Abstract
Eastern equine encephalitis virus (EEEV) is a mosquito-borne pathogen that cycles in birds but also causes severe disease in humans and horses. We examined patterns of avian host use by vectors of EEEV in Alabama from 2001 to 2009 using blood-meal analysis of field-collected mosquitoes and avian abundance surveys. The northern cardinal (Cardinalis cardinalis) was the only preferred host (fed on significantly more than expected based on abundance) of Culiseta melanura, the enzootic vector of EEEV. Preferred hosts of Culex erraticus, a putative bridge vector of EEEV, were American robin (Turdus migratorius), Carolina chickadee (Poecile carolinensis), barred owl (Strix varia), and northern mockingbird (Mimus polyglottis). Our results provide insight into the relationships between vectors of EEEV and their avian hosts in the Southeast and suggest that the northern cardinal may be important in the ecology of EEEV in this region.
Introduction
Birds are implicated as the primary reservoir hosts in transmission cycles of many mosquito-borne pathogens.1 Recent research has emphasized that some bird species are overused relative to their local availability by vector mosquitoes as blood-meal sources2–7 and that overuse of certain avian species could influence the transmission of pathogens.8 For example, temporal and spatial patterns of American robin (Turdus migratorius) abundance have been associated with variability of the rate of human cases of West Nile virus (WNV) and prevalence of WNV in mosquito populations.6,9 Such patterns have been attributed to a higher rate of feeding on American robins by foraging mosquitoes relative to other avian species,10 which leads to a higher probability of infection of robins, potentially causing robins to function as a superspreader of the virus.3
Studies of avian host use by mosquitoes are vital components to elucidating the ecology of arbovirus transmission. To date, however, such studies have been largely restricted to potential vectors of WNV.3–7 The extent to which observations from studies of WNV can be applied to other mosquito-borne pathogen systems for which birds serve as reservoir hosts is uncertain, because these pathogens vary in the ecological factors that influence transmission.11
The ecology of the transmission dynamics of eastern equine encephalitis virus (EEEV) is distinct from that of WNV. WNV is a periurban disease for which the primary enzootic vectors in the United States are Culex pipiens in the northeast and Cx. quinquefasciatus in the Southeast.12 In contrast, EEEV is endemic to bottomland hardwood swamps, and the primary enzootic vector over most of its range is the ornithophilic mosquito Culiseta melanura.13 Given the very different habitats in which the two viruses occur as well as the different vectors that transmit them, it seems likely that other aspects of the ecology of these viruses also differ.
In this study, we assessed patterns of avian host species use by mosquitoes by employing data collected over nearly a decade on the East Gulf Coastal Plain in Alabama. We determined the identity of avian blood meals in eight mosquito species: Cs. melanura, Cx. restuans, Aedes vexans, Coquillettidia perturbans, Cx. erraticus, Cx. peccator, Cx. territans, and Ochlerotatus sticticus. Five of these species have been implicated in transmission of EEEV, but the avian host preferences of these mosquitoes have not been characterized in detail. Cs. melanura is widely recognized as the primary enzootic vector of EEEV,14 and Cx. restuans has recently been proposed to function as an enzootic vector as well.15 Ae. vexans, Cq. perturbans, and Cx. erraticus have been proposed to play roles as bridge vectors.14,16 Cx. peccator, Cx. territans, and Oc. sticticus were also found to have bird blood meals during this study, but their roles in transmission of EEEV are uncertain.
In a previous study, we found that, collectively, mosquitoes at a study area in Tuskegee National Forest (TNF) feed on available vertebrate classes, with degree of catholicism varying by mosquito species.17 In the present study, we focus specifically on characterizing patterns of avian host use by mosquitoes in TNF. A preliminary analysis of the patterns of mosquito feeding on avian species in the same study area was published elsewhere.2 That study examined patterns of host use based on bird abundances estimated from a small number of point counts made within a limited portion of the study area. Moreover, the avian abundances used in the earlier analysis were based on estimates that did not account for imperfect detectability of avian species from point-count surveys. Here, we analyze multiple years of blood-meal data using an improved null model for mosquito use of avian hosts relative to availability that accounts for species detectability.18 Our goal was to produce comprehensive and accurate estimates of forage ratios of avian hosts for mosquito vectors of EEEV.
Materials and Methods
Mosquito surveys and blood-meal source identification.
We studied the blood-feeding patterns of mosquitoes over a 9-year period in a study area in TNF in Macon County, Alabama, by regularly collecting blood-engorged mosquitoes and using DNA analysis to identify the sources of their blood meals. This site has been the center of an ongoing study of the ecology of mosquitoes and their interactions with avifauna and herpetofauna in this focus of EEEV since 2001, and it is described more fully elsewhere.16,17,19,20 Briefly, the study site is a circular area encompassing 28 km2 predominated by a complex of forest, ponds, and wetlands. Much of the land is part of TNF, but it also extends into adjacent private lands.
Mosquitoes were collected annually between March and October from 2001 to 2009, except in 2005 when no mosquito sampling occurred. Mosquito collection entailed aspiration of individuals from natural resting sites and a variety of container types serving as artificial resting sites immediately surrounding each sampling location, with the container types used varying over the course of the study.17,19,20 After collection, mosquitoes were transported to the laboratory at Auburn University, sorted on a chill table by species, sex, and engorgement status, and stored at −70°C until processing for blood-meal identification.
The identity of host blood meals at the study site was determined by specific amplification of a portion of the vertebrate cytochrome B gene, as previously described.2,17,21,22 The identity of the amplicons was determined using a combination of heteroduplex analysis and direct DNA sequencing, as previously described.2,5,17
Avian surveys and modeling.
We conducted surveys of avian populations at 338 locations spread uniformly throughout the study area from May 15 to June in 2008. We used a systematic sampling design for the surveys, whereby survey points were located 250 m apart on vertices of a grid that covered the study area. Surveys were conducted during two non-overlapping rounds of sampling so that each point was visited two times during the summer. Estimating the probability of detection for each species requires repeated sampling of the same location, and therefore, during each of the two visits to a point-count location, three consecutive 4-minute counts were conducted. The species identification of all birds seen or heard within 100 m of the point-count location was recorded during each 4-minute count period. All sampling sessions occurred between 0500 and 1100 hours CDT.
Nocturnal bird surveys were also conducted at a subset of 50 of the bird point-count locations spaced 500 m apart. Nocturnal surveys were conducted using a combination of silent point counts and audio playback of the target species. On arriving at the survey location, the observer conducted three consecutive 3-minute counts of all individual birds detected within 200 m. The observer then played 20 seconds of chuck-will's-widow (Caprimulgus carolinensis) calls followed by a 1-minute count period. Next, the observer played 20 seconds of whip-poor-will (Caprimulgus vociferous) calls followed by a 1-minute count period. This procedure was then repeated for eastern screech owls (Megascops asio), barred owls (Strix varia), and great horned owls (Bubo virginianus), respectively, with each species' call being played followed by a 1-minute count period. Care was taken to make sure that the audio recordings were not audible more than 200 m away from the observer. Nocturnal surveys were conducted by a single observer between June 15 and July 3, 2009 from 2000 to 2400 and 0400 to 0500 hours CDT.
We estimated densities of avian hosts at mosquito sampling sites by applying predictive models of density for each bird species recorded during 2008 and 2009 point counts.18 We used N mixture models to incorporate heterogeneity in detectability of individual species into models of occupancy and abundance. We modeled mean density of each species as a linear combination of covariates describing the relative abundances of habitat types in 100-m buffers around bird point-count locations derived from the Alabama Gap Analysis Program (GAP) land-cover map23 and the National Land Cover Database Tree Canopy Cover Map24 and assumed a Poisson error distribution. Although such count data may follow a negative binomial distribution, the Poisson distribution has been found to be appropriate for many species detected during our surveys.25 All modeling was carried out using the program PRESENCE26 with further details of model development given elsewhere.27 Predicted densities of nocturnal species were standardized to a 100-m buffer, the area sampled for birds during diurnal avian surveys.
Because of practical considerations, we were forced to exclude a small number of avian species a priori from forage ratio calculations. First, house finches (Carpodacus mexicanus) were excluded from these analyses, because individuals of this spe'cies were housed in sentinel cages at the center of the study area from 2002 to 2004. Avian species from orders Ciconiiformes (herons) and Pelecaniformes (anhinga) were also excluded, because point-count methodologies do not provide accurate estimates of their densities,28 and we had no means to accurately census for these species. Inadequate numbers of wood ducks (Aix sponsa), chickens (Gallus gallus), and red-tailed hawks (Buteo jamaicensis) were detected frequently enough to model abundance, and thus, these three species were also excluded from forage ratio calculations.
Statistical analysis.
We estimated the rate of use of avian host species identified in blood meals collected between 2001 and 2009 relative to their availability by different mosquito species using the forage, or selection, ratio approach de'scribed elsewhere.29,30 With this approach, the ratio of the relative abundance of a host species in the blood-meal sample to its relative abundance in the avian community is a forage ratio. In the current study, the relative abundance of an avian host species in the blood-meal sample was calculated separately for Cx. erraticus, Cx. restuans, and Cs. melanura using blood-meal abundances summed across all study years. The relative abundance of a host species in the avian community was calculated separately for each of the three focal mosquito species using average estimated densities of avian hosts at all sites where individuals of each mosquito species were collected, respectively (Table 1).
Table 1.
Predicted relative abundances of avian species observed during point-count surveys in TNF and used in forage ratio calculations
| Species | Relative Abundance | ||
|---|---|---|---|
| Cx. erraticus | Cx. restuans | Cs. melanura | |
| Acadian flycatcher Empidonax virescens | 0.025 | 0.032 | 0.039 |
| American crow Corvus brachyrhychos | 0.022 | 0.016 | 0.015 |
| American robin Turdus migratorius | 0.003 | 0.003 | 0.003 |
| Barn swallow Hirundo rustica | 0.001 | 0.001 | 0.001 |
| Barred owl Strix varia | 0.006 | 0.006 | 0.008 |
| Blue grosbeak Guiraca cerulea | 0.004 | 0.003 | 0.004 |
| Blue jay Cyanocitta cristata | 0.030 | 0.027 | 0.027 |
| Blue-gray gnatcatcher Polioptila caerulea | 0.041 | 0.043 | 0.042 |
| Broad-winged hawk Buteo platypterus | 0.004 | 0.004 | 0.004 |
| Brown thrasher Toxostoma rufum | 0.017 | 0.011 | 0.010 |
| Brown-headed cowbird Molothrus ater | 0.009 | 0.008 | 0.008 |
| Brown-headed nuthatch Sitta pusilla | 0.011 | 0.008 | 0.007 |
| Carolina chickadee Poecile carolinensis | 0.030 | 0.030 | 0.029 |
| Carolina wren Thryothorus ludovivianus | 0.059 | 0.061 | 0.062 |
| Chipping sparrow Spizella passerina | 0.003 | 0.001 | 0.001 |
| Chuck-will's-widow Caprimulgus carolinensis | 0.001 | 0.000 | 0.000 |
| Common grackle Quiscalus quiscula | 0.002 | 0.001 | 0.001 |
| Common yellowthroat Geothlypis trichas | 0.006 | 0.007 | 0.008 |
| Downy woodpecker Picoides pubescens | 0.022 | 0.023 | 0.024 |
| Eastern bluebird Sialia sialis | 0.001 | 0.000 | 0.000 |
| Eastern kingbird Tyrannus tyrannus | 0.001 | 0.001 | 0.001 |
| Eastern phoebe Sayornis phoebe | 0.001 | 0.001 | 0.001 |
| Eastern towhee Pipilo erythrophthalmus | 0.011 | 0.007 | 0.007 |
| Eastern wood-pewee Contopus virens | 0.003 | 0.003 | 0.002 |
| Eurasian collared-dove Streptopelia decaocto | 0.000 | 0.000 | 0.000 |
| Field sparrow Spizella pusilla | 0.004 | 0.004 | 0.004 |
| Fish crow Corvus ossifragus | 0.004 | 0.004 | 0.004 |
| Gray Catbird Dumetella carolinensis | 0.003 | 0.003 | 0.003 |
| Great crested flycatcher Myiarchus crinitus | 0.020 | 0.019 | 0.019 |
| Hairy woodpecker Picoides villosus | 0.004 | 0.004 | 0.004 |
| Hooded warbler Wilsonia citrina | 0.038 | 0.042 | 0.039 |
| Indigo bunting Passerina cyanea | 0.016 | 0.013 | 0.013 |
| Kentucky warbler Opornis formosus | 0.007 | 0.008 | 0.008 |
| Lousiana waterthrush Parkesia motacilla | 0.005 | 0.007 | 0.008 |
| Mourning dove Zenaida macroura | 0.007 | 0.006 | 0.007 |
| Northern cardinal Cardinalis cardinalis | 0.111 | 0.109 | 0.104 |
| Northern mockingbird Mimus polyglottis | 0.003 | 0.001 | 0.002 |
| Northern parula Parula americana | 0.035 | 0.045 | 0.055 |
| Orchard oriole Icterus spurius | 0.001 | 0.000 | 0.000 |
| Pileated woodpecker Dryocopus pileatus | 0.012 | 0.014 | 0.015 |
| Pine warbler Dendroica pinus | 0.016 | 0.011 | 0.009 |
| Prairie warbler Dendroica discolor | 0.006 | 0.004 | 0.004 |
| Prothonotary warbler Protonotaria citrea | 0.005 | 0.006 | 0.008 |
| Purple martin Progne subis | 0.002 | 0.002 | 0.002 |
| Red-bellied woodpecker Melanerpes carolinus | 0.079 | 0.068 | 0.063 |
| Red-eyed vireo Vireo olivaceus | 0.061 | 0.067 | 0.062 |
| Red-headed woodpecker Melanerpes erythrocephalus | 0.005 | 0.004 | 0.004 |
| Red-shouldered hawk Buteo lineatus | 0.006 | 0.007 | 0.008 |
| Red-winged blackbird Agelaius phoeniceus | 0.002 | 0.001 | 0.002 |
| Ruby-throated hummingbird Archilochus colubris | 0.013 | 0.013 | 0.012 |
| Summer tanager Piranga rubra | 0.029 | 0.026 | 0.024 |
| Swainson's warbler Limnothlypis swainsonii | 0.004 | 0.005 | 0.005 |
| Tufted titmouse Baeolophus bicolor | 0.063 | 0.067 | 0.065 |
| White-eyed vireo Vireo griseus | 0.035 | 0.036 | 0.036 |
| Wild turkey Meleagris gallapavo | 0.001 | 0.001 | 0.001 |
| Wood thrush Hylocichla mustelina | 0.012 | 0.015 | 0.018 |
| Yellow-billed cuckoo Coccyzus americanus | 0.056 | 0.065 | 0.069 |
| Yellow-breasted chat Icteria virens | 0.007 | 0.005 | 0.005 |
| Yellow-shafted flicker Colaptes auratus auratus | 0.004 | 0.005 | 0.005 |
| Yellow-throated vireo Vireo flavifrons | 0.009 | 0.010 | 0.010 |
| Yellow-throated warbler Dendroica dominica | 0.002 | 0.002 | 0.001 |
Statistical significance of the forage ratio estimate for an avian species was based on overlap of the 95% confidence interval of the estimate with the value one.30 An avian species was considered to have been preferred if it was overused relative to its rate availability to a mosquito species, such that the lower 95% confidence limit for the forage ratio estimate was greater than one. A species was inferred to have been avoided if it was underused relative to its rate of availability, such that the upper 95% confidence limit for the forage ratio estimate was less than one. An avian species for which the 95% confidence interval for its forage ratio included one was considered to have been fed on opportunistically.30 We additionally estimated forage ratios for each avian host species using blood meals collected strictly between May 1 and August 15 to determine whether forage ratio estimates were biased by potentially non-constant relative abundances of avian species between March and October of each year.
The study area in and around TNF represents a rural environment undergoing no wide-scale alteration of habitats with stable bird populations between years. We, thus, assumed that the composition of the avian community had been stable over the course of the study period, such that it was reasonable to use point-count surveys in the TNF study area conducted during 2008 and 2009 as representative of the relative abundances of each species in the avian community over the course of the study. To formally test the validity of this assumption, we compared the avian community structure in and around TNF between 2001 and 2009 with data from the Breeding Bird Survey (BBS)31 along the Warrior Stand Route. The Warrior Stand Route runs through the TNF study area, and the BBS was conducted across the same years as mosquito surveys. This BBS route has been surveyed within 5 days of the same date under nearly identical weather conditions and by a single observer (G.E.H.) since 1998, and therefore, comparisons of abundances between years are not biased by heterogeneity in detectability of species because of season, weather, time of day, or observer effects.
We created a joint (2001 + 2009) dataset, with record entries indicating the species identification of individual birds observed during the 2001 and 2009 Warrior Stand Breeding Bird Surveys and the total number of records in the dataset equal to the total number of individuals observed in 2001 and 2009 (ntot = 1,707; n2001 = 856; n2009 = 851).32 We randomly sampled 856 individuals from this joint dataset and assigned them to the first simulated 2001 community; we assigned all remaining 851 individuals in the joint community dataset to the first simulated 2009 community. We then calculated the differences between these two simulated communities of the Shannon Index (H) and the Simpson Index (D), two common diversity indices used to assess community structure.33 We repeated this randomization and index calculation procedure to yield 10,000 estimates each of the differences in H and D between 2009 and 2001 simulated community pairs. We then calculated the proportions of the simulated community pairs for which the absolute values of the differences in D and H were greater than the absolute values of the observed differences in D and H between 2001 and 2009, respectively. We used these proportions as estimates of the P values for two-tailed tests of the null hypothesis that the avian community structure had not changed along the Warrior Stand Route between 2001 and 2009.32
Results
A total of 42 avian species were identified as the sources for 528 blood meals from nine species of mosquito in the TNF study area between 2001 and 2009 (Table 2). Cx. restuans and Cs. melanura fed primarily on perching birds (order Passeriformes), taking 72.0% and 77.4% of blood meals from perching birds, respectively, and secondarily on herons (family Ardeidae, order Ciconiiformes), taking 24.0% and 11.3% of blood meals from herons, respectively. Other avian hosts of these mosquitoes included yellow-billed cuckoos (order Cuculiformes), representing 4.0% and 5.66% of blood meals from Cx. restuans and Cs. melanura, respectively, and owls (order Strigiformes), representing 5.66% of Cs. melanura blood meals. Neither Cx. restuans nor Cs. melanura was found to feed on chickens or wild turkeys (order Galliformes), anhinga (order Pelecaniformes), raptors (order Falconiformes), ducks (family Anatidae, order Anseriformes), or hummingbirds (order Apodiformes). Cx. erraticus fed primarily on herons (64.3%) followed by birds from a wide variety of avian orders, including perching birds (24.7%), ducks (5.8%), owls (2.4%), gallinaceous birds (1.2%), cuckoos (1.0%), anhinga (0.5%), and hummingbirds (0.2%). The majority of avian blood meals were derived from birds that have established breeding populations in central Alabama (Table 2). Those species that do not have breeding populations in central Alabama overwinter, migrate through (e.g., American bittern Botaurus lentiginous), or are domesticated (chicken).
Table 2.
Total numbers of blood meals derived from avian species for mosquitoes collected in TNF between March and October from 2001 to 2009
| Ae. vexans | Cq. perturbans | Cx. erraticus | Cx. peccator | Cx. quinquefasciatus | Cx. restuans | Cx. territans | Cs. melanura | Oc. sticticus | |
|---|---|---|---|---|---|---|---|---|---|
| Acadian flycatcher Empidonax virescens | 1 | 2 | |||||||
| American bittern Botaurus lentiginous | 23 | 1 | 3 | 1 | |||||
| American crow Corvus brachyrhychos | 2 | ||||||||
| American robin Turdus migratorius | 14 | 1 | |||||||
| Anhinga Anhinga anhinga | 2 | ||||||||
| Barred owl Strix varia | 7 | 3 | |||||||
| Blue grosbeak Guiraca cerulea | 2 | ||||||||
| Blue jay Cyanocitta cristata | 3 | ||||||||
| Blue-gray gnatcatcher Polioptila caerulea | 2 | ||||||||
| Brown-headed cowbird Molothrus ater | 2 | ||||||||
| Carolina chickadee Poecile carolinensis | 1 | 16 | |||||||
| Carolina wren Thryothorus ludovivianus | 1 | 1 | |||||||
| Chicken Gallus gallus | 1 | ||||||||
| Common grackle Quiscalus quiscula | 2 | 4 | |||||||
| Common yellowthroat Geothlypis trichas | 3 | ||||||||
| Eastern screech owl Otus asio | 3 | ||||||||
| Eastern towhee Pipilo erythrophthalmus | 1 | ||||||||
| Great blue heron Ardea herodias | 1 | 141 | 9 | 2 | |||||
| Great egret Ardea alba | 1 | ||||||||
| Green heron Butorides virescens | 25 | 1 | 1 | 2 | |||||
| Gray catbird Dumetella carolinensis | 3 | 1 | |||||||
| Hooded warbler Wilsonia citrina | 1 | 2 | |||||||
| House finch Carpodacus mexicanus | 1 | 2 | 1 | 1 | |||||
| Kentucky warbler Opornis formosus | 1 | ||||||||
| Lousiana waterthrush Parkesia motacilla | 2 | 1 | |||||||
| Northern cardinal Cardinalis cardinalis | 3 | 22 | 4 | 6 | 1 | 20 | 1 | ||
| Northern mockingbird Mimus polyglottis | 13 | 1 | |||||||
| Orchard oriole Icterus spurius | 4 | ||||||||
| Pied-billed grebe Podilymbus podiceps | 1 | 7 | |||||||
| Pine warbler Dendroica pinus | 1 | ||||||||
| Red-eyed vireo Vireo olivaceus | 3 | ||||||||
| Red-tailed hawk Buteo jamaicensis | 1 | ||||||||
| Ruby-throated hummingbird Archilochus colubris | 1 | ||||||||
| Summer tanager Piranga rubra | 1 | ||||||||
| Tufted titmouse Baeolophus bicolor | 6 | 1 | 2 | ||||||
| White-eyed vireo Vireo griseus | 1 | 5 | 4 | 1 | |||||
| Wild turkey Meleagris gallapavo | 2 | 5 | |||||||
| Wood duck Aix sponsa | 17 | 1 | |||||||
| Wood thrush Hylocichla mustelina | 1 | 1 | 2 | ||||||
| Yellow-billed cuckoo Coccyzus americanus | 4 | 1 | 3 | ||||||
| Yellow-crowned night heron Nyctanassa violacea | 75 | 1 | 3 | ||||||
| Yellow-throated vireo Vireo flavifrons | 1 |
Cs. melanura significantly overused northern cardinal (Cardinalis cardinalis) relative to its rate of availability in the avian community, and thus the northern cardinal was inferred to have been a preferred host of Cs. melanura. Ninety-five percent confidence intervals of forage ratios for the 14 other bird species that were identified in Cs. melanura blood meals included a value of one, suggesting that these bird species were fed on opportunistically (Table 3 and Figure 1). Avian species inferred to have been preferred by Cx. erraticus included American robin, orchard oriole (Icterus spurious), northern mockingbird (Mimus polyglottis), wild turkey (Meleagris gallapavo), Carolina chickadee (Poecile carolinensis), barred owl, and northern cardinal. Carolina wren (Thryothorus ludovivianus) and hooded warbler (Wilsonia citrina) were both inferred to have been avoided by Cx. erraticus. Forage ratios of the remaining 13 species that Cx. erraticus fed on had 95% confidence intervals that included a ratio of one, suggesting that those species were fed on opportunistically (Table 3 and Figure 1). The 95% confidence intervals around the forage ratios of the eight bird species in the blood-meal sample from Cx. restuans included a ratio of one, indicating that these bird species were also fed on opportunistically by this mosquito species (Table 3 and Figure 1). In general, forage ratio estimates based on expected frequencies of less than five blood meals under the null model of opportunistic feeding should be viewed with caution.30 Avian species with expected frequencies greater than or equal to five in the blood-meal sample from Cx. erraticus were Carolina wren, yellow-billed cuckoo (Coccyzus amerianus), tufted titmouse (Baeolophus bicolor), and northern cardinal and northern cardinal in the blood-meal sample from Cs. melanura.
Table 3.
Forage ratios (95% confidence intervals) of the avian species from which blood meals were derived for Cx. erraticus, Cx. restuans, and Cs. melanura between March and October from 2001 to 2009
| Cx. erraticus | Cx. restuans | Cs. melanura | |
|---|---|---|---|
| Acadian flycatcher | 0.34 (−0.32, 1.00) | 1.11 (−0.40, 2.62) | |
| American crow | 6.70 (−2.08, 15.48) | ||
| American robin | 44.27 (22.51, 66.02)** | ||
| Barred owl | 10.08 (2.84, 17.32)* | 8.12 (−0.76, 17.01) | |
| Blue grosbeak | 4.55 (−1.70, 10.79) | ||
| Blue jay | 0.86 (−0.10, 1.83) | ||
| Blue-gray gnatcatcher | 1.03 (−0.37, 2.44) | ||
| Brown-headed cowbird | 1.84 (−0.69, 4.37) | ||
| Carolina chickadee | 4.60 (2.50, 6.69)** | ||
| Carolina wren | 0.15 (−0.14, 0.43)** | 0.35 (−0.33, 1.04) | |
| Common grackle | 19.65 (0.73, 38.58) | ||
| Common yellowthroat | 7.73 (−0.73, 16.19) | ||
| Eastern towhee | 3.28 (−3.08, 9.65) | ||
| Gray catbird | 8.69 (−1.02, 18.40) | 7.06 (−6.63, 20.74) | |
| Hooded warbler | 0.23 (−0.21, 0.67)** | 1.12 (−0.40, 2.64) | |
| Kentucky warbler | 1.15 (−1.10, 3.40) | ||
| Louisiana waterthrush | 15.88 (−4.94, 36.69) | 2.82 (−2.65, 8.3) | |
| Northern cardinal | 1.69 (1.05, 2.32)* | 2.89 (0.98, 4.81) | 4.18 (2.81, 5.56)** |
| Northern mockingbird | 34.59 (16.86, 52.32)** | ||
| Orchard oriole | 43.63 (1.61, 85.65)* | ||
| Pine warbler | 0.52 (−0.50, 1.55) | ||
| Red-eyed vireo | 1.04 (−0.10, 2.19) | ||
| Ruby-throated hummingbird | 0.67 (−0.64, 1.99) | ||
| Summer tanager | 2.00 (−1.81, 5.81) | ||
| Tufted titmouse | 0.81 (0.18, 1.44) | 0.78 (−0.71, 2.27) | 0.67 (−0.24, 1.58) |
| White-eyed vireo | 1.21 (0.17, 2.25) | 5.86 (0.76, 10.96) | 0.61 (−0.57, 1.79) |
| Wild turkey | 33.49 (4.77, 62.22)* | ||
| Wood thrush | 0.71 (−0.68, 2.11) | 6.92 (−2.15, 16.00) | |
| Yellow-billed cuckoo | 0.62 (0.02, 1.21) | 0.81 (−0.74, 2.36) | 0.94 (−0.09, 1.97) |
| Yellow-throated vireo | 2.24 (−2.10, 6.58) |
95% confidence intervals of forage ratios exclude 1.
99% confidence intervals of forage ratios exclude 1.
Figure 1.
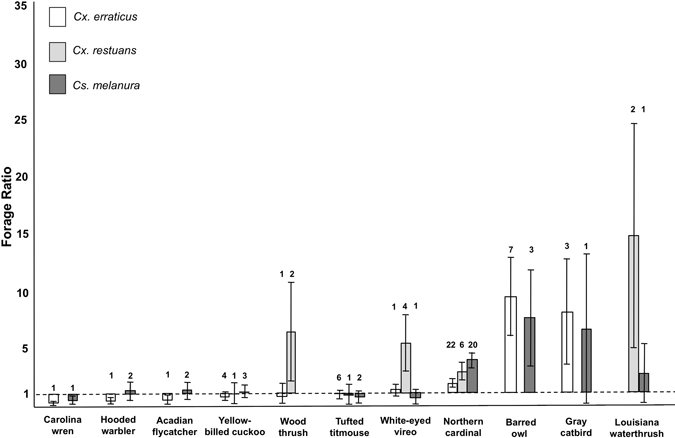
Forage ratios for avian species present in at least two of the total blood-meal samples collected from Cs. melanura, Cx. erraticus, and Cx. restuans in TNF between March and October from 2001 to 2009. Bars show estimated standard errors, and the numbers above the bars are sample sizes.
Comparisons of the forage ratios estimated from blood-meal data covering the entire March to October mosquito sampling period and blood-meal data from May 1 to August 15, when birds are not migrating in east-central Alabama, revealed a high degree of consistency for estimates between the two periods for both Cs. melanura and Cx. erraticus (Table 4). However, the confidence intervals of orchard oriole, wild turkey, and northern cardinal included one when forage ratios and associated standard errors for these three species were based on the blood meals collected strictly between May 1 and August 15. Comparison between the Cs. restuans samples were not made, because only four individuals of this species yielding avian-derived blood meals were collected between May 1 and August 15 over the 8 years of sampling.
Table 4.
Forage ratios of avian species using all blood meals collected between March and October or alternatively, strictly between May and August 15
| Cx. erraticus Forage ratio | Cs. melanura Forage Ratio | |||
|---|---|---|---|---|
| Mar–Oct | May–Aug 15th | Mar–Oct | May–Aug 15th | |
| Acadian flycatcher | 0.34 | 0.42 | 1.11 | 1.36 |
| American crow | ||||
| American robin | 44.27** | 46.43** | ||
| Barred owl | 10.08 * | 12.24* | 8.12 | 6.54 |
| Blue grosbeak | 4.55 | 2.79 | ||
| Blue jay | 0.86 | 1.05 | ||
| Blue-gray gnatcatcher | 1.03 | 0.62 | ||
| Brown-headed cowbird | 1.84 | |||
| Carolina chickadee | 4.60** | 4.62** | ||
| Carolina wren | 0.15** | 0.18** | 0.35 | 0.43 |
| Common grackle | 19.65 | 24.09 | ||
| Common yellowthroat | 7.73 | 6.25 | ||
| Eastern towhee | 3.28 | |||
| Gray catbird | 8.69 | 10.51 | 7.06 | |
| Hooded warbler | 0.23** | 0.28** | 1.12 | 1.37 |
| Kentucky warbler | 1.15 | 1.43 | ||
| Louisiana waterthrush | 2.82 | 3.43 | ||
| Northern cardinal | 1.69* | 1.61 | 4.18** | 4.55** |
| Northern mockingbird | 34.59** | 32.62** | ||
| Orchard oriole | 43.63* | 40.20 | ||
| Pine warbler | 0.52 | 0.66 | ||
| Red-eyed vireo | 1.04 | 1.27 | ||
| Ruby-throated hummingbird | 0.67 | 0.84 | ||
| Summer tanager | ||||
| Tufted titmouse | 0.81 | 0.67 | 0.67 | 0.82 |
| White-eyed vireo | 1.21 | 1.49 | 0.61 | 0.74 |
| Wild turkey | 33.49* | 24.69 | ||
| Wood thrush | 0.71 | 0.88 | ||
| Yellow-billed cuckoo | 0.62 | 0.57 | 0.94 | 0.76 |
| Yellow-throated vireo | 2.24 | 2.73 | ||
95% confidence intervals of forage ratios exclude 1 (not shown).
99% confidence intervals of forage ratios exclude 1 (not shown).
The observed difference in the Shannon Index between the 2001 and 2009 data from the BBS Warrior Stand Route was 0.022; the proportion of the community pairs for which the absolute value of the difference in H exceeded the absolute value of this observed value was 0.624. The observed difference in the Simpson Index between the 2001 and 2009 data from the BBS Warrior Stand Route was 0.003; the proportion of the community pairs for which the absolute value of the difference in H exceeded the absolute value of this observed value was 0.436. There was, thus no evidence to reject the null hypothesis of a stable avian community structure between 2001 and 2009 along the Warrior Stand Route using either the Shannon or Simpson Index as a measure of avian community structure and an α-cutoff of 0.05. Moreover, the rank of species abundances in the 2001 and 2009 samples was positively correlated (rS(49) = 0.90; P < 0.001). As such, there was strong support for the validity of our assumption that the relative abundances of avian species in the TNF study area estimated from point-count surveys during 2008 and 2009 were representative of their relative abundances over the course of the study period.
Discussion
Through comparisons of the sources of mosquito blood meals with the local avian community, we found that putative vectors of EEEV in the Southeast do not feed on birds opportunistically; rather, these mosquito species use some species of birds more or less than expected based on their relative abundance in the environment. Although a number of studies have previously shown similar heterogeneity in mosquito feeding patterns,2–7,34 our study is the first to show such heterogeneity at the host species level for Cs. melanura, the primary enzootic vector of EEEV. Our results provide evidence that the northern cardinal is a preferred host of Cs. melanura. As such, the northern cardinal will likely be exposed more frequently to EEEV than other avian species, and thus, we predict that it plays an important role in ecology of EEEV in the southeast.
In addition to the northern cardinal, 10 avian species—common yellowthroat (Geothlypis trichas), gray catbird (Dumetella carolinensis), eastern towhee (Pipilo erythrophthalmus), Louisiana waterthrush (Parkesia motacilla), yellow-throated vireo (Vireo flavifrons), barred owl (Strix varia), hooded warbler (Wilsonia citrina), Acadian flycatcher (Empidonax virescens), red-eyed vireo (Vireo olivaceus), and blue-gray gnatcatcher (Polioptila caerulea)— had forage ratio estimates for Cs. melanura that were greater than one, suggesting that these species may also be preferred hosts. Three of these species—barred owl, common yellowthroat, and gray catbird—were fed on much more than expected based on their relative abundances. Although the confidence interval for the forage ratios of these 10 species included one—making their overrepresentation in blood meals not statistically significant—standard error calculations were based on sample sizes that were too small to provide reliable estimates of confidence intervals.30 Despite low sample sizes and large standard errors, we suggest that northern cardinal, barred owl, common yellowthroat, and gray catbird have the highest probabilities for playing important roles in EEEV transmission among the avian species for which forage ratios were estimated in the current study.
The established model for EEEV transmission in the northeastern United States, which implicates Cs. melanura as the primary enzootic vector of the virus, is commonly extrapolated as an appropriate model for transmission of EEEV throughout North America. Recent studies, however, have suggested that this northeastern model may not accurately depict transmission of EEEV in southeastern foci and that other mosquito species, especially Cx. erraticus and Cx. restuans, may be important to enzootic transmission in the southeastern region.15,35 If Cx. erraticus or Cx. restuans plays a prominent role in EEEV transmission in the southeast, inferences about avian host preferences of these mosquito species become important. Cx. erraticus and Cx. restuans both had high forage ratios for northern cardinal, and northern cardinal was inferred to be a preferred host species of Cx. erraticus when forage ratios were calculated using the entire sample of blood meals collected between March and October.
We assumed that the avian community structure is most stable between May 1 and August 15, the period after spring migration and before late summer dispersal and migration of birds. When forage ratios were based on the restricted samples of blood meals collected during this period, neither Cx. erraticus nor Cx. restuans exhibited significant feeding preferences for northern cardinal. The few blood-meal samples available during this restricted period for Cx. restuans limit our ability to make inferences regarding significant rates of over- and underuse of avian hosts by this mosquito species. For Cx. erraticus, however, sample sizes of blood meals from May 1 to August 15 were adequate to make inferences, and we found that the American robin, Carolina chickadee, barred owl, and northern mockingbird are the preferred hosts of Cx. erraticus. As such, the northern cardinal may be less important in EEEV transmission compared with these species if Cx. erraticus is a more important enzootic vector of the virus in the Southeast. These results underscore the need for further research of the relative contribution of different mosquito species to EEEV in this region.
All of our conclusions regarding forage ratios must be considered in light of the fact that we had to exclude some species from our forage ratio analyses, because we were unable to accurately census these birds. Notable among these were herons, which comprised a large proportion of the blood meals from Cx. erraticus. The necessity of excluding herons from our analysis was unfortunate, because herons may also play an important role in the ecology of EEEV in the southeastern United States; additionally, herons comprised a large percentage of avian blood meals in our study. The fraction of total avian blood meals from herons varied by mosquito species, comprising 64.3% of the avian blood meals of Cx. erraticus but just 11.3% of Cs. melanura blood meals. This difference could reflect contact rates of mosquitoes and herons given differences in the ecologies of the mosquitoes and birds. Cx. erraticus breeds in permanent ponds, and the densities of Cx. erraticus females decline with distance from these breeding sites.20 Herons are water birds and thus, are also more likely to be found at permanent ponds. Cs. melanura, in contrast, breeds in water pockets associated with buttressed trees and temporary puddles of water created by uprooted trees that occur in swamp habitats36 but not necessarily near permanent water, and thus, it may encounter water birds less frequently than Cx. erraticus.
Given the high proportion of blood meals derived from herons, we would conclude that herons are likely to be frequently exposed to EEEV. Interestingly, in a study of exposure of different avian species to EEEV in Louisiana, 54.8% of heron species tested positive for EEEV neutralizing antibodies, whereas only 26.2% of passerine species were seropositive.37 Notable among the exposure rates of individual species was the high seroprevalence of yellow-crowned night herons (Nyctanassa violacea; 86.1%),37 the second most common avian host of Cx. erraticus in the current study. Several studies of defensive behaviors of birds to foraging mosquitoes found that some ciconiiforms, because of their stand and wait foraging technique, are highly susceptible to questing mosquitoes.38–40 Despite the high proportion of blood meals derived from herons, the high seroprevalence of EEEV in wild herons, and evidence that herons are important hosts for many of medically important mosquitoes, relatively little effort has been directed to quantifying the role of ciconiiform birds in the amplification of arboviruses relative to passerines. Results of the current study underscore the need for further research investigating the role of ciconiiform species in EEEV transmission.
The role that a preferred species plays in EEEV transmission follows directly from its forage ratio (i.e., the likelihood that an individual of that species will be fed on). Because they are more likely to be fed on in general, highly ranked (preferred) hosts have a higher likelihood of being fed on by an infected mosquito than lower ranked (less-preferred) hosts. It also follows that such highly ranked hosts, simply because they are a more probable hosts, are more likely to be fed on by a second uninfected mosquito that subsequently becomes infected. Consequently, assuming uniform reservoir competence (i.e., magnitude and duration of viremia), an individual of a more-preferred avian host species should be a more important virus amplifier than an individual from a less-preferred species. With all else equal, preferred host species will play a more important role in transmission dynamics than those that are less preferred.
One of the factors that could confound the relationship between vector feeding preference and host importance in virus amplification is reservoir competence. Preferred host species will have a more important role in transmission dynamics only if all avian host species are equivalent in terms of their reservoir competence. Conversely, if a preferred avian species is a poor reservoir host, then that species will act as a dilution host, reducing contact rates between vectors and competent reservoirs.41 Reservoir competence has been reported for a number of avian species,42 but it was not possible for us to infer interspecific differences in reservoir competence of birds based on such estimates. Further research is needed in this area to more accurately assess the influence of variability in reservoir competence of avian hosts on the transmission dynamics of EEEV.
The shift in inference in terms of preferred hosts of Cx. erraticus that occurred when forage ratio calculations were based on samples collected either from March to October or from May 1 to August 15 is not surprising given that the month of peak intensity of blood feeding in this species is August (Figure 2). Individuals of avian host species with a high relative abundance in the Cx. erraticus blood-meal samples collected between March and October may be more preferable hosts compared with individuals of other species, or they may simply have inflated forage ratios caused by changes in the avian community between May and the later weeks of August when post-breeding dispersal and migration begins. The latter possibility seems highly plausible, because resident birds make up an increasing proportion of the avian community as migratory species leave the study area beginning in August. Thus, northern cardinal and wild turkey, which are not long-distance migrants, are likely to have a greater rate of availability to Cx. erraticus from mid-August to October than is reflected in their relative abundances based on point-count surveys conducted during the breeding season. This confounding influence of an underestimated rate of availability of avian hosts was less likely to be present in the calculation of the forage ratio estimates of Cs. melanura, because this species has a peak intensity of blood feeding in TNF in May (Figure 2) when the structure of the avian host community as estimated from point-count surveys should be highly stable.
Figure 2.
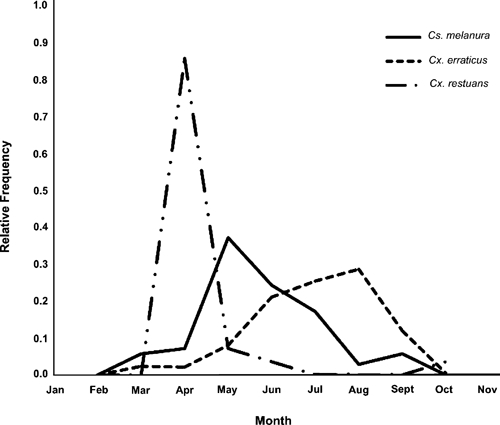
Relative frequencies of blood-engorged Cs. melanura (N = 70), Cx. erraticus (N = 1,457), and Cx. restuans (N = 28) collected in TNF each month between 2001 and 2009.
Our goal in conducting this study was to estimate forage ratios for avian species for putative vectors of EEEV. Such forage ratios represent potential proxy measures of the level of exposure to EEEV that individuals of different host species experience. An alternative approach to measuring EEEV exposure is to estimate the seroprevalence or seroconversion rate of EEEV directly in birds or to assay for antibodies of the virus. In studies in Michigan43 and New Jersey,44 northern cardinals and gray catbirds had high EEEV antibody seroprevalences when present in the sample of surveyed birds. Barred owls and common yellowthroats, the species with the highest Cs. melanura forage ratios in this study, were not present in samples from either of these studies. Overall, these studies confirm our assertion that northern cardinal, gray catbirds, and potentially, barred owls and common yellowthroats are exposed at a high rate to EEEV in regions where Cs. melanura is the primary enzootic vector of the virus given evidence from this study of the high Cs. melanura forage ratios for these species.
ACKNOWLEDGMENTS
The authors thank Jeremy Camp, Chris Cazalet, Xin Yue, Ashok Manoharan, Nathan Click, Katherine Gray, and Chris Porterfield for their assistance with the collection and identification of adult mosquitoes used in this study. We would also like to acknowledge Brian Rolek, Lisa McWilliams, and Tyler Eads for conducting bird surveys.
Footnotes
Financial support: Financial support for this study was provided by a grant from the Walter F. Coxe Research Fund of the Birmingham Audubon Society (to Christopher J. W. McClure) and a grant from the National Institute of Allergy and Infectious Diseases Project R01AI049724 (to Thomas R. Unnasch and Geoffrey E. Hill).
Authors' addresses: Laura K. Estep, Christopher J. W. McClure, and Geoffrey E. Hill, Department of Biological Sciences, Auburn University, Auburn, AL, E-mails: lke0001@auburn.edu, chrimcc@gmail.com, and hillgee@auburn.edu. Nathan D. Burkett-Cadena, Department of Entomology and Plant Pathology, Auburn University, Auburn, AL, E-mail: burkend@auburn.edu. Hassan. K. Hassan and Thomas R. Unnasch, Global Health Infectious Disease Research Program, Department of Global Health, College of Public Health, University of South Florida, Tampa, FL, E-mails: hhassan@health.usf.edu and tunnasch@health.usf.edu. Tyler L. Hicks, Department of Environmental and Natural Resource Sciences, Washington State University Vancouver, Vancouver, WA, E-mail: tyler_hicks@wsu.edu.
References
- 1.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 2.Hassan HK, Cupp EW, Hill GE, Katholi CR, Klingler K, Unnasch TR. Avian host preference by vectors of eastern equine encephalomyelitis virus. Am J Trop Med Hyg. 2003;69:641–647. [PubMed] [Google Scholar]
- 3.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proc R Soc Lond B Biol Sci. 2006;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrican LA, Hackett LE, Briggs JE, McGowan JW, Unnasch TR, Lee JH. Host-feeding patterns of Culex mosquitoes in relation to trap habitat. Emerg Infect Dis. 2007;13:1921–1923. doi: 10.3201/eid1312.070275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, Anderson M, Charnetzky D, McMillen L, Unnasch EA, Unnasch TR. Host choice and West Nile virus infection rates in blood fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee 2002–2003. Vector Borne Zoonotic Dis. 2007;7:365–386. doi: 10.1089/vbz.2006.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diuk-Wasser MA, Molaei G, Simpson JE, Folsom-O'Keefe CM, Armstrong PM, Andreadis TG. Avian communal roosts as amplification foci for West Nile virus in urban areas in northeastern United States. Am J Trop Med Hyg. 2010;82:337–343. doi: 10.4269/ajtmh.2010.09-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackay AJ, Kramer WL, Meece JK, Brumfield RT, Foil LD. Host feeding patterns of Culex mosquitoes (Diptera: Culicidae) in East Baton Rouge Parish, Louisiana. J Med Entomol. 2010;47:238–248. doi: 10.1603/me09168. [DOI] [PubMed] [Google Scholar]
- 8.Carver S, Bestall A, Jardine A, Ostfeld RS. Influence of hosts on the ecology of arboviral transmission: potential mechanisms influencing dengue, Murray Valley encephalitis, and Ross River virus in Australia. Vector-borne Zoonot. 2009;9:51–64. doi: 10.1089/vbz.2008.0040. [DOI] [PubMed] [Google Scholar]
- 9.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:606–610. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson JE, Folsom-O'Keefe CM, Childs JE, Simons LE, Andreadis TG, Diuk-Wasser MA. Avian host-selection by Culex pipiens in experimental trials. PLoS One. 2009;4:e7861. doi: 10.1371/journal.pone.0007861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuno G, Chang GJJ. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev. 2005;18:608–637. doi: 10.1128/CMR.18.4.608-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turrell MJ, Dohm DJ, Sardelis MR, O'Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- 13.Weaver SC. In: The Encyclopedia of Arthropod-Transmitted Infections. Service MW, editor. New York, NY: CABI Publishing; 2001. pp. 151–159. (Eastern equine encephalitis). [Google Scholar]
- 14.Scott TW, Weaver SC. Eastern equine encephalomyelitis virus: epidemiology and evolution of mosquito transmission. Adv Virus Res. 1989;37:277–328. doi: 10.1016/s0065-3527(08)60838-6. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SB, Lewoczko K, Huddleston DB, Moody E, Mukherjee S, Dunn JR, Jones TF, Wilson R, Moncayo AC. Host feeding patterns of potential vectors of eastern equine encephalitis virus at an epizootic focus in Tennessee. Am J Trop Med Hyg. 2009;81:452–456. [PubMed] [Google Scholar]
- 16.Cupp EW, Klinger K, Hassan HK, Viguers LM, Unnasch TR. Eastern equine encephalomyelitis virus transmission in central Alabama. Am J Trop Med Hyg. 2003;68:495–500. [PMC free article] [PubMed] [Google Scholar]
- 17.Burkett-Cadena ND, Graham SP, Hassan HK, Guyer C, Eubanks MD, Katholi CR, Unnasch TR. Blood feeding patterns of potential arbovirus vectors of the genus Culex targeting ectothermic hosts. Am J Trop Med Hyg. 2008;79:809–815. [PMC free article] [PubMed] [Google Scholar]
- 18.Royle JA. N-mixture models for estimating population size from spatially replicated counts. Biometrics. 2004;60:108–115. doi: 10.1111/j.0006-341X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 19.Burkett-Cadena ND, Eubanks MD, Unnasch TR. Preference of female mosquitoes for natural and artificial resting sites. J Am Mosq Control Assoc. 2008;24:228–235. doi: 10.2987/5662.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estep LK, Burkett-Cadena ND, Hill GE, Unnasch RS, Unnasch TR. Estimation of dispersal distances of Culex erraticus in a focus of eastern equine encephalitis virus in the southeastern United States. J Med Entomol. 2010;47:977–986. doi: 10.1603/me10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, Nasci RS. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Hassan H, Hill G, Cupp EW, Higazi TB, Mitchell CJ, Godsey MS, Jr, Unnasch TR. Identification of mosquito avian-derived blood meals by polymerase chain reaction-heteroduplex analysis. Am J Trop Med Hyg. 2002;66:599–604. doi: 10.4269/ajtmh.2002.66.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleiner KJ, Mackenzie MD, Silvano AL, Grand JA, Grand JB, Hogland J, Irwin ER, Mitchell MS, Taylor BD, Earnhardt T, Kramer E, Lee J, McKerrow AJ, Rubino MJ, Samples K, Terando A, Williams SG. GAP Land Cover Map of Ecological Systems for the State of Alabama (Provisional). Alabama Gap Analysis Project. 2007. www.auburn.edu\gap Available at. Accessed January 28, 2008. [Google Scholar]
- 24.Homer C, Huang C, Yang L, Wylie B, Coan M. Development of a 2001 National Landcover Database for the United States. Photogramm Eng Remote Sensing. 2004;70:829–840. [Google Scholar]
- 25.Chandler RB, King DI, Destefano S. Scrub-shrub bird habitat associations at multiple spatial scales in beaver meadows in Massachusetts. Auk. 2009;126:186–197. [Google Scholar]
- 26.Hines JE. Presence 2.1. Software to Estimate Patch Occupancy and Related Parameters. USGS-PWRC. 2006. www.mbr-pwrc.usgs.gov/software/presence.html Available at. Accessed December 1, 2009. [Google Scholar]
- 27.McClure CJW, Estep LK, Hill GE. Using public land cover data to determine habitat associations of breeding birds in Tuskegee National Forest, Alabama. South J Appl For. in press. [Google Scholar]
- 28.Johnson DH, Gibbs JP, Herzog M, Lor S, Niemuth ND, Ribic CA, Seamans M, Shaffer TL, Shriver WG, Stehman SV, Thompson WL. A sampling design framework for monitoring secretive marshbirds. Waterbirds. 2009;32:203–215. [Google Scholar]
- 29.Hess AD, Hayes RO, Tempelis CH. The use of the foraging ratio technique in mosquito host preference studies. Mosq News. 1968;28:386–389. [Google Scholar]
- 30.Manly BF, McDonald L, Thomas DL, McDonald TL, Erickson WP. Resource Selection by Animals: Statistical Design and Analysis for Field Studies. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2002. pp. 50–62. [Google Scholar]
- 31.Sauer JR, Hines JE, Fallon J. The North American Breeding Bird Survey, Results and Analysis 1966–2002. Laurel, MD: USGS Patuxent Wildlife Research Center; 2003. [Google Scholar]
- 32.Solow AR. A simple test for change in community structure. J Anim Ecol. 1993;62:191–193. [Google Scholar]
- 33.Peet RK. The measurement of species diversity. Annu Rev Ecol Syst. 1974;5:285–307. [Google Scholar]
- 34.Molaei G, Oliver J, Andreadis TA, Armstrong PM, Howard JJ. Molecular identification of blood-meal sources in Culiseta melanura and Culiseta morsitans from an endemic focus of eastern equine encephalitis virus in New York. Am J Trop Med Hyg. 2006;75:1140–1147. [PubMed] [Google Scholar]
- 35.Cupp EW, Tennessen KJ, Oldland WK, Hassan HK, Hill GE, Katholi CR, Unnasch TR. Mosquito and arbovirus activity during 1997–2002 in a wetland in northeastern Mississippi. J Med Entomol. 2004;41:495–501. doi: 10.1603/0022-2585-41.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayes RO, Maxfield HK. Interruption of diapause and rearing larvae of Culiseta melanura (Coq.) Mosq News. 1967;27:458–461. [Google Scholar]
- 37.Stamm DD. Studies on the ecology of equine encephalomyelitis. Am J Public Health. 1958;48:328–335. doi: 10.2105/ajph.48.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edman JD, Kale HW. Host behavior: its influence on the feeding success of mosquitoes. Ann Entomol Soc Am. 1971;64:513–516. [Google Scholar]
- 39.Webber LA, Edman JD. Anti-mosquito behavior of ciconiiform birds. Anim Behav. 1972;20:228–232. [Google Scholar]
- 40.Edman JD, Day JF, Walker ED. Field confirmation on the different antimosquito behavior of herons. Condor. 1984;86:91–121. [Google Scholar]
- 41.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komar N, Dohm DJ, Turell MJ, Spielman A. Eastern equine encephalitis virus in birds: relative competence of European starlings (Sturnus vulgaris) Am J Trop Med Hyg. 1999;60:387–391. doi: 10.4269/ajtmh.1999.60.387. [DOI] [PubMed] [Google Scholar]
- 43.McLean RG, Frier G, Parham GL, Francy DB, Monath TP, Campos EG, Therrien A, Kerschner J, Calisher CH. Investigations of the vertebrate hosts of eastern equine encephalitis during an epizootic in Michigan, 1980. Am J Trop Med Hyg. 1985;34:1190–1202. doi: 10.4269/ajtmh.1985.34.1190. [DOI] [PubMed] [Google Scholar]
- 44.Crans WJ, Caccamise DF, McNelly JR. Eastern equine encephalomyelitis virus in relation to the avian community of a coastal cedar swamp. J Med Entomol. 1994;31:711–728. doi: 10.1093/jmedent/31.5.711. [DOI] [PubMed] [Google Scholar]


