Abstract
Loiasis, which is caused by the filarial nematode Loa loa, affects millions of persons living in the rainforest areas and savannah regions of central Africa. Typical manifestations are calabar swellings and the eyeworm. We report a case of loiasis with unusual clinical complications: a peripheral neuropathy and focal hypo-echogenic lesions of the spleen, which disappeared after treatment with albendazole and ivermectin. The literature reports that L. loa infection can be associated with various manifestations, some of them being serious. More information is needed to better characterize the protean manifestations of the disease in loiasis-endemic areas to evaluate the true incidence of loiasis.
Introduction
Loa loa is widely distributed in the rain forests and some Guinean savannah areas of central Africa.1 Adult worms living in subcutaneous tissues and in the fascial layers overlying the somatic muscles produce microfilariae circulating in peripheral blood with a diurnal periodicity. Both parasite stages are able to produce a variety of clinical manifestations in different body locations. The so-called calabar swellings, corresponding to an angioedema of allergic nature,2 and the passage of an adult worm under the conjunctiva (eyeworm) are the manifestations most frequently reported. We report a case of L. loa infection with unusual manifestations.
Case Report
A 28-year-old woman from Italy was admitted to our Center for Tropical Diseases on June 16, 2008. She had traveled to Kenya seven years earlier (for one month), Mozambique four years earlier (for eight months), and Gabon three years earlier (for four months). On her return from Gabon in January 2006, she was subjected to routine blood tests, which showed 660 eosinophils/μL. In March 2006, she reported episodes of migrant transient edemas in both upper and lower limbs. In January 2007, she went to Ethiopia, where she is currently living. In May 2007, after having 10–15 calabar swellings within the last 14 months, a sudden sensory and motor deficit of the fourth and fifth fingers of the left hand developed. This deficit was not associated with an edema and after the onset of this neuropathy, no episodes of edema occurred. She was treated with corticosteroids and physiotherapy and showed slight improvement.
In June 2007, while in Italy, she was admitted to a neurology ward to investigate her neurologic signs: electromyography showed abnormalities at the level of the ulnar nerve with signs of denervation. She was discharged without therapy and with a diagnosis of sensory-motor neuropathy of the left brachial plexus. Leprosy was ruled out by a thorough clinical examination, which failed to show any skin lesion or involvement of other nerves. In March 2008, abdominal ultrasonography was performed to investigate marked and persistent hypereosinophilia (5,100/μL in September 2006, 1,870/μL in June 2007, and 2,410/μL in March 2008) of the patient. Ultrasonography showed multiple hypo-echogenic areas (maximum diameter = 1.5 cm) in the spleen, which were absent in June 2007. In May 2008, the patient was admitted to an infectious diseases ward in Italy to further study her hypereosinophilia. Blood smears and stool specimens were negative for parasites. Brain and spinal cord magnetic resonance imaging (MRI) did not show any pathologic changes, and an abdominal computed tomography scan and ultrasonography confirmed focal lesions in the spleen. Under suspicion of a lymphoma, a bone marrow biopsy was scheduled, but interrupted because of pain. A splenectomy was proposed to the patient but she preferred to consult our center.
In June 2008, the patient was afebrile and in good general conditions. Edema, lymphadenopathy, and organomegaly were not found. A neurologic examination showed hypotrophy and sensory-motor deficit of the left forearm and the fourth and fifth fingers of the left hand (Figure 1). Blood tests showed 3,300 eosinophils/μL. The C reactive protein level, erythrocyte sedimentation rate, and IgG level were normal; the gamma globulin level was slightly increased. Urine sediment was normal. Results of serial stool examinations and serologic tests for human immunodeficiency virus, hepatitis B and C viruses, Schistosoma, malaria, Strongyloides, Trypanosoma, and amoeba were all negative. A peripheral blood sample obtained at noon showed, by using the leuko-concentration method, 172 microfilariae in 13 mL of blood. The diagnosis of infection with L. loa was confirmed by examination of blood smears stained with Giemsa, which showed typical microfilariae, with nuclei extending to tip of tail (Figure 2).
Figure 1.
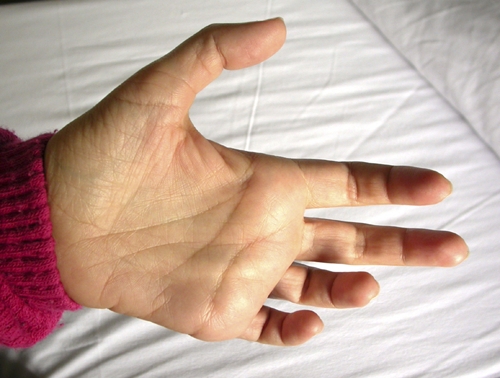
Affected hand of the patient, showing the clawing of the fourth and fifth fingers (ulnar clawing) and mild hypothenar muscle atrophy. This figure appears in color at www.ajtmh.org.
Figure 2.
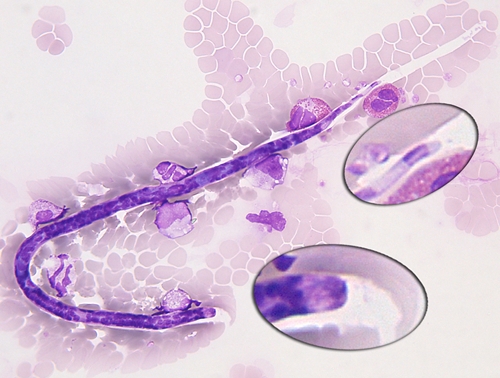
Loa loa microfilaria found in the bloodstream of the patient, showing the nuclei extending to tip of tail. This figure appears in color at www.ajtmh.org.
A chest radiograph was normal, and a CT scan showed negative results. Abdominal ultrasonography confirmed focal lesions (maximum diameter = 1.5 cm) in the spleen, whose size was otherwise within normal limits (longitudinal axis = 11 cm). Upper abdominal MRI showed hyperintense nodular lesions of the spleen in T1 fat saturation, delayed post-contrastographic phase (Figure 3), and an MRI of the brachial plexus did not show abnormalities. Electromyography confirmed severe abnormalities at the level of the ulnar nerve.
Figure 3.
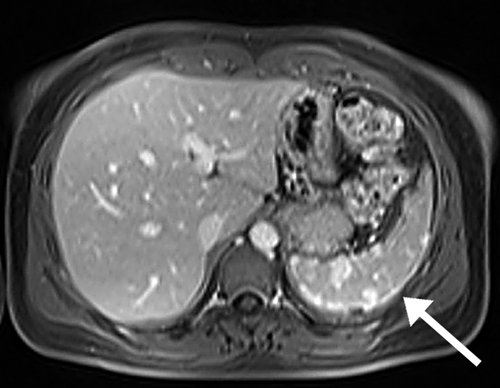
Upper abdominal magnetic resonance imaging of the patient, showing hyperintense lesions of the spleen in T1 fat saturation, delayed post-contrastographic phase.
The patient was treated with ivermectin (200 μg/kg in single dose), followed by albendazole (400 mg twice a day for 28 days) and prednisone (37.5 mg/day for 7 days and then decreasing doses for 3 weeks). The neurologic signs improved progressively during the month after the start of treatment. At follow-up six and twelve months after treatment the eosinophil count was within the normal range, the spleen lesions had disappeared when examined by ultrasonography, and the patient could move her fingers normally, needing only physiotherapy to increase muscle tone.
Discussion
If we consider the previous residence of the patient in a loiasis-endemic country (Gabon), persistence of a high eosinophil count, and the history of calabar swellings, we suspected loiasis, which was confirmed by microscopy. We wondered if the other key findings, the peripheral neuropathy, and the focal hypo-echogenic lesions of the spleen, could be attributed to the same cause. Although microfilariae were not isolated from the spleen, providing definitive evidence of splenic involvement in this case, the fact that the neurologic signs and the splenic lesions, which were present for at least one year before admission to our center, disappeared after treatment with albendazole and ivermectin supports the conclusion that they were caused by Loa infection. In addition, a literature review on rare clinical manifestations of loiasis shows that cases of Loa-related neuropathies and splenic lesions have been reported.
It is well known that antifilarial drugs, especially diethylcarbamazine (DEC) and ivermectin, can induce an encephalopathy in patients with high Loa microfilaremias.3,4 In addition, L. loa may also induce, even in absence of any treatment, various central neurologic manifestations, including unilateral or focal motor and/or sensory deficits,5–11 meningitis,12 encephalitis,13–17 or mental disturbances.8–10,18–21
Peripheral neurologic symptoms in patients with loiasis are less known. These symptoms were mentioned in earlier editions of Manson's Tropical Diseases22 and by Kivits who wrote in 1952, without further details, that “the only troubles caused by loiasis are calabar swellings, the unpleasant sensation of migratory movements of the adult worms, mainly under the conjunctiva, itching, and ‘phenomena of neuritis.’”13 Subsequently, six detailed cases were reported by various authors; one report described two cases.23 Similar to our patient, all patients were native to countries to which loiasis was not endemic. Four were from Europe,23–25 one from New Zealand,26 and one from Senegal.27 They had stayed for several months or years in Nigeria,23,26 Cameroon,24,25 or Gabon.26 In addition, all had a history of calabar swellings, sometimes fairly frequent. In four patients, neurologic manifestations occurred after appearance of a persistent swelling of one forearm or one wrist, sometimes extending to the hand, or after episodes of swellings repeated in quick succession at these locations.23,24,26,27 In the two other patients, neuropathy was induced by implementation of DEC treatment for loiasis, which also induced local edema.23,25 In all but one of the patients, signs consisted of motor and sensory deficits (in one case,25 the deficit was only sensory). Nerves affected were either the ulnar nerve,23,24 the median nerve,23,25,26 or both.27 Microfilariae were found in blood in three patients,23,24,27 and the other three patients were amicrofilaremic.23,25,26 One of the four patients with spontaneous loiasis was treated by surgical decompression of the transverse carpal ligament;26 the others three were treated with a course of DEC over several weeks.23,24,27 In the latter cases, treatment resulted in progressive improvement,27 and two patients had a full recovery within 1–3 months.23,24 No details were given about the outcome of one of the patients with DEC-induced neuropathy.25 In the other patient, improvement was only partial, and a slight motor deficit was still present 10 weeks after the appearance of neuropathy.23
On the basis of these cases, it appears that peripheral neurologic manifestations can occur either spontaneously, usually after a persistent calabar swelling, or as a result of treatment. Nutman and Kradin, who reported a case in a patient probably infected with L. loa and in whom edema and intermittent numbness and tingling in a leg developed, without frank deficit, highlight that when calabar swelling is sustained and particularly pronounced, paresthesia and entrapment neuropathies can result.28 Alternatively, close anatomic presence of worms (dead or encysted) near nerves has been advanced as a possible explanation for the peripheral neuropathies associated with loiasis.23 Such a mechanism probably occurred in other cases in which the filarial species involved was not L. loa.29–31
In our patient, edema had disappeared when the patient was examined in our center. Persistence of a sensory-motor deficit at that time may be attributed to neurologic signs accompanying compression neuropathies that can persist for a long time after treatment.32 Alternatively, it might have been caused by the presence of an adult worm remaining near the nerve, as suggested by Schofield.23 The latter mechanism would explain the fairly rapid effect of treatment on the neurologic signs. The effect of ivermectin on adult L. loa is not known, but the long-term effect of a single dose on microfilaremia indicates that the drug might be partially macrofilaricidal.33 Similarly, the kinetics of microfilarial clearance observed in loiasis patients treated with a three-week course of albendazole (200 mg twice a day, i.e. at lower doses than for our patient) might also reflect a macrofilaricidal effect of this regimen.34 Such an effect on the adult filariae has also been suggested for Wuchereria bancrofti.35 In our patient, disappearance of the adult worm after treatment would have reduced compression on the ulnar nerve and led secondarily to an improvement of the neurologic signs.
One striking feature is that all patients with Loa-related peripheral neuropathies reported were identified in expatriates. Because of lack of information on the denominator, the incidence of the condition cannot be compared between expatriates and the population living in loiasis-endemic areas. However, one may consider the possibility that these neuropathies are more frequent in persons exposed to the parasite at the adult age, as is the case for other manifestations of loiasis.36,37 The fact that calabar swellings, a factor that induces neuropathies, are more frequent and more severe in expatriates would explain the propensity of this group to have Loa-related neuropathies.
Hypo-echogenic spleen lesions had been reported in two patients with loiasis;38 both patients had splenectomies because of suspected lymphoma. However, histologic examination showed eosinophilic granulomata containing L. loa microfilariae. Splenectomy was also considered for our patient because of hypereosinophilia associated with hypo-echogenic spleen lesions. In all three patients, lesions were unexpectedly found in clinical imaging screenings more than two years after exposure. To our knowledge, our case is the first reported case of loiasis-related spleen lesions documented by MRI and found to have completely resolved after therapy.
Reports showed that physicians in tropical regions noticed the involvement of spleen in filarial diseases, including loiasis, even when ultrasonographic techniques were not available. However, information is scarce. Klotz described fibrotic spleen nodules in two patients in Nigeria who were infected with L. loa and had microfilariae in blood.39 Vascular channels within these nodules contained numerous eosinophils and more L. loa microfilariae than those of unaltered parts of the spleen. For the patient reported by Van Bogaert and others,40 who died after encephalopathy developed after DEC treatment, the spleen was found to be moderately enlarged. Foci of necrosis centered by large numbers of microfilariae and limited by a zone of fibrous reaction were found in the red pulp. Negesse and others reported their findings for a patient in whom encephalopathy developed whose spontaneous nature could not be ascertained.16 In this patient, the spleen red pulp was congested and contained many microfilariae; some of them were degenerating and had provoked granulomatous foci.
Paradoxically, more information about involvement of the spleen in Loa infection is available for monkeys experimentally infected with the parasite. It is known that in the drill and other susceptible monkeys, the increase in the microfilaremia occurring 150 days after infection is followed several weeks afterwards by a decrease to low levels, a phenomenon known as suppressed infection.41,42 At this time, the spleen appears enlarged and grossly nodular. If the primates are splenectomized at this stage, microfilaremia increases and remains at high levels. In another study, Duke showed that spleens of monkeys infected with Loa and treated with DEC are gradually restored to their previous condition.43
The role of spleen in human loiasis is not clear. Duke reported that the processes seen in monkeys would not occur in humans on the basis of the following considerations. 43 First, he believed that microfilariae tended to persist for years at a constant high level. Second, he shared the opinion of Raper44 that the fibrotic nodules described by Klotz 39 could be attributed to a different etiology, and that L. loa microfilariae in these nodules was probably incidental.44 We now know that many cases of loiasis are diagnosed with a low or even undetectable level of microfilaremia, particularly so in expatriates.36,37 Fain considered that in some cases microfilariae are killed by the host immune system, and quoting the considerations of Klotz and the experiments of Duke, speculated on the possible role of the spleen in the process.45 It has been shown that host genetic factors, probably associated with specific immunologic responses against the Loa microfilariae, predispose a person to become microfilaremic or amicrofilaremic.46 Such factors might explain rare occurrence of splenic involvement in loiasis in humans. As stated by Fain, histologic examinations of spleen tissue in persons infected with L. loa would shed light on this issue.
Although loiasis constitutes one of the most common reasons for medical consultations in some areas of West Africa, there is little interest in this neglected disease because it has long been regarded as benign. However, this parasite is so common and causes so much morbidity that it certainly deserves much more attention. Review of reported occasional or rare presentations clearly indicates that adult worms can be expected in any location, thus causing the most variable spectrum of clinical manifestations: deep organ involvement and serious sequelae can be expected especially when the diagnosis is delayed. Physicians and imaging specialists could make major contributions to solving these neglected aspects of the disease. More information is needed to better characterize the protean manifestations of this disease in loiasis-endemic areas to evaluate the true incidence of loiasis.
ACKNOWLEDGMENTS
We thank Professor Thomas Orihel for critical reading of the manuscript.
Footnotes
Authors' addresses: Federico Gobbi, Andrea Angheben, Maria Gobbo, Andrea Rossanese, and Zeno Bisoffi, Centro per le Malattie Tropicali, Ospedale Sacro Cuore-Don Calabria, Negrar, Verona, Italy, E-mails: federico.gobbi@sacrocuore.it, andrea.angheben@sacrocuore.it, maria.gobbo@sacrocuore.it, andrea.rossanese@sacrocuore.it, and zeno.bisoffi@sacrocuore.it. Michel Boussinesq, Unité Mixte de Recherche 145, Institut de Recherche pour le Développement and Université Montpellier 1, Montpellier, France, E-mail: michel.boussinesq@ird.fr. Marta Mascarello, Dipartimento di Malattie Infettive, Policlinico G. B. Rossi, Verona, Italy, E-mail: martamasca@yahoo.it. Manuel Corachán, Centre de Recerca en Epidemiologia i Salut Internacional, Hospital Clinic (CRESIB), Barcelona, Spain, E-mail: manuel.corachan@mundivia.es.
References
- 1.Boussinesq M, Gardon J. Prevalences of Loa loa microfilaraemia throughout the area endemic for the infection. Ann Trop Med Parasitol. 1997;91:573–589. doi: 10.1080/00034989760671. [DOI] [PubMed] [Google Scholar]
- 2.Chandler AC, Milliken G, Schuhardt VT. The production of a typical clabar swelling in a Loa patient by injection of a Dirofilaria immitis antigen, and some comments on the nature of Calabar swellings. Am J Trop Med. 1930;10:345–351. [Google Scholar]
- 3.Carme B, Boulesteix J, Boutes H, Puruehnce MF. Five cases of encephalitis during treatment of loiasis with diethylcarbamazine. Am J Trop Med Hyg. 1991;44:684–690. doi: 10.4269/ajtmh.1991.44.684. [DOI] [PubMed] [Google Scholar]
- 4.Boussinesq M, Gardon J, Gardon-Wendel N, Chippaux JP. Clinical picture, epidemiology and outcome of Loa-associated serious adverse events related to mass ivermectin treatment of onchocerciasis in Cameroon. Filaria J. 2003;2((Suppl)):S4. doi: 10.1186/1475-2883-2-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertrand-Fontaine M. La filaire de l'oeil (filaria loa) peut-elle déterminer des complications cérébrales? Gaz Hebdom Sci Med (Bordeaux) 1913;30:351–354. [Google Scholar]
- 6.Bertrand-Fontaine M, Schneider J, Wolfromm R, Cagnard V. Un cas de filariose cérébrale (double hémiplégie au cours d'une filariose à F. Loa) Bull Mem Soc Med Hop Paris. 1948;64:1092–1095. [Google Scholar]
- 7.Browne SG. Nematodosis of the central nervous system. J Trop Med Hyg. 1954;57:229–233. [PubMed] [Google Scholar]
- 8.Gallais P, Collomb H, Guedel J. Les manifestations neuro-psychiques des filarioses. Med Trop. 1954;14:663–677. [PubMed] [Google Scholar]
- 9.Bauer H, Bischoff A, Hansen J, Magun R. Loa loa filariosis mit cerebralen komplikationen als berufskrankheit. Arch Gewerbepathol Gewerbehyg. 1957;15:429–439. [PubMed] [Google Scholar]
- 10.Alajouanine T, Castaigne P, Lhermitte F, Cambier J. Encéphalite puis endocardite fibroblastique d'origine filarienne. Rev Neurol (Paris) 1959;101:656–660. [PubMed] [Google Scholar]
- 11.Langlois M, Perrouty B, Daoulas R, Berton M. Filariose loa, thrombose de l'artère centrale de la rétine et syndrome cérébelleux. Rev Neurol (Paris) 1962;107:381–385. [Google Scholar]
- 12.Bonnet M. Réflexions sur un cas de méningite aiguë à Microfilaria loa. Med Trop. 1943;3:273–277. [Google Scholar]
- 13.Kivits M. Quatre cas d'encéphalite mortelle avec invasion du liquide céphalo-rachidien par Microfilaria loa. Ann Soc Belg Med Trop. 1952;32:235–242. [PubMed] [Google Scholar]
- 14.Cauchie C, Rutsaert J, Thys O, Bonnyns M, Perier O. Encéphalite à Loa-loa traitée par l'association de cortisone et de carbamazine. Rev Belg Pathol Med Exp. 1965;31:232–244. [PubMed] [Google Scholar]
- 15.Same Ekobo A, Same-Voisin C, Eben-Moussi E, Ongmagne MJ. A propos d'un cas de méningo-encéphalite filarienne à Loa loa. Rappels des critères de diagnostic de certitude. Afr Med. 1981;20:359–361. [Google Scholar]
- 16.Negesse Y, Lanoie LO, Neafie RC, Connor DH. Loiasis: “calabar” swellings and involvement of deep organs. Am J Trop Med Hyg. 1985;34:537–546. doi: 10.4269/ajtmh.1985.34.537. [DOI] [PubMed] [Google Scholar]
- 17.Lukiana T, Mandina M, Situakibanza NH, Mbula MM, Lepira BF, Odio WT, Kamgno J, Boussinesq M. A possible case of spontaneous Loa loa encephalopathy associated with a glomerulopathy. Filaria J. 2006;10:5–6. doi: 10.1186/1475-2883-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenney M, Hewitt R. Psychoneurotic disturbances in filariasis, and their relief by removal of adult worms or treatment with Hetrazan. Am J Trop Med Hyg. 1950;30:895–899. doi: 10.4269/ajtmh.1950.s1-30.895. [DOI] [PubMed] [Google Scholar]
- 19.Juillet P, Savelli A, Bouvier S, Rigal J, Elis B. Manifestations psychopathologiques au cours des filarioses (à propos de trois cas) Ann Med Psychol (Paris) 1964;122:72–81. [PubMed] [Google Scholar]
- 20.Sizaret P, Degiovanni A, Degiovanni E, Simon JP. Filariose et troubles psychiques (à propos d'un cas) Ann Med Psychol (Paris) 1970;128:736–739. [PubMed] [Google Scholar]
- 21.Pays JF, Escalle JC, Cornet A, Brumpt L. Manifestations neuro-psychiques de la loase. A propos d'un cas de potomanie. Bull Soc Pathol Exot. 1976;69:265–272. [PubMed] [Google Scholar]
- 22.Manson-Bahr P. Manson's Tropical Diseases. 16th edition. London: Bailliere, Tindall & Cassell; 1966. p. 694. [Google Scholar]
- 23.Schofield FD. Two cases of loiasis with peripheral nerve involvement. Trans R Soc Trop Med Hyg. 1955;49:588–589. doi: 10.1016/0035-9203(55)90033-x. [DOI] [PubMed] [Google Scholar]
- 24.Sarkany I. Loiasis with involvement of peripheral nerves. Trans St Johns Hosp Dermatol Soc. 1959;42:49–51. [Google Scholar]
- 25.Bourgeade A, Nosny Y, Olivier-Paufique M, Faugère B. A propos de 32 cas d'oedèmes localisés récidivants au retour des tropiques. Bull Soc Pathol Exot. 1989;82:21–28. [PubMed] [Google Scholar]
- 26.Scott JAG, Davidson RN, Moody AH, Bryceson ADM. Diagnosing multiple parasitic infections: trypanosomiasis, loiasis and schistosomiasis in a single case. Scand J Infect Dis. 1991;2:777–780. doi: 10.3109/00365549109024307. [DOI] [PubMed] [Google Scholar]
- 27.MacLean I, Dumitru D, Robinson LR. July 1996 EMG Case of the Month. 1996. pp. 1–9. AAPM&R 1996 July.
- 28.Nutman TB, Kradin RL. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 1-2002. A 24-year-old woman with paresthesias and muscle cramps after a stay in Africa. N Engl J Med. 2002;346:115–122. doi: 10.1056/NEJMcpc010140. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher B, Khalifa M, Van Heerden P, Elbardisy N. Acute carpal tunnel syndrome due to filarial infection. Pathol Res Pract. 2002;198:65–67. doi: 10.1078/0344-0338-00187. [DOI] [PubMed] [Google Scholar]
- 30.Beaver PC, Horner GS, Bilos JZ. Zoonotic onchocercosis in a resident of Illinois and observations on the identification of Onchocerca species. Am J Trop Med Hyg. 1974;23:595–607. doi: 10.4269/ajtmh.1974.23.595. [DOI] [PubMed] [Google Scholar]
- 31.Simmons EH, Van Peteghem K, Trammell TR. Onchocerciasis of the flexor compartment of the forearm: a case report. J Hand Surg Am. 1980;5:502–504. doi: 10.1016/s0363-5023(80)80085-2. [DOI] [PubMed] [Google Scholar]
- 32.Bland JDP. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36:167–171. doi: 10.1002/mus.20802. [DOI] [PubMed] [Google Scholar]
- 33.Gardon J, Kamgno J, Folefack G, Gardon-Wendel N, Bouchité B, Boussinesq M. Marked decrease in Loa loa microfilaraemia six and twelve months after a single dose of ivermectin. Trans R Soc Trop Med Hyg. 1997;91:593–594. doi: 10.1016/s0035-9203(97)90041-9. [DOI] [PubMed] [Google Scholar]
- 34.Klion AD, Massougbodji A, Horton J, Ekoué S, Lanmasso T, Ahouissou NL, Nutman TB. Albendazole in human loiasis: results of a double-blind, placebo-controlled trial. J Infect Dis. 1993;168:202–206. doi: 10.1093/infdis/168.1.202. [DOI] [PubMed] [Google Scholar]
- 35.Jayakody RL, De Silva CS, Weerasinghe WM. Treatment of bancroftian filariasis with albendazole: evaluation of efficacy and adverse reactions. Trop Biomed. 1993;10:19–24. [Google Scholar]
- 36.Nutman TB, Miller KD, Mulligan M, Ottesen EA. Loa loa infection in temporary residents of endemic regions: recognition of a hyperresponsive syndrome with characteristic clinical manifestations. J Infect Dis. 1986;154:10–18. doi: 10.1093/infdis/154.1.10. [DOI] [PubMed] [Google Scholar]
- 37.Klion AD, Massougbodji A, Sadeler BC, Ottesen EA, Nutman TB. Loiasis in endemic and nonendemic populations: immunologically mediated differences in clinical presentation. J Infect Dis. 1991;163:1318–1325. doi: 10.1093/infdis/163.6.1318. [DOI] [PubMed] [Google Scholar]
- 38.Burchard GD, Reimold-Jehle U, Bürkle V, Kretschmer H, Vierbuchen M, Racz P, Lo Y. Splenectomy for suspected malignant lymphoma in two patients with loiasis. Clin Infect Dis. 1996;23:979–982. doi: 10.1093/clinids/23.5.979. [DOI] [PubMed] [Google Scholar]
- 39.Klotz O. Nodular fibrosis of the spleen associated with filaria Loa. Am J Trop Med. 1930;10:57–64. [Google Scholar]
- 40.Van Bogaert L, Dubois A, Janssens PG, Radermecker J, Tverdy G, Wanson M. Encephalitis in Loa-loa filariasis. J Neurol Neurosurg Psychiatry. 1955;18:103–119. doi: 10.1136/jnnp.18.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duke BO. Studies on loiasis in monkeys. II. The population dynamics of the microfilariae of Loa in experimentally infected drills (Mandrillus leucophaeus) Ann Trop Med Parasitol. 1960;54:15–31. [PubMed] [Google Scholar]
- 42.Orihel TC, Moore PJ. Loa loa: experimental infection in two species of African primates. Am J Trop Med Hyg. 1975;24:606–609. doi: 10.4269/ajtmh.1975.24.606. [DOI] [PubMed] [Google Scholar]
- 43.Duke BO. Studies on loiasis in monkeys. III. The pathology of the spleen in drills (Mandrillus leucophaeus) infected with Loa. Ann Trop Med Parasitol. 1960;54:141–146. [PubMed] [Google Scholar]
- 44.Raper AB. Fibrotic nodules of the spleen. J Pathol Bacteriol. 1959;78:1–16. [PubMed] [Google Scholar]
- 45.Fain A. Les problèmes actuels de la loase. Bull World Health Organ. 1978;56:155–167. [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia A, Abel L, Cot M, Richard P, Ranque S, Feingold J, Demenais F, Boussinesq M, Chippaux JP. Genetic epidemiology of host predisposition microfilaraemia in human loiasis. Trop Med Int Health. 1999;4:565–574. doi: 10.1046/j.1365-3156.1999.00442.x. [DOI] [PubMed] [Google Scholar]


