graph). *P = 0.03. (B) ELISA of IL-10 in supernatants from naïve T cells activated as in (A) in 10% FCS/RPMI in the presence of anti-inducible T-cell co-stimulator (ICOS). Results are expressed as the mean ± SD of 3 different donors. (C) ELISA of IL-17, IFN-γ, and IL-10 in supernatants from naïve T cells activated as in (A) in serum-free conditions in the presence of IL-10 Abs. (D) Intracellular staining of cells cultured under Th17 polarizing conditions in the presence of IL-27 and anti-IL-10. One of 3 representative donors is shown.
Since IL-10 was produced under Th17 polarizing conditions with IL-27, we investigated whether IL-10 was involved in mediating the IL-27-induced inhibition of IL-17. Naïve T cells were activated with CD3/CD28, the Th17 polarizing cytokines, and IL-27 with and without a neutralizing IL-10 Ab. IL-27 significantly inhibited T cell differentiation into Th17 cells (Fig. 5C). Addition of anti-IL-10 did not restore IL-17 to levels detected without IL-27, although completely blocking the IL-10 produced in the cultures. Likewise, the control IL-4 Ab had no effect on the IL-27-mediated inhibition of IL-17. Intracellular cytokine staining of the cultured cells confirmed the ELISA data (Fig. 5D).
IL-27 inhibits production of IL-17 by memory T cells
We next investigated whether IL-27 could regulate IL-17 production by CD4+ memory T cells. We demonstrated that CD3/CD28 ligation of CD4+ memory T cells results in IL-17 production that is further upregulated by IL-1β and IL-23 (Liu and Rohowsky-Kochan 2008). Activation of CD4+CD45RO+ T cells with CD3/CD28 resulted in production of IL-17, IFN-γ, and IL-10 (Fig. 6A). Addition of IL-27 to the cultured T cells resulted in a significant reduction (P = 0.002) of IL-17 with a slight but not significant increase in IFN-γ and IL-10. In the presence of IL-1β and IL-23, there was a >3-fold increase in IL-17 (P = 0.0003) with modest changes in IFN-γ and IL-10 production. Addition of IL-27 significantly diminished (P = 0.0007) the IL-1β/IL-23-induced secretion of IL-17. Intracellular cytokine staining of the memory T cells showed that IL-27 suppressed the percent IL-17+ T cells both in the absence and presence of IL-1β and IL-23, confirming the ELISA data (Fig. 6B). IL-17 and RORC gene expression was markedly decreased in the presence of IL-27 (Fig. 6C). To determine whether IL-10 was involved in mediating the IL-27-induced inhibition of IL-17 by memory T cells, we activated CD4+CD45RO+ T cells with CD3/CD28 in the absence and presence of IL-27 and a neutralizing IL-10 antibody. Addition of the anti-IL-10 did not reverse the IL-27-induced inhibition of IL-17 secretion (Fig. 6D).
FIG. 6.
IL-27 inhibits IL-17 production by memory T cells. Memory CD4+CD45RO+ T cells were activated with CD3/CD28 in the absence and presence of IL-1β and IL-23 with and without IL-27 for 6 days in 10% FCS/RPMI. (A) ELISA of IL-17, IFN-γ, and IL-10 in cell-free culture supernatants. Data are expressed as the mean ± SEM of 9 different healthy individuals. *P = 0.002 in comparison to cultures without IL-27; **P = 0.0007 in comparison to cultures without IL-27; and ***P = 0.0003 in comparison to cultures without IL-1β and IL-23. (B) Intracellular staining of IL-17 and IFN-γ in the cells in (A). Numbers in quadrants indicate percent cells in the CD4+CD45RO+ gate. One of 8 representative donors is shown. (C) Real-time polymerase chain reaction of IL-17 and RORC gene expression. Data from one of 2 donors are depicted. (D) ELISA of IL-17 and IL-10 in supernatants from memory T cells cultured as described in (A) in the presence of anti-IL-10 (left graph). Data are expressed as the mean ± SD of 4 different donors. ELISA of IL-17 and IFN-γ in supernatants from memory T cells cultured as described in (A) in the absence and presence of rIL-10 for 6 days (middle and right graphs). Data are expressed as the mean ± SD of 3 different donors.
Since blocking IL-10 did not reverse the IL-27-mediated inhibition of IL-17, we tested whether rIL-10 had any effect on IL-17 production. Activation of memory T cells in the presence of rIL-10 resulted in a slight although not significant reduction in IL-17 regardless whether IL-1β and IL-23 were added (Fig. 6D). In contrast, addition of rIL-10 markedly decreased IFN-γ production.
Discussion
In this study, we demonstrate that IL-27 suppresses the development of human Th17 cells by downregulating RORC expression in an IL-10-independent manner. These studies propose that the intracellular signaling factors STAT1 and the suppressor protein SOCS1 may participate in this inhibition. IL-27 inhibits differentiation of naïve T cells into IL-17+ T cells under different Th17 polarizing conditions. IL-27 suppresses the production of some Th17 subset cytokines such as IL-22 and IL-21 but not TNF-α. Moreover, we also show that IL-27 inhibits IL-17 production by committed Th17 memory cells, which is independent of IL-10.
Human Th17 differentiation is promoted by different cytokines depending upon the culture conditions. We demonstrate that IL-27 markedly suppresses the differentiation of IL-17+ T cells in serum-containing and serum-free Th17 polarizing conditions. One reason for testing whether the inhibitory effect of IL-27 was similar in both polarizing conditions was that some initial experiments (Figs. 1A and 5B) were performed in FCS-containing media (before the report on Th17 differentiation in serum-free conditions). Moreover, it was uncertain whether the presence of TGF-β would have an effect on the inhibitory effect of IL-27. Since IL-27 had similar effects on Th17 development in both culture conditions, these experiments were not repeated in serum-free media. IL-17 levels were not dramatically disparate under the different polarizing conditions, although a >10-fold reduction in IFN-γ levels was observed under serum-free conditions. The reason for this difference is unclear but may be attributed to the addition of exogenous TGF-β with the serum-free media.
Our results demonstrate that the IL-27-mediated inhibition of IL-17 is seen at the protein level, in the frequency of cytokine-producing T cells and at the transcription of the IL-17 gene and of the transcription factor RORC. We observed that in addition to IL-17, IL-27 suppressed the Th17 subset-associated cytokines IL-21 and IL-22 but not TNF-α secretion. Our studies support published findings showing that IL-27 inhibits human IL-17F and IL-22 protein (Diveu and others 2009) and gene transcription (Murugaiyan and others 2009) and extend them to include IL-21 and TNF-α. These studies corroborate those in mice showing that IL-27 does not downregulate TNF-α production (Stumhofer and others 2006). The IL-27 inhibition of IL-17 responses is dose dependent as is the upregulation of IFN-γ production and both are optimal at 100 ng/mL.
We further demonstrate that IL-27 induces STAT1 and STAT3 phosphorylation in human T cells as well as expression of the downstream STAT1 target gene SOCS1. Our studies show that IL-27 induces rapid phosphorylation of STAT1 similar to that induced by IFN-γ and that the strength and duration of phosphorylation is greater than that induced by either IFN-γ or IL-6. IL-27 also induces rapid phosphorylation of STAT3 that is weaker than that induced by IL-6. These results suggest that IL-27 has a STAT1-dominant effect on gene activation during Th17 differentiation of human naïve T cells. Our data are in agreement with a study showing that IL-27 induces STAT1 and STAT3 in T cells (Hibbert and others 2003) and further show the kinetics and strength of this activation. In mice, IL-27-mediated IL-17 inhibition was shown to be STAT1 dependent (Batten and others 2006; Stumhofer and others 2006; Diveu and others 2009; El-behi and others 2009). The role of STAT3 in the IL-27-mediated inhibition of IL-17 is uncertain since this transcription factor is both necessary for IL-17 transcription and is induced by IL-27. It is tempting to speculate that similar to the murine system, human IL-27-mediated inhibition of IL-17 is STAT1 dependent. Further studies using siRNA to silence STAT1 in human T cells are required to confirm the role of STAT1 in the ability of IL-27 to dampen IL-17 production.
Cytokine signaling through the STAT pathway is negatively regulated by SOCS proteins. IL-27 suppresses CD28-mediated IL-2 production that is correlated with the induction of SOCS3 expression in CD4+ T cells (Owaki and others 2006; Villarino and others 2006). In the present study, we show that inhibition of Th17 differentiation by IL-27 is associated with the upregulation of SOCS1 expression. Levels of IL-17 protein in the culture supernatants are inversely associated with SOCS1 expression. Expression of SOCS3 is slightly modulated by IL-27. Induction of SOCS expression in human T cells by IL-27 is a salient finding and is in concert with activation of STAT1 and SOCS1 in human macrophages (Kalliolias and Ivashkiv 2008). Whether the IL-27-mediated IL-17 inhibition is attributed to a SOCS1-mediated dampening of STAT1 or STAT3 and the role of SOCS3 remains to be determined. Studies utilizing siRNA to silence SOCS1 or SOCS3 in human T cells may elucidate the role of these proteins in mediating the IL-27-induced inhibition of IL-17.
In mice, IL-27 is able to induce IL-10 production in T cells; however, whether the immunosuppressive effect of IL-27 on IL-17 is mediated by IL-10 is controversial (Awasthi and others 2007; Fitzgerald and others 2007; McGeachy and others 2007; Stumhofer and others 2007; Batten and others 2008). In humans, one study showed that IL-27 promotes the development of IL-10-secreting Tr1 cells while inhibiting the generation of Th17 cells (Murugaiyan and others 2009). Others reported that the IL-27-induced IL-10 production by human T cells was not consistently observed and did not reach statistical significance (Diveu and others 2009). Our results demonstrate that IL-27 does not induce IL-10 production in naïve CD4+ T cells activated with CD3/CD28 in either serum-containing or serum-free media and support those by Diveu and others. Additional crosslinking of ICOS had no effect on the ability of IL-27 to induce IL-10 production in naïve CD4+ T cells. The reason for the discrepancy in results with the study by Murugaiyan and others is most likely due to differences in culture conditions, as our cells were activated in the absence of exogenous IL-2 and presence of antibodies to IFN-γ and IL-4 as both these cytokines are known to inhibit IL-17 development. Similar to the results of their study, we observed that IL-10 did not mediate the IL-27-induced inhibition of IL-17 as addition of an IL-10 neutralizing Ab did not reverse the IL-27-mediated inhibition of IL-17 as seen both at the protein level and by intracellular flow cytometry. We observed slightly different effects on IL-10 depending on the culture media used. In serum-containing media, Th17 polarizing cytokines (IL-1β and IL-23) did not have any effect on IL-10 production and an increase though not significant in IL-10 was seen when IL-27 was added. In serum-free media, Th17 polarizing cytokines (IL-1β, IL-6, IL-23, and TGF-β) significantly increased IL-10 production, most likely due to the TGF-β and addition of IL-27 had no effect on IL-10. Our data are similar to a study showing that addition of IL-27 to cultures of Th17 polarizing cells had little effect on IL-10 production, which already was elevated in these cells (Stumhofer and others 2007).
A critical finding of this article is that IL-27 inhibits IL-17 production by committed memory T cells. We observed that IL-27 inhibits IL-17 protein and gene expression as well as RORC gene expression in purified memory T cells activated in the absence and presence of IL-1β and IL-23, cytokines that were shown to upregulate IL-17 in human memory T cells (Liu and Rohowsky-Kochan 2008). Similar to the results with naïve T cells, IL-10 is not involved in mediating the IL-27-induced suppression of Th17 responses. IL-27 was shown to inhibit IL-17 production by total T cells (Murugaiyan and others 2009); the present study specifically characterizes the effect on memory T cells. Our studies are in contrast to studies in mice showing that IL-27 has differential regulatory activities on naïve versus memory T cells (Diveu and others 2009; El-behi and others 2009). These results have important implications for the therapeutic regulation of inflammatory, autoimmune diseases, where the pathogenic Th17 cells have already developed.
Increasing evidence suggests that Th17 cells are critical in human inflammatory and autoimmune responses. Upregulated IL-17 expression is found in diseased tissue and blood of patients with multiple sclerosis, rheumatoid arthritis, psoriasis, scleroderma, and Crohn's disease (Bettelli and others 2007). Hence, understanding how IL-17 is regulated in humans is critical. Our studies indicate that IL-27 antagonizes human Th17 development and production by memory T cells and therefore may be a potential therapeutic tool for the treatment of inflammatory autoimmune disease associated with Th17 cells.
Acknowledgments
These studies were supported by a grant from the National Institutes of Health NS34245 to Christine Rohowsky-Kochan.
Author Disclosure Statement
No competing financial interests exist.
References
- Acosta-Rodriguez EV. Napolitani G. Lanzavecchia A. Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Awasthi A. Carrier Y. Peron JPS. Bettelli E. Kamanaka M. Flavell RA. Kuchroo VK. Oukka M. Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- Batten M. Kljavin NM. Li J. Walter MJ. de Sauvage FJ. Ghilardi N. Cutting Edge: IL-27 is a potent inducer of IL-10 but not Foxp3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- Batten M. Li J. Yi S. Kljavin NM. Danilenko DM. Lucas S. Lee J. de Sauvage FJ. Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- Bettelli E. Oukka M. Kuchroo VK. Th-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Chen Z. Laurence A. Kanno Y. Pacher-Zavisin M. Zhu BM. Tato C. Yoshimura A. Hennighausen L. O'Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L. De Palma R. Santarlasci V. Maggi L. Capone M. Frosali F. Rodolico G. Querci V. Abbate G. Angeli R. Berrino L. Fambrini M. Caproni M. Tonelli F. Lazzeri E. Parronchi P. Liotta F. Maggi E. Romagnani S. Annunziato F. Human interleukin-17-producing cells originate from a CD161 CD4 T-cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diveu C. McGeachy MJ. Boniface K. Stumhofer JS. Sathe M. Joyce-Shaikh B. Chen Y. Tato CM. McClanahan TK. de Waal Malefyt R. Hunter CA. Cua DJ. Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- El-behi M. Ciric B. Yu S. Zhang G-X. Fitzgerald D. Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DC. Zhang GX. El-Behi M. Fonseca-Kelly Z. Li H. Yu S. Saris CJ. Gran B. Ciric B. Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- Harrington LE. Hatton RD. Mangan PR. Turner H. Murphy TL. Murphy KM. Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1131. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hibbert L. Pflanz S. de Waal Malefyt R. Kastelein RA. IL-27 and IFN-α signal via Stat1 and Stat3 and induce T-bet and IL-12Rβ2 in naïve T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- Ivanov II. McKenzie BS. Zhou L. Tadokoro CE. Lepelley A. Lafaille JJ. Cua DJ. Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kalliolias GD. Ivashkiv LB. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J Immunol. 2008;180:6325–6333. doi: 10.4049/jimmunol.180.9.6325. [DOI] [PubMed] [Google Scholar]
- Liu H. Rohowsky-Kochan C. Regulation of IL-17 in human CCR6+effector memory T cells. J Immunol. 2008;180:7948–7957. doi: 10.4049/jimmunol.180.12.7948. [DOI] [PubMed] [Google Scholar]
- Lucas S. Ghilardi N. Li J. de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naïve CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N. Unutmaz D. Littman DR. The differentiation of human Th-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR. Harrington LE. O'Quinn DB. Helms WS. Bullard DC. Elson CO. Hatton RD. Wahl SM. Schoeb TR. Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ. Bak-Jensen KS. Chen Y. Tato CM. Blumenschein W. McClanahan T. Cua DJ. TGF-b and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell–mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- Murugaiyan G. Mittal A. Lopez-Diego R. Maier LM. Anderson DE. Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufert C. Becker C. Wirtz S. Fantini MC. Weigmann B. Galle PR. Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- Owaki T. Asakawa M. Kamiya S. Takeda K. Fukai F. Mizuguchi J. Yoshimoto T. IL-27 suppresses CD28-mediated IL-2 production through suppressor of cytokine signaling 3. J Immunol. 2006;176:2772–2780. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- Park H. Li Z. Yang XO. Chang SH. Nurieva R. Wang YH. Wang Y. Hood L. Zhu Z. Tian Q. Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanz S. Hibbert L. Mattson J. Rosales R. Vaisberg E. Bazan J. Kastelein R. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- Pflanz S. Timans JC. Cheung J. Rosales R. Kanzler H. Gilbert J. Hibbert L. Churakova T. Travis M. Vaisberg E. Blumenschein WM. Mattson JD. Wagner JL. To W. Zurawski S. McClanahan TK. Gorman DM. Bazan JF. de Waal Malefyt R. Rennick D. Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naïve CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS. Laurence A. Wilson EH. Huang E. Tato CM. Johnson LM. Villarino AV. Huang Q. Yoshimura A. Sehy D. Saris CJ. O'Shea JJ. Hennighausen L. Ernst M. Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS. Silver JS. Laurence A. Porrett PM. Harris TH. Turka LA. Ernst M. Saris CJ. O'Shea JJ. Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- Takeda A. Hamano S. Yamanaka A. Hanada T. Ishibashi T. Mak TW. Yoshimura A. Yoshida H. Cutting Edge: role of IL-27 WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- Veldhoen M. Hocking RJ. Atkins CJ. Locksley RM. Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Villarino A. Hibbert L. Lieberman L. Wilson E. Mak T. Yoshida H. Kastelein RA. Saris C. Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- Villarino AV. Stumhofer J. Saris CJM. Kastelein RA. de Sauvage FJ. Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- Volpe E. Servant N. Zollinger R. Bogiatzi S. Hupe P. Barillot E. Soumelis V. A critical function for transfroming growth factor-β, interleukin-23 and proinflammatory cytokines in driving and modulating human Th-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- Wilson NJ. Boniface K. Chan JR. McKenzie BS. Blumenschein WM. Mattson JD. Basham B. Smith K. Chen T. Morel F. Lecron JC. Kastelein RA. Cua DJ. McClanahan TK. Bowman EP. de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T. Yoshimoto T. Yasuda K. Mizuguchi J. Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokine production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179:4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]