Abstract
Integrated vector management is a pillar of the South Asian visceral leishmaniasis (VL) elimination program, but the best approach remains a matter of debate. Sand fly seasonality was determined in 40 houses sampled monthly. The impact of interventions on Phlebotomus argentipes density was tested from 2006–2007 in a cluster-randomized trial with four arms: indoor residual spraying (IRS), insecticide-treated nets (ITNs), environmental management (EVM), and no intervention. Phlebotomus argentipes density peaked in March with the highest proportion of gravid females in May. The EVM (mud plastering of wall and floor cracks) showed no impact. The IRS and ITNs were associated with a 70–80% decrease in male and female P. argentipes density up to 5 months post intervention. Vector density rebounded by 11 months post-IRS, whereas ITN-treated households continued to show significantly lower density compared with households without intervention. Our data suggest that both IRS and ITNs may help to improve VL control in Bangladesh.
Introduction
In 2005, the governments of India, Bangladesh, and Nepal signed a memorandum of understanding stating their commitment to the elimination of visceral leishmaniasis (VL) as a public health problem in the subcontinent.1 The Indian subcontinent contains the largest VL focus in the world, with an estimated annual incidence of 200,000–300,000 clinical cases and more than 100 million people at risk.2,3 In this region, the disease is caused by the protozoan parasite Leishmania donovani and transmitted by the sand fly Phlebotomus argentipes.4 The most severe clinical syndrome is also called kala-azar (“black fever”) and is characterized by fever, wasting, hepatosplenomegaly, hemorrhagic, and infectious complications, and more than 90% case fatality in the absence of treatment.4,5
Integrated vector management is one of the stated pillars of the South Asian VL elimination program, but the best approach to effective, sustained vector control is still a matter of debate. In addition, centralized vector control activities in Bangladesh suffer from lack of coordination and unreliable procurement of insecticide.6 Insecticide-treated nets (ITNs) may provide an alternative or adjunct to centrally controlled indoor residual spraying (IRS) for vector control. The major aim of this study was to compare the entomological effect of IRS, ITNs, and environmental management to a control group with no intervention. Monthly sand fly density data in the absence of vector control were collected previously from one of the study communities and are presented to provide a context for the vector intervention data.
Methods
Monthly sand fly collections 2002–2003.
From January 2002 to April 2004, an epidemiologic study was carried out in one village in Fulbaria subdistrict, Mymensingh District, chosen on the basis of a reported high incidence of VL in government surveillance data.7 The household census conducted for the epidemiological study was used as the basis for a random stratified sample of houses for entomological sampling. On the basis of the census, the study area consisted of 506 houses in three paras (neighborhoods). The largest para was divided in half geographically, giving four strata containing between 108 and 141 houses; 10 houses were then selected at random from each stratum. Sand flies were collected two nights per month from each of the 40 houses from September 2002 to August 2003. American Biophysics Corp. (ABC) portable light traps (Clarke Mosquito Control, Chicago, IL) were hung at dusk in a corner inside the house 8 to 15 cm above the floor and a minimum of 5 cm from the nearest wall. The traps were collected the following morning, the flies collected from the inside of the traps by a mechanical aspirator, killed using chloroform-soaked cotton, and preserved in 70% ethanol. Male and female sand flies were separated in the field office. Sand flies were dissected to identify the genus (Phlebotomus or other). Monthly climate data for 2002–2003 were collected from the district weather station in Mymensingh.
Cluster randomized trial of vector intervention methods.
The current study is one of four parallel studies in India, Nepal, and Bangladesh that used similar methods and design.8 The intervention trial was conducted in Fulbaria subdistrict, Mymensingh District, from October 2006 to September 2007. Sand flies were collected before and after introduction of the interventions and compared with the control area. The study villages were chosen on the basis of the incidence of kala-azar in the previous 3 years of Bangladesh national passive surveillance data (Director General Health Services). The passive surveillance system reports cases treated in the government health facilities.
The necessary sample size was estimated through simulations using data from the 2002 to 2003 monthly sand fly collections described previously. The expected effect of the intervention was included in the simulation by decreasing the observed number of flies in the simulated intervention households by 70%. Sand fly distributions were assumed to follow a negative binomial regression.8,9 The minimum sample size to provide 80% power and 95% significance was estimated to be six clusters per arm.
Village and household selection.
Four villages were randomly selected from the 20 villages with the highest VL incidence and a minimum of 300 households. Each selected village was then divided into six geographic clusters of at least 50 houses with 50 meters of distance between clusters. All houses in the 24 selected clusters were allocated to a vector control intervention arm or the control arm (no intervention). Five households were selected from each of the clusters by simple random sampling, yielding a subset of 120 houses that participated in the vector collection. Each household was assigned a code indicating the subdistrict, village, cluster, and house, which was recorded on the door of the house.
Baseline insecticide susceptibility testing.
Pre-intervention deltamethrin susceptibility testing was conducted using sand flies collected from randomly selected households. Susceptibility was tested in tube bioassays, using the World Health Organization Pesticide Evaluation Scheme (WHOPES) standard chamber method.10 In each assay, 15–20 unfed, non-gravid female P. argentipes were introduced into a susceptibility chamber lined with paper impregnated with 0.05% deltamethrin. Five replicates with 15–20 flies each and one control with unimpregnated paper and 20 P. argentipes were run. After 1 hour of exposure, percentage knockdown was recorded before the P. argentipes were taken out of the test chamber, placed into a 150-mL paper cup that was covered with netting, and maintained for 24 hours at 27 ± 2°C and 80% ± 10% relative humidity, with a small cotton-wool swab soaked in 10% (w/v) sucrose solution placed on the netting top. Percentage mortality was recorded 24 hours post exposure.
Sand fly collections.
Sand fly collections were conducted using ABC light traps and followed the same methods described for the 2002–2003 study. Trapping occurred on two consecutive nights in each house for each collection cycle. The pre-intervention vector collections were carried out in October 2006. Post-intervention collections were conducted in December 2006, January, March, April, and October 2007. Morphological sex and species identification (P. argentipes versus other species) was carried out using a phase contrast microscope and based on a standard sand fly identification manual.11
Household assignment and vector interventions.
On the basis of the baseline collections carried out in October 2006, each cluster was identified as high, intermediate, or low density. Assignment to intervention arms was then stratified by the average vector density to provide comparable vector density distribution in each arm: (A) indoor residual spray (IRS), (B) long-lasting insecticide-treated nets (ITNs), (C) environmental management (EVM), and (D) control (see Table 1). Pre-intervention evaluations showed that 90% of households owned and used a locally manufactured bed net, and that 89% of households used mud plastering of their walls and floors.
Table 1.
Number of study households by geographic cluster and stratum of sand fly density in the baseline survey, Fulbaria, Mymensingh, Bangladesh, October 2006
| Cluster | IRS* | ITN† | EVM‡ | CON§ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Households in each stratum | Households in each stratum | Households in each stratum | Households in each stratum | |||||||||
| High¶ | Med | Low | High | Med | Low | High | Med | Low | High | Med | Low | |
| 1 | 5 | 5 | 5 | 5 | ||||||||
| 2 | 5 | 5 | 5 | 5 | ||||||||
| 3 | 5 | 5 | 5 | 5 | ||||||||
| 4 | 5 | 5 | 5 | 5 | ||||||||
| 5 | 5 | 5 | 5 | 5 | ||||||||
| 6 | 5 | 5 | 5 | 5 | ||||||||
Arm A: indoor residual spraying.
Arm B: insecticide-treated nets.
Arm C: environmental management.
Arm D: control.
Stratum definitions: high 131–182, medium 97–125, low 37–95 sand flies per trap night.
The IRS was carried out using deltamethrin (K-Othrine 5%, Aventis Bayer, Bayer CropScience AG, Monheim am Rhein, Germany) with a target concentration of 20 mg active ingredient/m2. Spray teams were locally hired by the research team and given 2 days of training by the investigators. Household contents were removed before spraying. The insecticide was applied with Hudson Expert pumps (H.D. Hudson Manufacturing Company, Chicago, IL) to the interior and exterior walls, targeting cracks and crevices in and around peridomestic sites. Insecticide was applied to the inside surfaces of all structures (human dwelling, animal sheds, and other peridomestic structures). All houses in the selected clusters were sprayed.
The ITN used in the study was the PermaNet 2.0 (second generation, Vestergaard Frandsen, Lausanne, Switzerland) made of polyester blended with deltamethrin 55 mg/m2. Sufficient ITNs were distributed to all houses in the selected clusters to provide space for all family members to sleep under a net. Two sizes (160 × 180 × 150 cm and 100 × 180 × 150 cm) of ITNs were used, depending upon the number of family members and sleeping pattern. A total of 489 nets (216 double size and 273 single size) were distributed to 296 households (mean 1.65 nets per household).
For the environmental management arm, the research team trained community mobilizers who conducted weekly home visits and educated household members. The major activity was filling cracks and crevices in the walls and floors of human dwellings, detached kitchens, cattle sheds, and other structures such as cattle troughs with mud plaster. In addition, the team promoted cleaning up debris from the environment. Household incentives were offered, consisting of a pen, pencil, and notebook for children attending school, or soap if there were no schoolchildren in the household. Houses were visited monthly to encourage compliance.
Statistical analysis.
Poisson regression models were used to examine the effects of treatments on the rates of sand flies trapped at the different collection times. Male and female P. argentipes sand flies were modeled separately as well as combined, for a total of three models. In all models, the outcome of interest was the mean number of sand flies trapped per household per collection time, and a random effect was included to account for the correlation between observations from the same households over time. A random effect allowing observations from households within the same cluster to be correlated was also included. Treatment arms IRS, ITN, and EVM were compared with the control arm at baseline and at the four post-intervention collection times using rate ratios calculated from the three models. Rate ratios were also used to compare the IRS and ITN treatment arms at the different collection times. All analyses were performed using the GLIMMIX procedure in SAS 9.2 (SAS Institute Inc., Cary, NC).
Ethical approvals and informed consent.
The 2002–2003 sand fly collections were carried out under a study protocol approved by Centers for Disease Control and Prevention (CDC) and International Center for Diarrheal Disease Research, Bangladesn (ICDDR,B) ethics review committees. Written informed consent was obtained from the head of each participating household. The 2006–2007 vector control intervention protocol was approved by the Ethical Review Committee of WHO/Special Program for Research and Training in Tropical Diseases (TDR) and the Bangladesh Medical Research Council. Written informed consent was obtained from the head of each participating household. After completion of the intervention study all 300 households in the control arm received a PermaNet ITN.
Results
Seasonality data.
A total of 17,189 Phlebotomus spp. (8,985 male and 8,204 female including 3,037 gravid and 247 blood-fed females) were trapped during the monthly collections of 2002–2003. In addition, 1,838 Sergentomyia spp. flies (1,237 female and 601 male) were collected. In March, the peak month, a mean of 50 Phlebotomus spp. were captured per house; more than 10 Phlebotomus spp. were trapped per house every month except December through February, when ambient temperatures were lowest (Figure 1A and B). The peak of female Phlebotomus spp. density occurred in March, but the highest proportion gravid was seen in May 2003.
Figure 1.
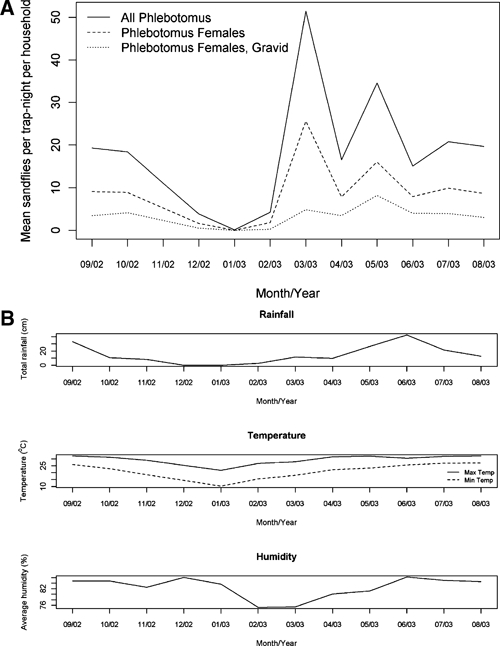
(A) Sand fly density per household trap night by month from September 2002 to August 2003. (B) Mean minimum and maximum temperature, humidity, and rainfall by month in Mymensingh, Bangladesh, from September 2002 to August 2003.
Comparison of vector control interventions.
The households chosen for sand fly trapping in each arm of the trial were evenly distributed among sand fly density strata (Table 1). In the baseline insecticide susceptibility testing, the sand fly knockdown rate at 1 hour was 88%, and mortality at 24 hours was 100%. On the basis of the initial trapping, the strata were defined as high (131–182), medium (97–125), and low (37–95) sand flies per trap night. Sand fly density in the baseline survey in October 2006 was not significantly different between arms (P = 0.82). Of 9,766 sand flies trapped over the course of the intervention study, 8,295 (84.9%) were identified as P. argentipes, 900 (9.2%) as other Phlebotomus spp., and 571 (5.8%) as Sergentomyia spp. (Table 2). Among P. argentipes, 3,877 (46.7%) were female, including 967 (24.9%) gravid females and 32 (0.8%) blood-fed females.
Table 2.
Sand flies collected in all study households by species, sex, gravidity, feeding status, and month of collection, Fulbaria, Mymensingh, Bangladesh. Interventions instituted November 2006
| Species | Gender | Status | Month of collection | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Oct 2006 | Jan 2007 | Mar 2007 | Apr 2007 | Oct 2007 | ||||
| Phlebotomus argentipes | ||||||||
| Female | 1163 | 47 | 1237 | 622 | 808 | 3877 | ||
| Gravid | 398 | 4 | 99 | 193 | 273 | 967 | ||
| Fed | 14 | 0 | 4 | 1 | 13 | 32 | ||
| Neither | 751 | 43 | 1134 | 428 | 522 | 2878 | ||
| Male | 1378 | 70 | 1296 | 764 | 910 | 4418 | ||
| Total | 2541 | 117 | 2533 | 1386 | 1718 | 8295 | ||
| Sergentomyia spp. | 80 | 6 | 131 | 296 | 58 | 571 | ||
| Other Phlebotomus spp. | 81 | 0 | 155 | 558 | 106 | 900 | ||
| Total | 2702 | 123 | 2819 | 2240 | 1882 | 9766 | ||
The absolute numbers, species, and status of sand flies collected in the households in each arm of the study are shown in Table 3 A and D. In March and April 2007, indoor residual spraying and ITNs were associated with statistically significant decreases in the density of both female and male P. argentipes compared with the control arm (Figure 2A and B, and Table 4A and B; rate ratios ranging from 0.22 to 0.31 for females and 0.20 to 0.21 for males). Within the ITN arm, there was no difference in the rate ratio for female versus male P. argentipes for the first three post-intervention time points, but the rate ratio was lower for male than female P. argentipes in October 2007 (P = 0.04). There was no significant difference in P. argentipes density between the IRS and ITN arms at any time point from October 2006 through April 2007. In October 2007 (11 months post intervention), P. argentipes density remained significantly lower in the ITN arm than in the control arm (rate ratios 0.47 for females and 0.34 for males). However, the rate ratio for the IRS arm was greater than 1.0, indicating a significantly higher density of flies compared with the control arm. In October 2007, the density in the IRS arm was also significantly higher than in the ITN arm. The proportion of gravid female P. argentipes ranged from 28% to 40% in the baseline survey (Figure 3). Post intervention, the proportion of gravid returned to baseline levels in the IRS, EVM, and control arms, but remained below 20% in the ITN arm.
Table 3A.
Sand flies collected in households assigned to indoor residual spraying (IRS) intervention arm, by species, sex, gravidity, feeding status, and month of collection, Fulbaria, Mymensingh, Bangladesh. Interventions instituted November 2006
| Species | Gender | Status | Month of collection | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Oct 2006 | Jan 2007 | Mar 2007 | Apr 2007 | Oct 2007 | ||||
| Phlebotomus argentipes | ||||||||
| Female | 276 | 6 | 145 | 64 | 305 | 796 | ||
| Gravid | 100 | 0 | 19 | 19 | 92 | 230 | ||
| Fed | 2 | 0 | 0 | 0 | 4 | 6 | ||
| Neither | 174 | 6 | 126 | 45 | 209 | 560 | ||
| Male | 319 | 1 | 116 | 65 | 302 | 803 | ||
| Total | 595 | 7 | 261 | 129 | 607 | 1599 | ||
| Sergentomyia spp. | 22 | 1 | 15 | 39 | 16 | 93 | ||
| Other Phlebotomus spp. | 16 | 0 | 9 | 54 | 21 | 100 | ||
| Total | 633 | 8 | 285 | 222 | 644 | 1792 | ||
Table 3D.
Sand flies collected in households assigned to the control (CON) arm, by species, sex, gravidity, feeding status, and month of collection, Fulbaria, Mymensingh, Bangladesh. Interventions instituted November 2006
| Species | Gender | Status | Month of collection | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Oct 2006 | Jan 2007 | Mar 2007 | Apr 2007 | Oct 2007 | ||||
| Phlebotomus argentipes | ||||||||
| Female | 277 | 16 | 525 | 295 | 181 | 1294 | ||
| Gravid | 110 | 2 | 44 | 99 | 63 | 318 | ||
| Fed | 2 | 0 | 0 | 0 | 4 | 6 | ||
| Neither | 165 | 14 | 481 | 196 | 114 | 970 | ||
| Male | 355 | 35 | 615 | 357 | 228 | 1590 | ||
| Total | 632 | 51 | 1140 | 652 | 409 | 2884 | ||
| Sergentomyia spp. | 24 | 3 | 37 | 48 | 10 | 122 | ||
| Other Phlebotomus spp. | 27 | 0 | 42 | 211 | 32 | 312 | ||
| Total | 683 | 54 | 1219 | 911 | 451 | 3318 | ||
Figure 2.
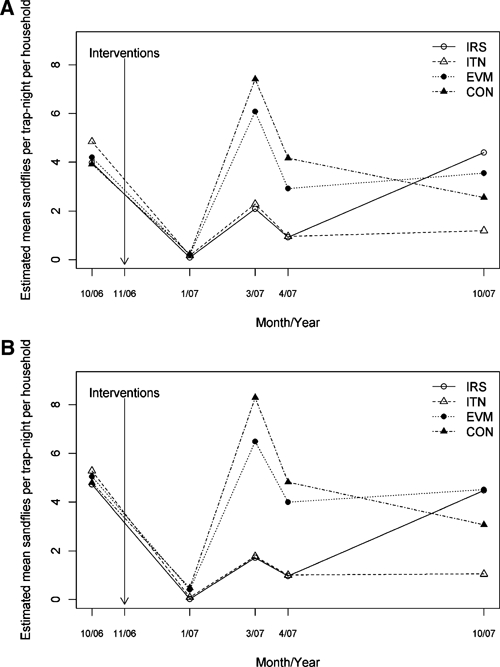
Mean household density of (A) female and (B) male Phlebotomus argentipes in each study arm before the intervention (October 2006) and at four points in time post intervention (January, March, April, and October 2007). IRS = indoor residual spraying; ITN = insecticide-treated net; EVM = environmental management; CON = control arm.
Table 4A.
Change in number of female Phlebotomus argentipes collected in households in each intervention arm compared with control arm expressed as rate ratios by month of collection, Fulbaria, Mymensingh, Bangladesh. Interventions instituted November 2006
| Month | IRS* | ITN | EVM |
|---|---|---|---|
| Rate ratio (95% CI) | Rate ratio (95% CI) | Rate ratio (95% CI) | |
| Oct 2006 | 1.02 (0.69–1.50) | 1.24 (0.84–1.82) | 1.07 (0.73–1.58) |
| Jan 2007 | 0.38 (0.10–1.50) | 0.73 (0.23–2.25) | 0.91 (0.31–2.63) |
| Mar 2007 | 0.28 (0.19–0.42)† | 0.31 (0.21–0.46)† | 0.82 (0.57–1.17) |
| Apr 2007 | 0.22 (0.14–0.36)† | 0.23 (0.14–0.38)† | 0.70 (0.47–1.05) |
| Oct 2007 | 1.72 (1.15–2.58)‡ | 0.47 (0.29–0.76)† | 1.39 (0.92–2.10) |
IRS = indoor residual spraying; ITN = insecticide-treated nets; EVM = environmental management.
Difference significant at P < 0.05 with rate ratio < 1.0 (decreased sand fly density) compared with control.
Difference significant at P < 0.05 with rate ratio > 1.0 (increased sand fly density) compared with control.
Table 4B.
Change in number of male Phlebotomus argentipes collected in households in each intervention arm compared with control arm expressed as rate ratios by month of collection, Fulbaria, Mymensingh, Bangladesh. Interventions instituted November 2006
| Month | IRS* | ITN | EVM |
|---|---|---|---|
| Rate ratio (95% CI) | Rate ratio (95% CI) | Rate ratio (95% CI) | |
| Oct 2006 | 0.99 (0.68–1.43) | 1.10 (0.76–1.60) | 1.05 (0.73–1.53) |
| Jan 2007 | 0.03 (0.00–0.53)† | 0.19 (0.05–0.66)† | 0.86 (0.40–1.86) |
| Mar 2007 | 0.21 (0.14–0.31)† | 0.21 (0.14–0.32)† | 0.78 (0.55–1.11) |
| Apr 2007 | 0.20 (0.12–0.32)† | 0.21 (0.13–0.34)† | 0.83 (0.57–1.21) |
| Oct 2007 | 1.45 (0.98–2.15)‡ | 0.34 (0.21–0.55)† | 1.46 (0.99–2.16) |
IR = indoor residual spraying; ITN = insecticide-treated nets; EVM = environmental management.
Difference significant at P < 0.05 with rate ratio < 1.0 (decreased sand fly density) compared with control.
Difference significant at P < 0.05 with rate ratio > 1.0 (increased sand fly density) compared with control.
Figure 3.
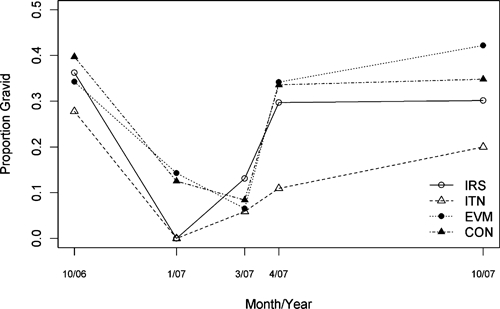
Proportion of female Phlebotomus argentipes found to be gravid in collections from each study arm before the intervention (October 2006) and at four points in time post intervention (January, March, April, and October 2007). IRS = indoor residual spraying; ITN = insecticide-treated net; EVM = environmental management; CON = control arm.
Discussion
Our seasonality data were consistent with published data from India and Nepal,12,13 showing a peak in Phlebotomus spp. density in March, with the highest proportion of gravid females in May. Because 76–100% of the Phlebotomus spp. collected at each time point in the trial households without intervention were P. argentipes (Table 3D), we believe that this pattern reflects the seasonality of the VL vector. In our intervention trial, both IRS and ITNs were associated with a 70–80% decrease in the density of P. argentipes 4 to 5 months after the intervention. Vector density had rebounded 11 months post-IRS, whereas households in the ITN arm continued to show significantly lower vector density compared with the control arm. Our data suggest that consideration should be given to the potential roles of both IRS and ITNs in improving VL control in Bangladesh.
Recent publications on vector control methods in the Indian subcontinent have presented a mixed picture with conflicting data, especially with regard to ITNs. A study of locally impregnated nets in Bangladesh showed ∼60% decrease in overall sand fly density, very similar in magnitude to the current results.14 The pooled analysis of data from the current study and its sister studies in Nepal and India showed inconsistent results for ITNs, but that analysis examined overall sand fly density rather than impact on P. argentipes.8 In the pooled data, IRS resulted in significant sand fly density reductions in all sites, whereas ITNs produced significant decreases in India and Bangladesh, but not the two Nepal sites.8 Although the EVM arm in our study, which promoted filling in cracks and crevices in the walls and floors with mud plaster showed no impact on P. argentipes density, the Indian site and one of the two Nepal sites showed some impact on overall sand fly density from the use of mud plaster mixed with lime.8 A study in Bihar, India, which examined the impact of two different brands of ITN found a small (11%) but significant decrease in household density of male P. argentipes, but no significant decrease for female P. argentipes.15 In contrast to the Bihar results, we found that the densities of both sexes of P. argentipes showed large and significant decreases compared with the control arm at every post-intervention sampling point. One methodological difference is that the Bihar study distributed nets only to the sampled households; the current study distributed nets to all 50 households in the selected clusters, not just the six sampled households.15 Yet another study conducted in sites in India and Nepal reported a 24.9% decrease in P. argentipes density resulting from cluster-wide ITN distribution; although this decline was statistically significant, it was much more modest than the 70–80% decrease seen in our data.16 It seems unlikely that the fundamental biology of P. argentipes is substantially different in Bangladesh compared with endemic areas of India and Nepal a few hundred miles to the west. More plausible explanations for differences in findings between sites include the differences in the rigor of study designs, trapping and other field work, net storage conditions, compliance with net usage, and variation in vector insecticide exposure and susceptibility.
The vector control argument is often framed in terms of IRS versus ITNs, but if the current effort to eliminate VL as a public health problem in the Indian subcontinent is to have a chance of success, it may be more productive to evaluate the possibility that the two modalities could be used in a complementary fashion. Both entomological and disease control impacts will need to be assessed. Our entomological data highlight the significant but transient effect of IRS on P. argentipes density; 11 months post-application vector density had fully rebounded. The ITN arm of the study, by contrast, still showed more than 60% lower density of P. argentipes compared with control 11 months after the nets were first hung (Table 4C). Appropriate delivery of IRS requires a technically strong, organized central program, and to date, Bangladesh has made little progress toward mounting spray campaigns.6 The ITNs can circumvent this barrier through community-based programs, but their impact on disease transmission is likely to depend on individual initiative to use them consistently, especially during the hot season (March–June) when sand fly populations and the proportion gravid of females are at their highest, and when the association of bed net use with protection from kala-azar is strongest.7
Table 4C.
Change in number of Phlebotomus argentipes (both sexes) collected in households in each intervention arm compared with control arm expressed as rate ratios by month of collection, Fulbaria, Mymensingh, Bangladesh. Interventions instituted November 2006
| Month | IRS* | ITN | EVM |
|---|---|---|---|
| Rate ratio (95% CI) | Rate ratio (95% CI) | Rate ratio (95% CI) | |
| Oct 2006 | 0.99 (0.70–1.41) | 1.16 (0.81–1.64) | 1.06 (0.75–1.51) |
| Jan 2007 | 0.15 (0.05–0.46)† | 0.36 (0.15–0.83)† | 0.88 (0.45–1.69) |
| Mar 2007 | 0.24 (0.17–0.35)† | 0.26 (0.18–0.37)† | 0.80 (0.57–1.12) |
| Apr 2007 | 0.21 (0.14–0.32)† | 0.22 (0.14–0.33)† | 0.77 (0.54–1.10) |
| Oct 2007 | 1.57 (1.09–2.25)‡ | 0.40 (0.26–0.60)† | 1.43 (1.00–2.06)‡ |
IRS = indoor residual spraying; ITN = insecticide-treated nets; EVM = environmental management.
Difference significant at P < 0.05 with rate ratio < 1.0 (decreased sand fly density) compared with control.
Difference significant at P < 0.05 with rate ratio > 1.0 (increased sand fly density) compared with control.
The best evidence we have for the feasibility of elimination of VL as a public health problem rests on the experience of the global malaria eradication program of the 1950s–1960s, when VL cases dropped close to zero and molecular data show that L. donovani passed through a tight genetic bottleneck reflecting near elimination.17,18 We hypothesize that a more sustained impact may be achieved if IRS is used to effect a rapid decrease in sand fly populations, followed by wide distribution of ITNs to prevent high rates of leishmania transmission when the sand fly populations rebound. This hypothesis deserves to be rigorously tested. A successful VL control program will require deployment of all the tools at our disposal.
Table 3B.
Sand flies collected in households assigned to insecticide-treated nets (ITN) intervention arm, by species, sex, gravidity, feeding status, and month of collection, Fulbaria, Mymensingh, Bangladesh. Interventions instituted November 2006
| Species | Gender | Status | Month of collection | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Oct 2006 | Jan 2007 | Mar 2007 | Apr 2007 | Oct 2007 | ||||
| Phlebotomus argentipes | ||||||||
| Female | 324 | 11 | 153 | 64 | 80 | 632 | ||
| Gravid | 90 | 0 | 9 | 7 | 16 | 122 | ||
| Fed | 6 | 0 | 0 | 1 | 0 | 7 | ||
| Neither | 228 | 11 | 144 | 56 | 64 | 503 | ||
| Male | 358 | 6 | 120 | 68 | 71 | 623 | ||
| Total | 682 | 17 | 273 | 132 | 151 | 1255 | ||
| Sergentomyia spp. | 18 | 1 | 46 | 144 | 19 | 228 | ||
| Other Phlebotomus spp. | 24 | 0 | 42 | 134 | 19 | 219 | ||
| Total | 724 | 18 | 361 | 410 | 189 | 1702 | ||
Table 3C.
Sand flies collected in households assigned to environmental management (EVM) intervention arm, by species, sex, gravidity, feeding status, and month of collection, Fulbaria, Mymensingh, Bangladesh. Interventions instituted November 2006
| Species | Gender | Status | Month of collection | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Oct 2006 | Jan 2007 | Mar 2007 | Apr 2007 | Oct 2007 | ||||
| Phlebotomus argentipes | ||||||||
| Female | 286 | 14 | 414 | 199 | 242 | 1155 | ||
| Gravid | 98 | 2 | 27 | 68 | 102 | 297 | ||
| Fed | 4 | 0 | 4 | 0 | 5 | 13 | ||
| Neither | 184 | 12 | 383 | 131 | 135 | 845 | ||
| Male | 346 | 28 | 445 | 274 | 309 | 1402 | ||
| Total | 632 | 42 | 859 | 473 | 551 | 2557 | ||
| Sergentomyia spp. | 16 | 1 | 33 | 65 | 13 | 128 | ||
| Other Phlebotomus spp. | 14 | 0 | 62 | 159 | 34 | 269 | ||
| Total | 662 | 43 | 954 | 697 | 598 | 2954 | ||
ACKNOWLEDGMENTS
We thank Mustakim Ali, Nazrul Islam, Masud Uddin, Yukiko Wagatsuma, Robert Breiman, James H. Maguire, Vashkar Chowdhury, Mohammed Lal Mia, Uzzali Bishwas, Popy Bishwas, Nurjahan Akhter, Arifa Khatun, Sharifa Khatun, Salim Uddin, the staff of the Fulbaria Upazila Health Complex, and the study participants.
Footnotes
Financial support: The 2002–2003 study was funded by a grant from the Centers for Disease Control and Prevention Emerging Infections Initiative. The 2006–2007 study was funded by the Special Programme for Research and Training in Tropical Diseases, World Health Organization.
Authors' addresses: Rajib Chowdhury, Regional Office for South-East Asia, World Health Organization, Indraprastha Estate, Mahatma Gandhi Marg, New Delhi, India, E-mail:rajib478@yahoo.com. Ellen Dotson, Anna J. Blackstock, Shannon McClintock, and Caryn Bern, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention (DPD/CDC), Atlanta, GA, E-mails: ebd6@cdc.gov, hyp9@cdc.gov, smcclin@emory.edu, and cxb9@cdc/gov. Narayan P. Maheswary, Shyla Faria, Saiful Islam, and Shireen Akhter, National Institute of Preventive and Social Medicine, Dhaka, Bangladesh, E-mails: narayanmaheswary@yahoo.com, shylafaria@yahoo.com, tapu_fh@yahoo.com, and shireen_nipsom@yahoo.com. Tangin Akter, Department of Zoology, University of Dhaka, Dhaka, Bangladesh, E-mail: aktertl@yahoo.com. Axel Kroeger, Special Programme for Research and Training in Tropical Diseases, World Health Organization, Geneva, Switzerland, E-mail: kroegera@who.int.
References
- 1.Bhattacharya SK, Sur D, Sinha PK, Karbwang J. Elimination of leishmaniasis (kala-azar) from the Indian subcontinent is technically feasible & operationally achievable. Indian J Med Res. 2006;123:195–196. [PubMed] [Google Scholar]
- 2.World Health Organization . The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 3.Alvar J, Aparicio P, Aseffa A, den Boer M, Cañavate C, Dedet J, Gradoni L, ter Horst R, López-Vélez R, Moreno J. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeronimo SM, de Queiroz Sousa A, Pearson RD. In: Tropical Infectious Diseases: Principles, Pathogens and Practice. Guerrant RL, Walker DH, Weller PF, editors. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2006. pp. 1095–1113. (Leishmaniasis). [Google Scholar]
- 5.Sen Gupta PC. History of kala-azar in India. Ind Med Gaz. 1947;82:281–286. [PMC free article] [PubMed] [Google Scholar]
- 6.Mondal D, Alam MS, Karim Z, Haque R, Boelaert M, Kroeger A. Present situation of vector-control management in Bangladesh: a wake up call. Health Policy. 2008;87:369–376. doi: 10.1016/j.healthpol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Bern C, Hightower AW, Chowdhury R, Ali M, Amann J, Wagatsuma Y, Haque R, Kurkjian K, Vaz LE, Begum M, Akter T, Cetre-Sossah CB, Ahluwalia IB, Dotson E, Secor WE, Breiman RF, Maguire JH. Risk factors for kala-azar in Bangladesh. Emerg Infect Dis. 2005;11:655–662. doi: 10.3201/eid1105.040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi AB, Das ML, Akhter S, Chowdhury R, Mondal D, Kumar V, Das P, Kroeger A, Boelaert M, Petzold M. Chemical and environmental vector control as a contribution to the elimination of visceral leishmaniasis on the Indian subcontinent: cluster randomized controlled trials in Bangladesh, India and Nepal. BMC Med. 2009;7:54. doi: 10.1186/1741-7015-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ordonez Gonzalez J, Kroeger A, Avina AI, Pabon E. Wash resistance of insecticide-treated materials. Trans R Soc Trop Med Hyg. 2002;96:370–375. doi: 10.1016/s0035-9203(02)90363-9. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors. Geneva, Switzerland: WHO; 1998. pp. 1–45. [Google Scholar]
- 11.Lewis D. A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae) Bull Br Mus. 1982;45:121–209. [Google Scholar]
- 12.Dinesh DS, Ranjan A, Palit A, Kishore K, Kar SK. Seasonal and nocturnal landing/biting behaviour of Phlebotomus argentipes (Diptera: Psychodidae) Ann Trop Med Parasitol. 2001;95:197–202. doi: 10.1080/00034980120041071. [DOI] [PubMed] [Google Scholar]
- 13.Picado A, Das ML, Kumar V, Dinesh DS, Rijal S, Singh SP, Das P, Coosemans M, Boelaert M, Davies C. Phlebotomus argentipes seasonal patterns in India and Nepal. J Med Entomol. 2010;47:283–286. doi: 10.1603/me09175. [DOI] [PubMed] [Google Scholar]
- 14.Mondal D, Chowdhury R, Huda MM, Maheswary NP, Akther S, Petzold M, Kumar V, Das ML, Gurung CK, Ghosh D, Kroeger A. Insecticide-treated bed nets in rural Bangladesh: their potential role in the visceral leishmaniasis elimination programme. Trop Med Int Health. 2010;15:1382–1389. doi: 10.1111/j.1365-3156.2010.02635.x. [DOI] [PubMed] [Google Scholar]
- 15.Dinesh DS, Das P, Picado A, Davies C, Speybroeck N, Ostyn B, Boelaert M, Coosemans M. Long-lasting insecticidal nets fail at household level to reduce abundance of sandfly vector Phlebotomus argentipes in treated houses in Bihar (India) Trop Med Int Health. 2008;13:953–958. doi: 10.1111/j.1365-3156.2008.02096.x. [DOI] [PubMed] [Google Scholar]
- 16.Picado A, Das ML, Kumar V, Kesari S, Dinesh DS, Roy L, Rijal S, Das P, Rowland M, Sundar S, Coosemans M, Boelaert M, Davies CR. Effect of village-wide use of long-lasting insecticidal nets on visceral leishmaniasis vectors in India and Nepal: a cluster randomized trial. PLoS Negl Trop Dis. 2010;4:e587. doi: 10.1371/journal.pntd.0000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam MZ, Kuhls K, Schweynoch C, Sundar S, Rijal S, Shamsuzzaman AK, Raju BV, Salotra P, Dujardin JC, Schonian G. Multilocus microsatellite typing (MLMT) reveals genetic homogeneity of Leishmania donovani strains in the Indian subcontinent. Infect Genet Evol. 2009;9:24–31. doi: 10.1016/j.meegid.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Bora D. Epidemiology of visceral leishmaniasis in India. Natl Med J India. 1999;12:62–68. [PubMed] [Google Scholar]


