Abstract
Interferon (IFN)-β in preclinical studies, compared to IFN-α2, bound with higher affinity to its receptor, induced to higher levels of IFN-stimulated gene products, induced more apoptosis in melanoma cells, and had antitumor effects against melanoma. A maximally tolerated dose of 12 × 106 international units/m2 after 2 weeks subcutaneously daily with dose escalation to 18 × 106 international units/m2 was thus used in a phase II trial of IFN-β1a in cutaneous metastatic melanoma (n = 17) and uveal melanoma (n = 4). It resulted in expected but reversible drug-related severe (grade 3) adverse events in 13/21 patients; anorexia and fatigue were mostly of mild or moderate severity and infrequently needed dose reduction. Although a single patient had a sustained regression, overall IFN-β1a did not have clinical benefit (response rate <10%; median progression-free survival 1.8 months). Effective and potent induction in peripheral blood cells and into serum of products of IFN-stimulated genes such as the pro-apoptotic cytokine, TRAIL, and the immunomodulatory and anti-angiogenic chemokines, CXCL10 and CCL8, confirmed gene regulatory actions. To probe further anti-angiogenic mechanisms, both VEGF-A and CXCL-5 were assessed; compared to before treatment, both proteins decreased. Continued improvements in understanding of antitumor mechanisms will enhance usefulness of IFNs for nodal or distant metastases from melanoma.
Introduction
Interferon (IFN)-α2 for metastatic melanoma has resulted in disease regression in 15% of patients with metastatic disease and prolongation of disease-free and overall survival in patients with newly diagnosed disease at risk for recurrence (Kirkwood and others 2002; Ascierto and Kirkwood 2008; Balch and others 2009). Different IFNs have different biological effects on cell proliferation, virus replication, and immune cell function (Pestka and others 2004; Honda and others 2005; Borden and others 2007). IFN-β shares, however, only 30% amino acid homology with IFNs-α and although binding to the same receptor does so with higher affinity and with a likely differing steric interaction and signaling effects (Ruzicka and others 1987; Pestka and others 2004; Borden and others 2007). Possibly, as a result of the differing receptor interaction, IFN-β induces more strongly the transcriptionally regulated IFN-stimulated genes (ISGs) (Der and others 1988; Leaman and others 2003). Furthermore, it more potently induces antiproliferative effects and apoptosis in tumor cells in vitro than does IFN-α2 (Schiller and others 1986; Krasagakis and others 1991; Chawla-Sarkar and other 2001).
In murine studies, IFN-β has resulted in antitumor effects for transplantable syngeneic melanomas (Ida and others 1982; Ryuke and others 2003), and in the nude mouse, human IFN-β resulted in greater antitumor effects than IFN-α2 for melanoma xenografts (Gomi and others 1984; Johns and others 1992). A deficiency in humans in IFN-β production has been identified in the epidermis overlying melanomas with a concomitant increase in angiogenesis (McCarty and others 2003). Since IFN-β has antiangiogenic effects in the nude mouse (Sidky and Borden 1987), these antitumor effects may result from vascular inhibition in addition to direct effects on tumor. However, a high dose daily infusion of IFN-β (60 million units) for 4 days resulted in partial responses in 3/15 patients with metastatic melanoma (Abdi and others 1988).
A phase I trial of IFN-β1a had identified a maximally tolerated dose of 12 × 106 international units (IU)/m2 with escalation to 18 × 106 IU/m2 daily (Ravandi and others 1999). Although in humans a low dose of IFN-β1 intravenously twice weekly was ineffective as was continuous infusion (Sarna and others 1987), with the need for improved treatments for metastatic melanoma, a phase II trial of IFN-β1a was initiated. Objectives were to identify antitumor effects, adverse events, and gene regulatory actions at the maximally tolerated dose.
Methods
Inclusion and exclusion criteria
All patients met the following eligibility criteria: histological diagnosis of malignant melanoma, measurable disease as defined by the National Cancer Institute (NCI) response evaluation criteria in solid tumors guidelines, performance status Eastern Cooperative Oncology Group (ECOG) of ≤2, recovered >2 weeks from the toxicity of palliative (small port) radiation therapy, ≤1 prior systemic regimen (chemotherapeutic or biological) for metastatic disease, not have received any adjuvant or metastatic disease IFN-α2 ≤12 months prior, and no major surgery within 28 days, together with granulocyte count ≥1.2 × 109 L−1, platelets ≥100 × 109 L−1, hemoglobin ≥9.5 gm/100 mL, creatinine ≤1.5 mg/dL, and bilirubin (total) ≤1.5 mL/dL, and must have provided written informed consent as to the investigative nature of treatment in accordance with institutional and federal guidelines. Excluded were patients with uncontrolled central nervous system (CNS) metastases in the prior 6 months, chronic infections, history of serious cardiac arrhythmia, congestive heart failure, pregnant or lactating women, fertile women or men unless surgically sterile or using effective contraception, serologic evidence of HIV or HBsAg, organ allografts, high dose glucocorticoids, age <18, or history of severe psychiatric disorders. Although trial and institutional procedures were subsequently improved, 2 patients in the first 5 entered (1 with anemia from chronic bleeding from gastrointestinal metastases and 1 with prior but asymptomatic atherosclerotic cardiac disease) had eligibility parameters outside of specified; neither, however, experienced any treatment-related adverse events and were included in all analyses. Patients were assessed for adverse events weekly for 4 weeks, biweekly for 2 weeks, and then monthly. Measureable disease was assessed at each visit and by imaging every 2 months.
Treatment plan
Patients received IFN-β1a provided as Rebif® by Merck Serono. Recombinant human IFN-β1a (Rebif) is composed of the native amino acid sequence of natural human IFN-β. It is produced in mammalian cells (Chinese hamster ovary cells) and glycosylated like the natural protein. In its physicochemical and biological properties it has not been distinguishable from human fibroblast-derived natural IFN-β (Frone®; Serono). It was administered at 12 × 106 IU/m2 subcutaneously (sq) daily with dose escalation after 14 days and if no adverse events >grade 2 to 18 × 106 IU/m2. Patients received IFN-β1a until disease progression or a dose-limiting toxicity supervened. Near study end an amendment added topical retinoid vitamin A crème 0.05% applied once daily for 4 final patients with cutaneous metastases.
ELISA and multiplex assays for ISG products
β2-microglobulin (R&D Systems, Minneapolis, MN) was quantitated in patients' sera using a competitive binding enzyme immunoassay. TRAIL, CXCL10 (IP-10), FGFβ, and CCL20 (MIP-3α) (R&D Systems), TRAIL-R1 and TRAIL-R4 (Gen-Probe, Inc. Canton,MA), and CCL8 (MCP-2) (RayBiotech, Raitan, NJ) were quantitated in frozen stored patients' sera using individual quantitative sandwich enzyme immunoassays for batched samples. IL-1RA, VEGF-A, TNF-α, CSF-G, IL-1α IL-1β, CCL-2 (MCP-1), IL-2, IL-6, IL-17, IFN-γ, CXCL8 (IL-8), CXCL5 (ENA-78), CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL5 (RANTES) were quantitated in patients' sera using the Luminex multi-analyte technology (Luminex Corp., Austin, TX). All assays were performed according to manufacturer's instructions. The lower limits of sensitivity for all of the ELISAs were 0.4 μg/mL for β2 microglobulin, 0.5 ng/mL for neopterin, 0.3 ng/mL (TRAILR1), and 0.3–15 pg/mL for all other proteins. The lower limits of sensitivity for all products measured using the Luminex technology was 10-15pg/mL.
RNA collection and analyses
Blood was collected in PAX tubes (PreAnalytiX, Inc., Franklin Lakes, NJ) before treatment and day 8, and RNA was prepared using the PreAnalytiX Blood RNA kit according to manufacturer's instructions. cDNA was prepared using the SuperScript III First-Strand Synthesis System (Invitrogen, Inc. Carlsbad, CA) according to instructions of the manufacturer. Selected gene expression was assessed by TaqMan Gene Expression probes, TaqMan Universal polymerase chain reaction (PCR) MasterMix, and the ABI 7500 Cycler (Applied Biosystems, Branchburg, NJ) according to the manufacturer. GAPH was used to normalize CT values and fold expression was calculated based on pretreatment CT values.
Biostatistics
Data were calculated at each day as mean and standard deviation. Repeated measures analysis of variance was used to determine if ISGs differed among days. Pairwise comparisons between days were performed using a Bonferroni correction. All statistical tests were 2-sided; P < 0.05 and P < 0.017 were used to indicate significance of the overall test and pairwise comparisons, respectively. Data were analyzed using SAS® software (SAS Institute, Inc., Cary, NC).
Results
Patient characteristics and treatment administration
Entered on this trial were 21 patients with metastatic melanoma (17 cutaneous and 4 uveal primaries) of median age 61 (range 30–81), more male gender (n = 16), and 20 Caucasians. Metastatic disease at the time of study enrollment included cutaneous or soft tissues (n = 10), nodes (6), and visceral sites (n = 16). Only 1 patient had previously treated brain metastasis but did not have active disease at the time of study entry. All patients were ambulatory (ECOG performance status was 0 or 1) except 1 (performance status 2). Only 4 patients had had prior systemic therapy for metastatic disease, 7 had received IFN-α2 after surgery for the primary disease more than 12 months before, and 3 patients had had prior radiation.
Nine patients received 1 cycle of therapy, 9 patients received 2, and 1 patient each received 3, 6, and 12 cycles. Toleration after 2 weeks allowed protocol defined dose escalation of IFN-β1a to 18 × 106 units/m2 in 11 of the 21 patients with no unexpected or atypical adverse events. Eleven patients (52%) had dose reductions (n = 8) and/or delays (n = 10) at some point during treatment. All patients have had IFN-β1a discontinued and all but 1 have expired from progressive disease. Treatment was discontinued because of progressive disease in 17 patients; 2 for patient request (1 after 12 months) and 1 each for recurring protocol defined toxicity (grade 3 hepatic transaminases).
Side effects
The most commonly reported adverse events, possibly, probably, or definitely associated with IFN-β1a, were the flu-like and constitutional symptoms associated with IFNs and injection-site reactions (Table 1). Flu-like symptoms of mild or moderate fever (≤40°C) or chills were experienced with the first injection by 17/21 patients but was mild (≤39°C) in 15. Almost all (19/21) patients had mild (n = 16) to moderate (n = 3) injection-site reactions. The latter were qualitatively more marked than expected based upon experience with a lower SQ dose of IFN-α2.
Table 1.
Side Effects with Administration of Interferon-β1a
| |
Toxicity grade (CTC Version 2.0) |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Fever (without neutropenia) | 11 | 3 | 0 | 0 |
| Chills/rigors | 15 | 2 | 0 | 0 |
| Weight loss | 3 | 1 | 0 | 0 |
| Anorexia | 7 | 3 | 0 | 0 |
| Fatigue | 7 | 9 | 2 | 0 |
| Injection site reactions | 16 | 3 | 0 | 0 |
| Leukopenia | 4 | 8 | 0 | 0 |
| Lymphopenia | 0 | 11 | 4 | 0 |
| Thrombocytopenia | 6 | 2 | 2 | 0 |
| AST increased | 4 | 3 | 1 | 0 |
| ALT increased | 3 | 6 | 1 | 0 |
| Hypophosphatemia | 0 | 6 | 1 | 0 |
Fatigue was reported by 18/21 patients during treatment but was graded as only mild or moderate (<1 performance level decline) in 16. Anorexia occurred in 10/21 patients but was never graded as worse than moderate. Despite having progressive malignancy, only 4 patients lost more than 5% (grade 1) from starting weight during the period of administration of IFN-β and in only 1 patient did weight loss exceed 10%. Overall, the side effects of fatigue and anorexia associated with high doses of IFN-α2 in melanoma seemed quantitatively less severe and bothersome.
No patient had leukopenia <2,000 mm−3. Severe (n = 4; <500 mm−3) lymphopenia and thrombocytopenia (n = 4; <50,000 mm−3) did occur. AST elevations occurred in 12/21 and in 6 patients at the escalated dose was severe (>5× normal) leading to dose alteration. Temporally associated hypophosphatemia of <2.5 mg/dL, a side effect not previously related to IFNs, resulted in 7/21 patients, including 1 patient with <2.0 mg/dL. The hypophosphatemia had no identified clinical association or complication.
Overall, 62% (13/21) of patients had at least one grade 3 event that resulted in protocol-defined alterations in dose. Except for 2 instances of fatigue, all grade 3 adverse events were hepatic transaminase elevations or hematologic count suppression. Adverse events considered drug related were all reversible and without clinically identifiable or continuing sequelae. In 6/16 instances the grade 3 adverse events occurred when dose was escalated to18 × 106 units/m2. No life-threatening adverse events occurred.
Response and survival
One patient with cutaneous melanoma had a partial and sustained response in skin in the lower leg and lymph nodes (pelvic). This patient had biopsied progressive cutaneous nodules, a previously resected, progressive, painful large (>7 cm) pelvic mass and pulmonary metastases. After 3 months of IFN-β, the cutaneous and pulmonary disease had completely regressed and the pelvic mass had decreased and eventually regressed on IFN-β1a to fibrotic but nonmeasureable pelvic mass on CT scans over 8 months. With disappearance of his cutaneous disease, the patient asked to withdraw from IFN-β1a after 13 months, but since an abnormal mass, possibly fibrosis, persisted at the site, he was considered only a partial response. He has now remained off treatment for >3 years without disease recurrence. All other patients progressed in <6 months; median progression-free survival was 7 weeks and median overall survival was 7 months. The last 4 patients entered onto the study had retinoid crème additionally used topically without regression of cutaneous metastases.
ISG product induction
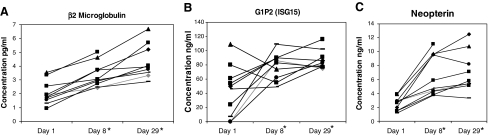
To confirm biological activity of the subcutaneous IFN-β1a and to probe mechanism of action, 10 random patients had representative ISG protein products quantitated in serum. These were assessed by ELISA or multiplex assay before treatment, after 1 week, and after 1 month of daily injections. Consistent with other studies of IFNs (Goldstein and others 1989; D'Cunha and others 1996; Ravandi and others 1999; Buchwalder and others 2000; Borden 2005; Masci and others 2007), immunoregulatory IFN-stimulated proteins increased. Although an expected inter-patient variability occurred, particularly in baseline levels, β2-microglobulin, guanosine triphosphate (GTP) cyclohydrolase (measured as its product neopterin), and ISG15 (G1P2) increased significantly (P < 0.01) and in almost every patient (Fig. 1A–C). All increases in these ISG protein products were sustained after 1 month of IFN-β1a. β2-microglobulin increased further when the level at d29 was compared to d8 (P < 0.001). Even after 1 month of daily treatment, however, no significant (P < 0.05) changes were identified in CSF-GM, IL-2, IL-6, IL-17, or IFN-γ [in 3/10 patients IFN-γ did increase from undetectable (<10 pg/mL) to measurable (19-54pg/mL)].
FIG. 1.
Serum samples from melanoma patients were analyzed for β2 microglobulin (A), G1P2 (ISG15) (B), and neopterin (C) expression at day 1 (pretreatment), day 8, and day 29 after interferon (IFN)b-1a treatment by ELISAs. Significant increases (*P < 0.001) in β2 microglobulin, G1P2 (ISG15), and neopterin were identified at days 8 and 29. All patients received 22 × 106 international units (IU)/m2 on days 1–14 and 18 × 106 IU/m2 on days 15–29. IFN, interferon; ISG, IFN-stimulated genes.
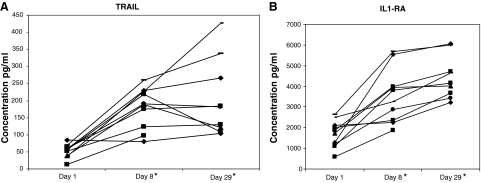
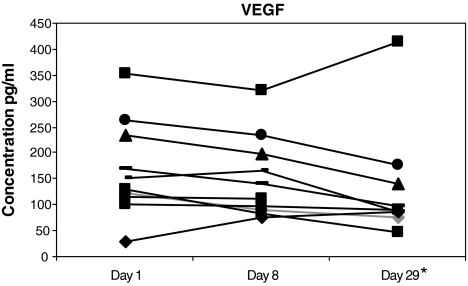
Cytokines with both pleiotropic and more restricted-lineage cellular effects were also assessed. Significantly increased (P < 0.001) were TRAIL and IL-1RA (Fig. 2A, B). The patient with the clinical response had the highest level of the pro-apoptotic TRAIL gene product of any patient following the escalated dose on d29. VEGF-A overall decreased modestly (P < 0.03, unadjusted for multiple comparisons) after 1 month of treatment in 9/10 patients (Fig. 3). Without significant (P < 0.05) change were TRAIL-R4 (a decoy receptor), TNF-α, and FGF-β (data not shown).
FIG. 2.
Serum samples from melanoma patients were analyzed for TRAIL (A) and IL-1RA (B) expression at day 1 (pretreatment), day 8 and day 29 after IFNb-1a treatment. The level of TRAIL was determined using an ELISA and the level of IL-1RA was determined using the Luminex multi-analyte technology. Expression of both TRAIL and IL-1RA was significantly (*P < 0.001) increased after treatment. All patients received 22 × 106 IU/m2 on days 1–14 and 18 × 106 IU/m2 on days 15–29.
FIG. 3.
Serum samples from melanoma patients were analyzed for VEGFa expression at day 1 (pretreatment), day 8, and day 29 after IFNb-1a treatment. The level of VEGFa was determined using the Luminex multi-analyte technology. A decrease in VEGFa (*P < 0.03) was identified at day 29. All patients received 22 × 106 IU/m2 on days 1–14 and 18 × 106 IU/m2 on days 15–29.
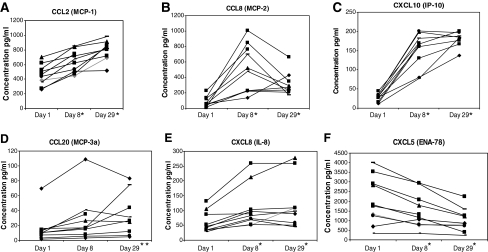
Chemokines that increased most markedly (P < 0.001) were CCL2 (MCP-1), CCL8 (MCP-2), and CXCL10 (IP-10) (Fig. 4A–C). CCL8 and β2 microglobulin were the only ISG products to be further significantly (P < 0.01) stimulated from d8 to d29. Less marked but significant increases after 29 days of treatment (P < 0.01) resulted in CCL20 (MIP-3α) and CXCL8 (IL-8) (Fig. 4D, E). Identified unexpectedly was a decrease in the proangiogenic CXCL5 (ENA-78) (Fig. 4F). No changes resulted in CCL3 (MIP-1α), CCL4 (MIP-1β), or CCL5 (RANTES) (data not shown).
FIG. 4.
Serum samples from melanoma patients were analyzed for CCL2 (MCP-1), CCL8 (MCP-2), CXCL10 (IP-10), CCL20 (MCP-3a), CXCL5 (ENA-78), and CXCL8 (IL-8) expression (A–F) at day 1 (pretreatment), day 8, and day 29 after IFNb-1a treatment. The levels of CCL2, CCL8, CXCL5 (ENA-78), and CXCL8 were determined using the Luminex multi-analyte technology, and the levels of CXCL10 and CCL20 were determined using ELISAs. Significant increases (*P < 0.001) in CCL2, CCL8, CXCL8, and CXCL10 were identified at days 8 and 29. A significant increase (**P < 0.05) in CCL20 was identified at day 29. A significant decrease (*P < 0.001) was identified at days 8 and 29 in CXCL5 (ENA-78). All patients received 22 × 106 IU/m2 on days 1–14 and 18 × 106 IU/m2 on days 15–29.
To further confirm that increases in proteins resulted from ISGs, RNA was purified from peripheral white cells drawn on days 1 and 8 and specific gene products assessed with 37 different probes. Substantial relative increases occurred in specific RNA after a week of IFN-β when assessed by quantitative (q) real time (RT)-PCR in 3 patients (Table 2). In a fourth patient (data not shown) slight increases (6-fold) in IFI27 and ISGp27 also resulted without increases in other ISGs (this patient did have expected increases in protein products of ISGs, suggesting thus RNA loss during storage or extraction). Specific mRNA increases in ISG15, CCL8, CXCL10, and TRAIL confirmed protein product increases of the same genes in the 3 patients.
Table 2.
Fold Change in Interferon-Stimulated Genes RNA After Interferon-b Treatment
| Genes | Patient #1 | Patient #2 | Patient #3 |
|---|---|---|---|
| IFI27 | 313 | 173 | 340 |
| G1P2 | 204 | 94 | 116 |
| CCL8 | 161 | 24 | 161 |
| CXCL10 | 120 | 43 | 41 |
| G1P3 | 62 | 600 | 37 |
| MX1 | 59 | 13 | 35 |
| XAF1 | 28 | 20 | 19 |
| IRF7 | 23 | 112 | 11 |
| CXCL11 | 11 | 2 | 14 |
| IL22RA1 | 9.8 | 1 | 3.1 |
| TRAIL | 9.3 | 39 | 6.7 |
| BST2 | 8.3 | 5.4 | 6.1 |
| AIM2 | 7.5 | 2.9 | 8.5 |
| CCL7 | 3.6 | 0.9 | 2 |
| STAT1 | 3.4 | 4.4 | 4.9 |
| FasL | 3 | 2.7 | 1.8 |
| IFITM2 | 2.3 | 0.9 | 3.8 |
Peripheral blood was collected pretreatment and on day 8 of treatment in PAX tubes. RNA was prepared using the PreAnalytiX kit according to manufacturer's instructions. cDNA was prepared with SuperScript III First-Strand Synthesis system, quantitative real time (RT)-polymerase chain reaction was performed with ABI probes on an ABI 7500 Cycler, CT values were normalized with GAPH, and fold change was calculated based on pretreatment CT values. All patients received 22 × 106 international units/m2 on days 1–14 and 18 × 106 international units/m2 on days 15–29.
All other gene products, identified as increased more than 2 × by qRT-PCR in at least 2 of the 3 patients (Table 2), were ISGs known from in vitro studies (Der and others 1988; Leaman and others 2003; Borden and others 2007) but most not previously identified as increased by IFNs in patients. Conversely, no increases resulted in genes such as CSF-GM, SHP1, TRAIL-R1, PTEN, or TSP1 that are not identified ISGs (data not shown).
Discussion
IFN-β1a at a maximally tolerated dose daily did little to induce tumor regression in metastatic cutaneous melanoma in patients with good performance status and limited prior therapy (only 1/17 objective regressions). The high dose resulted in expected severe (grade 3 but no life-threatening grade 4) but reversible bone marrow suppression, transaminase elevations or fatigue in 13/21 patients. The short progression-free and overall survival of on this trial was unfortunately quite consistent with a recent review of international results for patients with metastatic melanoma on phase II trials of new agents (in which median progression-free survival was 1.7 months and overall survival was 6.2 months (Korn and others 2008). A new IFN of clinical benefit would likely need to achieve a response rate of at least 20% to be of interest for additional study; with 1/17 responses, a type II error of ∼ 0.10 for a response rate exceeding this level was calculated (90% confidence interval = 0.1% − 25%).
Since IFNs are a component of host response to pathogens and tumors, immunomodulation has had prominence as an underlying mechanism for antitumor actions of IFNs (Dunn and others 2006; Ferrantini and others 2007). Confirming this effect, increases in β2-microglobulin, ISG-15, and the monocyte activation product neopterin (Fig. 1A–C) occurred. These ISG products may reflect increased innate immunity or are ones that are components of the MHC complex, which also increases with IFNs (Goldstein and others 1989; Borden and others 2007). Although absence of detectable levels can always reflect lability or technical factors, no significant increases were identifiable in the proteins of nonspecific immune cell activation (IL-1α, IL-β, or CSF-GM) or specific immunity (IL-2, IL-6, IL-17, or IFN-γ). Other studies have failed to detect changes in the soluble IL-2R or T cell subsets in patients in response to IFN-β (Ravandi and others 1999; Reder and others 2008). In support, however, of immunomodulatory actions were also induction of the dendritic cell and T cell chemoattractants, CCL8 and CCL20.
Apoptosis and antiproliferative effects, particularly with IFN-β, have been recognized in vitro in an increasing diversity of cell lines, including melanoma, colorectal, and bronchogenic carcinomas (Chawla-Sarkar and others 2002, 2003). Even when genes were induced by IFN-α2, IFN-β on a comparative basis was almost always more potent in inducing expression (Der and others 1988; Leaman and others 2003). Prominent among these were genes whose products induce apoptosis, including TRAIL, which was confirmed subsequently as contributing to apoptosis in melanoma cells after IFN-β (Chawla-Sarkar and others 2002, 2003; Reder and others 2008). In contrast to IFN-α2 (Ji and others 2003; Zimmer and others 2008), with IFN-β, TRAIL increased in serum of almost all treated patients (Fig. 2A) with the highest level occurred in the patient who had the sustained tumor regression.
However, TRAILR1 may be silenced by methylation of its promoter (Bae and others 2008). Resistance to TRAIL has been attributed to expression of decoy receptors or to overexpression of inhibitory proteins (Ashkenazi 2008). Our patients had no evidence for change in expression of receptor TRAIL- R1 by qRT-PCR or for the decoy receptor TRAIL-R4 by ELISA (Wicovsky and others 2005).
A marked reduction in the level of the proangiogenic chemokine, CXCL5, was identified (Fig. 4F). This reduction in CXCL5 was in accord with reduction of secretion by monocytes in vitro (Schnyder-Candrian and others 1995). Furthermore, although relatively of less magnitude, possibly reflecting its longer serum t1/2, a reduction in serum VEGF-A by 28 days resulted from IFN-β (Fig. 4), as with IFN-α2 (Yurkovetsky and others 2007). Post-translational mechanisms that decrease expression of VEGF and potentially CXCL5 by IFNs have begun to be elucidated (Ray and others 2009). Consistent with post-translational inhibition was a lack of decrease (or increase) in specific gene transcripts for VEGF (data not shown). Conversely, the anti-angiogenic CXCL10 was potently increased (Fig. 4C). CXCL10 has both direct inhibitory effects on endothelial cell proliferation and may suppress VEGF expression in murine breast carcinomas (Angiolillo and others 1995; Feldman and others 2006; Taylor and other 2008; Aronica and others 2009).
In contrast to other studies (Ji and others 2003; Zimmerer and others 2008; Rani and others 2009), our focus was protein products of ISGs. However, transcriptional upregulation of specific mRNA for 4 of the assessed protein products in serum was confirmed by qRT-PCR (Table 2). Several other ISGs, often identified in vitro (Der and others 1998; Leaman and others 2003; Borden and others 2007), were confirmed as also increased in patients; these included IFI27, G1P3, XAF1, IRF7, IL22RA, TRAIL, BST2, AIM2, CCL7, FASL, and IFITM2 (Table 2). Each of the gene products increased by IFN-β, whether protein or RNA has at least 1 consensus sequence for STAT1 and/or IRF1 within its promoter region predicted by a transcription factor analysis tool (www.sabiosciences.com). The only exception to this was CXCL8, which has no STAT1 or IRF1 site within its promoter region. Furthermore, like CXCL5, production of CXCL8 by stimulated human monocytes was decreased rather than increased by an IFN-α and by IFN-γ (Schnyder-Candrian and others 1995; Strieter and others 2004). Since CXCL8 is secreted by melanoma cells and increases in progression of metastatic melanoma (Ugurel and others 2001; Brennecke and others 2005), its rise (Fig. 4E) probably thus reflected disease progression rather than induction by IFN-β1a.
IFNs have potent and pleiotropic gene regulatory effects in melanoma, antitumor activity for syngeneic murine melanomas, human melanoma xenografts, and effectiveness in patients with melanoma primaries resected primaries at high risk for recurrence (Borden and others 2007; Ascierto and Kirkwood 2008). They thus remain a lead for continuing investigation of therapeutic use with isoforms, long-acting formulations, or as part of combinations. Identifying and dissecting the relative role of the many genes that modulate pleiotropic effects of IFNs remains largely unresolved (Borden and Williams 2010). Understanding of actions of these gene products regulated by IFNs will remain critical to improved clinical use.
Acknowledgments
Contributions of Ms. Denise Robson to clinical data verification and MerckSerono (Andrew Galazka, M.D., Geneva) to supply IFN-β1a are gratefully recognized. The cytokine data were generated using Luminex multi-analyte technology in collaboration with Tracey L. Bonfield, Ph.D., D(ABMLI), from the Inflammatory Mediator CORE, Case Western Reserve University. The study was supported by RO1CA090914 and the Taussig Cancer Institute.
Author Disclosure Statement
No competing financial interests exist.
References
- Abdi EA. Tan YH. McPherson TA. Natural human interferon-beta in metastatic malignant melanoma. A phase II study. Acta Oncol. 1988;27(6b):815–817. doi: 10.3109/02841868809094364. [DOI] [PubMed] [Google Scholar]
- Angiolillo AL. Sgadari C. Taub DD. Liao F. Farber JM. Maheshwari S. Kleinman HK. Reaman GH. Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182(1):155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica SM. Raiber L. Hanzly M. Kisela C. Antitumor/antiestrogenic effect of the chemokine interferon inducible protein 10 (IP-10) involves suppression of VEGF expression in mammary tissue. J Interferon Cytokine Res. 2009;29(2):83–92. doi: 10.1089/jir.2008.0034. [DOI] [PubMed] [Google Scholar]
- Ascierto PA. Kirkwood JM. Adjuvant therapy of melanoma with interferon: lessons of the past decade. J Transl Med. 2008;6:62. doi: 10.1186/1479-5876-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7(12):1001–1012. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- Bae SI. Cheriyath V. Jacobs BS. Reu FJ. Borden EC. Reversal of methylation silencing of Apo2L/TRAIL receptor 1 (DR4) expression overcomes resistance of SK-MEL-3 and SK-MEL-28 melanoma cells to interferons (IFNs) or Apo2L/TRAIL. Oncogene. 2008;27(4):490–498. doi: 10.1038/sj.onc.1210655. [DOI] [PubMed] [Google Scholar]
- Balch CM. Gershenwald JE. Soong SJ. Thompson JF. Atkins MB. Byrd DR. Buzaid AC. Cochran AJ. Coit DG. Ding S. Eggermont AM. Flaherty KT. Gimotty PA. Kirkwood JM. McMasters KM. Mihm MC., Jr Morton DL. Ross MI. Sober AJ. Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden EC. Sen GC. Uze G. Silverman RH. Ransohoff RM. Foster GR. Stark GR. Interferons at age 50: Past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6(12):975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden EC. Williams BR. Interferon-stimulated genes and their protein products: What and how? J Interferon Cytokine Res. 2011;31(1):1–4. doi: 10.1089/jir.2010.0129. [DOI] [PubMed] [Google Scholar]
- Borden EC. Review: Milstein Award lecture: Interferons and cancer: Where from here? J Interferon Cytokine Res. 2005;25(9):511–527. doi: 10.1089/jir.2005.25.511. [DOI] [PubMed] [Google Scholar]
- Brennecke S. Deichmann M. Naeher H. Kurzen H. Decline in angiogenic factors, such as interleukin-8, indicates response to chemotherapy of metastatic melanoma. Melanoma Res. 2005;15(6):515–522. doi: 10.1097/00008390-200512000-00006. [DOI] [PubMed] [Google Scholar]
- Buchwalder PA. Buclin T. Trinchard I. Munafo A. Biollaz J. Pharmacokinetics and pharmacodynamics of IFN-beta 1a in healthy volunteers. J Interferon Cytokine Res. 2000;20(10):857–866. doi: 10.1089/10799900050163226. [DOI] [PubMed] [Google Scholar]
- Chawla-Sarkar M. Leaman DW. Borden EC. Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: Correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res. 2001;7(6):1821–1831. [PubMed] [Google Scholar]
- Chawla-Sarkar M. Leaman DW. Jacobs BS. Borden EC. IFN-beta pretreatment sensitizes human melanoma cells to TRAIL/Apo2 ligand-induced apoptosis. J Immunol. 2002;169(2):847–855. doi: 10.4049/jimmunol.169.2.847. [DOI] [PubMed] [Google Scholar]
- Chawla-Sarkar M. Lindner DJ. Liu YF. Williams BR. Sen GC. Silverman RH. Borden EC. Apoptosis and interferons: Role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8(3):237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- D'Cunha J. Ramanujam S. Wagner RJ. Witt PL. Knight E., Jr. Borden EC. In vitro and in vivo secretion of human ISG15, an IFN-induced immunomodulatory cytokine. J Immunol. 1996;157(9):4100–4108. [PubMed] [Google Scholar]
- de Veer MJ. Holko M. Frevel M. Walker E. Der S. Paranjape JM. Silverman RH. Williams BR. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69(6):912–920. [PubMed] [Google Scholar]
- Der SD. Zhou A. Williams BR. Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1988;95(26):15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME. Yang JC. Sherry R. Hughes MS. Royal R. Kammula U. Robbins PF. Huang J. Citrin DE. Leitman SF. Wunderlich J. Restifo NP. Thomasian A. Downey SG. Smith FO. Klapper J. Morton K. Laurencot C. White DE. Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–52339. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP. Koebel CM. Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6(11):836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- Falschlehner C. Schaefer U. Walczak H. Following TRAIL's path in the immune system. Immunology. 2009;127(2):145–154. doi: 10.1111/j.1365-2567.2009.03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman ED. Weinreich DM. Carroll NM. Burness ML. Feldman AL. Turner E. Xu H. Alexander HR., Jr. Interferon gamma-inducible protein 10 selectively inhibits proliferation and induces apoptosis in endothelial cells. Ann Surg Oncol. 2006;13(1):125–133. doi: 10.1245/ASO.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Ferrantini M. Capone I. Belardelli F. Interferon-alpha and cancer: Mechanisms of action and new perspectives of clinical use. Biochimie. 2007;89(6–7):884–893. doi: 10.1016/j.biochi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Goldstein D. Sielaff KM. Storer BE. Brown RR. Datta SP. Witt PL. Teitelbaum AP. Smalley RV. Borden EC. Human biologic response modification by interferon in the absence of measurable serum concentrations: A comparative trial of subcutaneous and intravenous interferon-beta serine. J Natl Cancer Inst. 1989;81(14):1061–1068. doi: 10.1093/jnci/81.14.1061. [DOI] [PubMed] [Google Scholar]
- Gomi K. Morimoto M. Nakamizo N. Antitumor effect of human recombinant interferon-beta against human melanomas transplanted into nude mice. J Pharmacobiodyn. 1984;7(12):951–961. doi: 10.1248/bpb1978.7.951. [DOI] [PubMed] [Google Scholar]
- Griffith TS. Chin WA. Jackson GC. Lynch DH. Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161(6):2833–2840. [PubMed] [Google Scholar]
- Honda K. Takaoka A. Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25(3):349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Honda K. Yanai H. Takaoka A. Taniguchi T. Regulation of the type I IFN induction: A current view. Int Immunol. 2005;17(11):1367–1378. doi: 10.1093/intimm/dxh318. [DOI] [PubMed] [Google Scholar]
- Hosseini-Moghaddam SM. Mousavi A. Alavian SM. Is {beta}-interferon a promising therapeutic option for the management of hepatitis C? J Antimicrob Chemother. 2009;63(6):1097–1103. doi: 10.1093/jac/dkp092. [DOI] [PubMed] [Google Scholar]
- Ida N. Uenishi N. Kajita A. Satoh Y. Antitumor effect of human fibroblast interferon on the growth of human melanoma cells implanted in nude mice. Gann. 1982;73(6):952–960. [PubMed] [Google Scholar]
- Ikeda K. Arase Y. Saitoh S. Kobayashi M. Suzuki Y. Suzuki F. Tsubota A. Chayama K. Murashima N. Kumada H. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32(2):228–232. doi: 10.1053/jhep.2000.9409. [DOI] [PubMed] [Google Scholar]
- Ji X. Cheung R. Cooper S. Li Q. Greenberg HB. He XS. Interferon alfa regulated gene expression in patients initiating interferon treatment for chronic hepatitis C. Hepatology. 2003;37(3):610–621. doi: 10.1053/jhep.2003.50105. [DOI] [PubMed] [Google Scholar]
- Johns TG. Mackay IR. Callister KA. Hertzog PJ. Devenish RJ. Linnane AW. Antiproliferative potencies of interferons on melanoma cell lines and xenografts: higher efficacy of interferon beta. J Natl Cancer Inst. 1992;84(15):1185–1190. doi: 10.1093/jnci/84.15.1185. [DOI] [PubMed] [Google Scholar]
- Kirkwood JM. Bender C. Agarwala S. Tarhini A. Shipe-Spotloe J. Smelko B. Donnelly S. Stover L. Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. J Clin Oncol. 2002;20(17):3703–3718. doi: 10.1200/JCO.2002.03.052. [DOI] [PubMed] [Google Scholar]
- Kirkwood JM. Strawderman MH. Ernstoff MS. Smith TJ. Borden EC. Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14(1):7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- Korn EL. Liu PY. Lee SJ. Chapman JA. Niedzwiecki D. Suman VJ. Moon J. Sondak VK. Atkins MB. Eisenhauer EA. Parulekar W. Markovic SN. Saxman S. Kirkwood JM. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- Krasagakis K. Garbe C. Krüger S. Orfanos CE. Effects of interferons on cultured human melanocytes in vitro: Interferon-beta but not-alpha or -gamma inhibit proliferation and all interferons significantly modulate the cell phenotype. J Invest Dermatol. 1991;97(2):364–372. doi: 10.1111/1523-1747.ep12480767. [DOI] [PubMed] [Google Scholar]
- Leaman DW. Chawla-Sarkar M. Jacobs B. Vyas K. Sun Y. Ozdemir A. Yi T. Williams BR. Borden EC. Novel growth and death related interferon-stimulated genes (ISGs) in melanoma: Greater potency of IFN-beta compared with IFN-alpha2. J Interferon Cytokine Res. 2003;23(12):745–756. doi: 10.1089/107999003772084860. [DOI] [PubMed] [Google Scholar]
- Masci P. Olencki T. Wood L. Rybicki L. Jacobs B. Williams B. Faber P. Bukowski R. Tong K. Borden EC. Gene modulatory effects, pharmacokinetics, and clinical tolerance of interferon-alpha1b: A second member of the interferon-alpha family. Clin Pharmacol Ther. 2007;81(3):354–361. doi: 10.1038/sj.clpt.6100081. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Bielenberg DR. Nilsson MB. Gershenwald JE. Barnhill RL. Ahearne P. Bucana CD. Fidler IJ. Epidermal hyperplasia overlying human melanoma correlates with tumour depth and angiogenesis. Melanoma Res. 2003;13(4):379–387. doi: 10.1097/00008390-200308000-00007. [DOI] [PubMed] [Google Scholar]
- Pestka S. Krause CD. Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Rani MR. Xu Y. Lee JC. Shrock J. Josyula A. Schlaak J. Chakraborthy S. Ja N. Ransohoff RM. Rudick RA. Heterogeneous, longitudinally stable molecular signatures in response to interferon-beta. Ann N Y Acad Sci. 2009;1182:58–68. doi: 10.1111/j.1749-6632.2009.05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravandi F. Estrov Z. Kurzrock R. Breitmeyer JB. Maschek BJ. Talpaz M. A phase I study of recombinant interferon-beta in patients with advanced malignant disease. Clin Cancer Res. 1999;5(12):3990–3998. [PubMed] [Google Scholar]
- Ray PS. Jia J. Yao P. Majumder M. Hatzoglou M. Fox PL. A stress-responsive RNA switch regulates VEGFA expression. Nature. 2009;457(7231):915–919. doi: 10.1038/nature07598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reder AT. Velichko S. Yamaguchi KD. Hamamcioglu K. Ku K. Beekman J. Wagner TC. Perez HD. Salamon H. Croze E. IFN-beta1b induces transient and variable gene expression in relapsing-remitting multiple sclerosis patients independent of neutralizing antibodies or changes in IFN receptor RNA expression. J Interferon Cytokine Res. 2008;28(5):317–331. doi: 10.1089/jir.2007.0131. [DOI] [PubMed] [Google Scholar]
- Ruzicka FJ. Jach ME. Borden EC. Binding of recombinant-produced interferon beta ser to human lymphoblastoid cells. Evidence for two binding domains. J Biol Chem. 1987;262(33):16142–16149. [PubMed] [Google Scholar]
- Ryuke Y. Mizuno M. Natsume A. Suzuki O. Nobayashi M. Kageshita T. Matsumoto K. Saida T. Yoshida J. Growth inhibition of subcutaneous mouse melanoma and induction of natural killer cells by liposome-mediated interferon-beta gene therapy. Melanoma Res. 2003;13(4):349–356. doi: 10.1097/00008390-200308000-00003. [DOI] [PubMed] [Google Scholar]
- Sarna GP. Figlin RA. Pertcheck M. Phase II study of betaseron (beta ser17-interferon) as treatment of advanced malignant melanoma. J Biol Response Mod. 1987;6(4):375–378. [PubMed] [Google Scholar]
- Schiller JH. Willson JK. Bittner G. Wolberg WH. Hawkins MJ. Borden EC. Antiproliferative effects of interferons on human melanoma cells in the human tumor colony-forming assay. J Interferon Res. 1986;6(6):615–625. doi: 10.1089/jir.1986.6.615. [DOI] [PubMed] [Google Scholar]
- Schnyder-Candrian S. Strieter RM. Kunkel SL. Walz A. Interferon-alpha and interferon-gamma down-regulate the production of interleukin-8 and ENA-78 in human monocytes. J Leukoc Biol. 1995;57(6):929–935. doi: 10.1002/jlb.57.6.929. [DOI] [PubMed] [Google Scholar]
- Schonfeld A. Nitke S. Schattner A. Wallach D. Crespi M. Hahn T. Levavi H. Yarden O. Shoham J. Doerner T, et al. Intramuscular human interferon-beta injections in treatment of condylomata acuminata. Lancet. 1984;1(8385):1038–1042. doi: 10.1016/s0140-6736(84)91450-8. [DOI] [PubMed] [Google Scholar]
- Sidky YA. Borden EC. Inhibition of angiogenesis by interferons: effects on tumor- and lymphocyte-induced vascular responses. Cancer Res. 1987;47(19):5155–5161. [PubMed] [Google Scholar]
- Strieter RM. Starko KM. Enelow RI. Noth I. Valentine VG. Effects of interferon-gamma 1b on biomarker expression in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2004;170(2):133–140. doi: 10.1164/rccm.200312-1670OC. Idiopathic Pulmonary Fibrosis Biomarkers Study Group. [DOI] [PubMed] [Google Scholar]
- Stroncek DF. Basil C. Nagorsen D. Deola S. Aricó E. Smith K. Wang E. Marincola FM. Panelli MC. Delayed polarization of mononuclear phagocyte transcriptional program by type I interferon isoforms. J Transl Med. 2005;3:24. doi: 10.1186/1479-5876-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KL. Leaman DW. Grane R. Mechti N. Borden EC. Lindner DJ. Identification of interferon-beta-stimulated genes that inhibit angiogenesis in vitro. J Interferon Cytokine Res. 2008;28(12):733–740. doi: 10.1089/jir.2008.0030. [DOI] [PubMed] [Google Scholar]
- Ugurel S. Rappl G. Tilgen W. Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19(2):577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- Wicovsky A. Siegmund D. Wajant H. Interferons induce proteolytic degradation of TRAILR4. Biochem Biophys Res Commun. 2005;337(1):184–190. doi: 10.1016/j.bbrc.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Yurkovetsky ZR. Kirkwood JM. Edington HD. Marrangoni AM. Velikokhatnaya L. Winans MT. Gorelik E. Lokshin AE. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13(8):2422–2228. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
- Zimmerer JM. Lesinski GB. Ruppert AS. Radmacher MD. Noble C. Kendra K. Walker MJ. Carson WE., 3rd. Gene expression profiling reveals similarities between the in vitro and in vivo responses of immune effector cells to IFN-alpha. Clin Cancer Res. 2008;14(18):5900–5906. doi: 10.1158/1078-0432.CCR-08-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]