Abstract
Multiple linear regression models were fitted to look for associations between changes in the incidence rate of dengue fever and climate variability in the warm and humid region of Mexico. Data were collected for 12 Mexican provinces over a 23-year period (January 1985 to December 2007). Our results show that the incidence rate or risk of infection is higher during El Niño events and in the warm and wet season. We provide evidence to show that dengue fever incidence was positively associated with the strength of El Niño and the minimum temperature, especially during the cool and dry season. Our study complements the understanding of dengue fever dynamics in the region and may be useful for the development of early warning systems.
Introduction
Dengue fever (DF) is an infectious disease caused by the dengue virus, with mosquitoes acting as the vector. Symptoms include headaches, rashes, joint and muscle pains, and in a small proportion of cases, life-threatening complications such as dengue hemorrhagic fever and dengue shock syndrome.1 It is present in over 100 tropical and subtropical countries,2 and approximately 50–100 million cases are reported every year.3 The economic burden of the disease has been estimated in millions of international dollars (i.e., I$514 per ambulatory case and I$1,394 per hospitalized case)4 and thousands of disability adjusted life years (DALYs; i.e., 427 DALYs/million population).5 In Mexico, DF is endemic all over the country and presents a year-round transmission. Almost 60% of the cases occur in the southern part of the country, which is characterized by a warm and humid climate.
The absence of cold winters, the high concentration of population in urban areas,6 and the high marginalization7 observed in the region allow the development of the disease throughout the year.1 Behavioral and cultural factors also play a key role in the prevalence of the disease.1 For example, the close proximity and poor construction of houses and buildings in the cities, the use of natural ventilation instead of air conditioning, and low access to health services and health education interact to facilitate dengue transmission.1
Several studies indicate that DF shows a strong inter- and intra-annual variability8 that is the result of both extrinsic (e.g., climate variability) and intrinsic (e.g., host–virus interactions mediated by herd immunity and host susceptibility) factors.9 Both these factors drive the serotype-specific dynamics and may increase the incidence rate of the disease.10
Significant efforts have been made to understand the effects of climate variability on the transmission dynamics of DF. Increases in temperature, sea surface temperature, and precipitation and the presence of El Niño events have been associated with elevated DF incidence in Mexico11,12 and other countries.8,13,14
Previous studies relating climate variability to DF in Mexico have analyzed time series of DF cases up to 1 year,11,15,16 and only a few have analyzed series of up to 10 years.12,17 Such short series pose problems for disentangling the overall associations between climate variability and DF. Our study analyzes 23 years of reported DF cases to examine associations with temperature, precipitation, and El Niño events. We also analyze a larger geographical area than previous studies by including data from 12 provinces compared with previous studies that focused on smaller areas such as a few cities or municipalities.15,16
The aim of this paper was to examine if changes in the incidence rate of DF are associated with climate variability manifested in the presentation of El Niño events and variability in ambient temperature and precipitation in the warm and humid region of Mexico. The null hypothesis was that the cumulative incidence rate (CIR) of the disease is not statistically associated with climate variability.
Materials and Methods
Data.
Monthly DF notifications were obtained from the National System of Epidemiologic Surveillance (SINAVE) in Mexico for the period of January 1985 to December 2007 (Figure 1). DF notifications are defined as any legal notification of confirmed classic dengue fever and dengue hemorrhagic fever referred to SINAVE by the local health authorities.18 Because dengue hemorrhagic fever is only a severe presentation of DF, these cases were not analyzed separately. Data were obtained for the warm and humid provinces of Mexico (i.e., Campeche, Colima, Chiapas, Guerrero, Michoacán, Morelos, Nayarit, Oaxaca, Quintana Roo, Tabasco, Veracruz, and Yucatán). The classification was based on data provided by the National Institute of Statistics, Geography, and Informatics (INEGI). These provinces have a joint population of over 31 million people. This climatic region contains the majority (59.8%) of the total of DF cases occurring in Mexico. A total of 249,618 DF cases were reported in the warm and humid region of Mexico over the period from 1985 to 2007.
Figure 1.
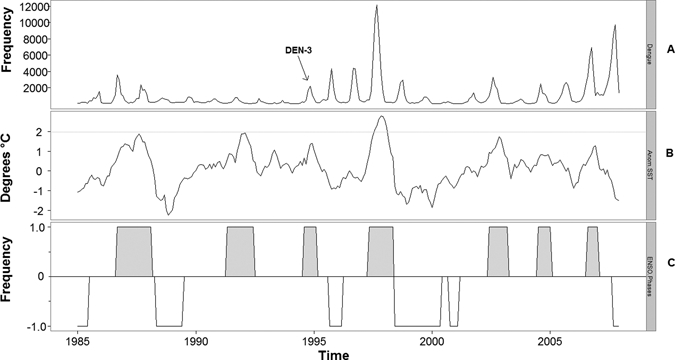
(A) Reported cases of dengue fever (DF). (B) Sea surface temperature (SST) anomalies in degrees Celcius. Notice that only the 1997–1998 El Niño exceeds the 2°C threshold (gray line). (C) El Niño (filled bars) and La Niña (white bars) events (zero frequencies indicate neutral phases).
To obtain an estimate of the strength of El Niño, we obtained monthly sea surface temperature (SST) data from the Climate Prediction Center of the National Oceanic and Atmospheric Administration.19 We chose the Niño Region 3.4 (5°N–5°S, 120°–170°W), because it is one of the most robust indexes for defining the evolution of El Niño events.20 These data are presented in Figure 1. We identified seven El Niño and six La Niña events in the series (Figure 1). Periods in between events were classified as neutral. Explanations on what constitutes an El Niño or La Niña event can be found elsewhere.21
Monthly minimum and maximum temperatures and precipitation per province were obtained from the Mexican National Meteorological Service. The provincial data were averaged to provide overall temperature and precipitation time series data for the region. The number of DF cases within each province was summed to provide overall time series data for the region. Within this warm and humid region, our data show that the year-round climate has two distinct seasons: cool and dry from November to May and warm and wet from June to October (Figure 2). During the warm and wet season, accumulated precipitation averages 200 mm, maximum temperature (Tmax) is 31°C, and minimum temperature (Tmin) is 18°C. In the cool and dry season, precipitation averages 42 mm, Tmax is 30°C, and Tmin is 14°C.
Figure 2.
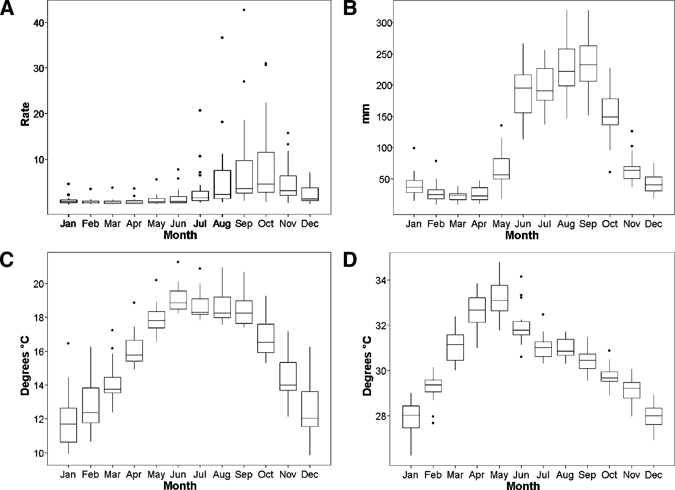
Box plots of the seasonal pattern of (A) DF incidence, (B) precipitation, (C) minimum temperature (Tmin), and (D) maximum temperature (Tmax) in the warm and humid region of Mexico for the period from 1985 to 2007.
The DF data were converted to a CIR based on the region population (cases per 100,000 population). Population size per province was obtained from INEGI for 1990, 1995, 2000, and 2005. Population size for the intervening years was estimated assuming linear growth. We stabilized the variance in the series taking the natural logarithm of the CIR (Ln-CIR).
Temporal analyses of the aggregated DF cases (including all serotypes) revealed interannual fluctuations with a strong seasonal component. The seasonality of the transmission shows a peak occurring in October (Figure 2). The aggregation of the in- and out-of-phase interannual cycles of each serotype and their seasonal components may be responsible for the seasonal peaks9 observed in the aggregated serotype time series.
Data analysis.
The first stage of the analysis was to establish the associations between the Ln-CIRs with the various lags of the explanatory variables to determine the optimal time lag for the final models. DF shows a non-linear dynamics, with strong seasonality and interannual oscillations22 that may result from the interaction of intrinsic and extrinsic factors.23
In these regression models, we controlled for long-term trends by including an index variable of time in the model, and we controlled for seasonal effects by including 0,1 dummy variables for each month. This is because long-term changes in DF incidence may result from non-climatic factors such as changes in the reporting practices. Similarly, seasonal changes may be caused by non-climatic factors such as holidays. A similar approach has been used in previous studies.15,24
Many lags of the explanatory variables were significantly correlated with the dependent variable (Table 1). Including all the related lags in a model would have led to significant collinearity. Consequently, we created new explanatory variables using the mean values of the two lags with the highest significant regression coefficients. The new variables were Tmin lagged 1 and 2 months (Tmin1,2), Tmax and SST in the current and previous months (Tmax0,1 and SST0,1), and precipitation lagged 6 and 7 months (precipitation6,7).
Table 1.
Regression coefficients of climatic variables versus the Ln-CIR
| Lag (months) | SST (°C) | Tmin (°C) | Tmax (°C) | Precipitation (dm) |
|---|---|---|---|---|
| 0 | 0.24* | 0.44* | 0.47* | −0.31 |
| −1 | 0.23* | 0.46* | 0.44* | −0.36 |
| −2 | 0.22† | 0.46* | 0.41* | −0.40 |
| −3 | 0.20† | 0.46* | 0.43* | −0.53‡ |
| −4 | 0.17‡ | 0.44* | 0.41* | −0.68† |
| −5 | 0.14‡ | 0.39* | 0.33† | −0.71* |
| −6 | 0.11 | 0.34* | 0.28‡ | −0.73* |
| −7 | 0.10 | 0.31* | 0.22 | −0.81* |
| −8 | 0.10 | 0.31* | 0.14 | −0.54‡ |
| −9 | 0.10 | 0.35* | 0.20 | −0.40 |
| −10 | 0.10 | 0.36* | 0.22 | −0.37 |
| −11 | 0.09 | 0.40* | 0.19 | −0.38 |
| −12 | 0.10 | 0.46* | 0.13 | −0.28 |
Models were controlled for time and season. Ln-CIR = natural logarithm of the cumulative incidence rate of DF.
Significant at the 0.001 level.
Significant at the 0.01 level.
Significant at the 0.05 level.
Before using these variables in the final models, non-stationarity was examined, because the statistical properties of epidemiological time series commonly vary with time8 and may lead to spurious regression problems.2 We conducted Phillips–Perron tests to verify the stationarity of all variables. The dependent variable and all four independent variables were stationary and consequently, were used in regression models.
We fitted different multiple linear regression models using the Ln-CIR as the dependent variable. The normal distribution of the series was corroborated with a Jarque–Bera test of composite normality. All models were adjusted for long-term trend and seasonality as described earlier. These were included in the model even if not significant. Analyses were conducted in SPSS version 16.0 and R version 2.9.2.25
The final models were produced in a series of stages. Initial models included SST0,1 as the explanatory variable. The next stage of models examined the impact of weather on the Ln-CIR by fitting monthly Tmin1,2, Tmax0,1, and precipitation6,7 as explanatory variables. In all models, Durbin–Watson tests were conducted to detect autocorrelation in the residuals. When the results were autocorrelated, an adjustment was included incorporating the Ln-CIR lagged by 1 month. Higher order lags were not required. However, this could not be achieved for models including SST, because this variable is highly autocorrelated. Including an adjustment for autocorrelation would have removed all the variability coming from the El Niño signal. We stratified the models for the presence of El Niño events and the rainy season to assess if the association between the variables increased during these events.
El Niño modulates variations in ambient temperature and precipitation in Mexico.26 Having produced models for first SST and subsequently, temperature and precipitation, the final stage was to fit models with SST and any weather variable significant in the earlier models. This would examine whether the effects of SST on DF are fully explained by weather.
In the dataset, we observed a significant increase in incidence (from ~6,700 cases per year from 1985 to 1996 to ~45,100 in 1997) coinciding with the strongest El Niño event (1997–1998) in our record. This period also saw the introduction of the DEN-3 serotype, which may have led to elevated DF because of lower herd immunity. We tested the influence of this extreme event on the model results by excluding the 13 months coinciding with this period from the series and fitting regression models as before.
Results
The averaged CIR increases from 1.99 cases per 100,000 population during the neutral period to 5.52 (ratio = 2.8) during the El Niño events and 3.10 (ratio = 1.6) during La Niña events (Table 2). Excluding the months coinciding with the 1997–1998 El Niño decreases the CIR to 4.10 (26% reduction) during the El Niño events. These changes in CIR are significant (P = 0.02).
Table 2.
CIR differences and ratios per SST period (per 100,000 population)
| Parameters | CIR difference | CIR ratio |
|---|---|---|
| Including 1997–1998 El Niño | ||
| El Niño vs. neutral period | 5.52–1.99 | 2.77 |
| El Niño vs. La Niña | 5.52–3.10 | 1.78 |
| La Niña vs. neutral period | 3.10–1.99 | 1.56 |
| Not including 1997–1998 El Niño | ||
| El Niño vs. neutral period | 4.10–1.99 | 2.06 |
| El Niño vs. La Niña | 4.10–3.10 | 1.32 |
| La Niña vs. neutral period | 3.10–1.99 | 1.56 |
The CIR increases from 1.6 cases per 100,000 population in the cool and dry season to 5.5 cases per 100,000 population (ratio = 3.4) during the rainy season (Table 3). The seasonality of the disease is similar to precipitation (Figure 2).
Table 3.
CIR differences and ratios per rainy season (per 100,000 population)
| Parameters | CIR difference | CIR ratio |
|---|---|---|
| Warm and wet season vs. cool and dry season | 5.5–1.6 | 3.4 |
The results from the first multiple regression are presented in Table 4 and indicate that a 1°C increase in SST0,1 results in monthly increases in the Ln-CIR (B = 0.238; P < 0.001). When the model was stratified into El Niño months, SST0,1 was significantly associated with the Ln-CIR during the El Niño months (B = 0.463; P = 0.028) but not during the non-El Niño periods (B = –0.126; P = 0.301). However, after removing the data during the 1997–1998 El Niño, SST0,1 was not significantly associated with DF. This observation was consistent for models non-stratified (B = 0.125; P = 0.095) and stratified by El Niño (B = –0.216; P = 0.512) as well as non-El Niño months (B = −0.133; P = 0.275), indicating that the previously observed association is highly influenced by the 1997–1998 event. The introduction of the serotype DEN-3 to the country in 1995 (Figure 1) and the lack of serotype identification of each case makes it almost impossible to disentangle whether the El Niño or the introduction of a new DF serotype is responsible for this peak in incidence.
Table 4.
Regression coefficients of the Ln-CIR versus SST0,1
| Model | N | SST0,1 | Adjusted R2 | |
|---|---|---|---|---|
| B (P < |t|) | 95% CI | |||
| Including 1997–1998 El Niño | ||||
| Whole year | 276 | 0.24 (0.00) | 0.11–0.37 | 0.46 |
| Stratified by El Niño | ||||
| Present | 75 | 0.46 (0.03) | 0.05–0.88 | 0.56 |
| Absent | 201 | −0.13 (0.30) | −0.36–0.11 | 0.38 |
| Not including 1997–1998 El Niño | ||||
| Whole year | 263 | 0.13 (0.10) | −0.02–0.27 | 0.44 |
| Stratified by El Niño | ||||
| Present | 62 | −0.22 (0.51) | −0.87–0.44 | 0.54 |
| Absent | 201 | −0.13 (0.28) | −0.37–0.11 | 0.38 |
Models were controlled for time and season. CI = confidence interval.
Table 5 examines the impact of weather (Tmin1,2, Tmax0,1, and precipitation6,7) on DF and indicates that monthly increases in the Ln-CIR (B = 0.079; P = 0.019) result after every 1°C increase in Tmin1,2. Such increases can be expected during the non-El Niño months (B = 0.108; P = 0.008) and the cool and dry season (B = 0.094; P = 0.015). Similar results were obtained after removing months during the 1997–1998 El Niño. No significant associations were found between the Ln-CIR and Tmax0,1 or precipitation6,7 in the models (Table 5). Previous studies used shorter lags for assessing the association between DF incidence and precipitation.12,17 However, the use of shorter precipitation lags for higher biological plausibility (i.e., precipitation2,3 and precipitation4,5) did not affect the results of the analyses.
Table 5.
Regression coefficients of the Ln-CIR versus Tmin1,2, Tmax0,1, and precipitation6,7
| Model | N | Tmin1,2 | Tmax0,1 | Precipitation6,7 | Adjusted R2 | |||
|---|---|---|---|---|---|---|---|---|
| B (P < |t|) | 95% CI | B (P < |t|) | 95% CI | B (P < |t|) | 95% CI | |||
| Including 1997–1998 El Niño | ||||||||
| Whole year | 274 | 0.08 (0.02) | 0.03–0.15 | 0.12 (0.07) | −0.01–0.24 | −0.26 (0.06) | −0.53–0.01 | 0.88 |
| Stratified by El Niño | ||||||||
| Present | 75 | 0.00 (0.97) | −0.14–0.14 | 0.16 (0.19) | −0.08–0.40 | −0.32 (0.31) | −0.94–0.31 | 0.87 |
| Absent | 199 | 0.11 (0.01) | 0.03–0.19 | 0.10 (0.22) | −0.06–0.26 | −0.27 (0.10) | −0.60–0.06 | 0.87 |
| Stratified by season | ||||||||
| Warm wet | 115 | 0.04 (0.55) | −0.09–0.18 | 0.00 (0.99) | −0.25–0.24 | −0.87 (0.04) | −1.70–0.05 | 0.85 |
| Cool dry | 159 | 0.09 (0.02) | 0.02–0.17 | 0.15 (0.05) | −0.00–0.30 | −0.21 (0.16) | −0.51–0.09 | 0.85 |
| Not including 1997–1998 El Niño | ||||||||
| Whole year | 261 | 0.11 (0.00) | 0.04–0.18 | 0.13 (0.06) | −0.00–0.26 | −0.26 (0.07) | −0.53–0.02 | 0.87 |
| Stratified by El Niño | ||||||||
| Present | 62 | 0.11 (0.35) | −0.12–0.34 | 0.22 (0.13) | −0.07–0.51 | −0.32 (0.36) | −1.07–0.37 | 0.84 |
| Absent | 200 | 0.11 (0.01) | 0.03–0.19 | 0.10 (0.23) | −0.06–0.25 | −0.27 (0.10) | −0.60–0.05 | 0.87 |
| Stratified by season | ||||||||
| Warm wet | 110 | 0.16 (0.06) | −0.00–0.32 | 0.03 (0.80) | −0.21–0.27 | −0.64 (0.13) | −1.47–0.18 | 0.83 |
| Cool dry | 152 | 0.09 (0.03) | 0.01–0.18 | 0.16 (0.05) | −0.00–0.32 | −0.21 (0.20) | −0.53–0.11 | 0.84 |
Models were controlled for time, season, and autocorrelation. CI = confidence interval.
Table 5 shows associations between Tmin1,2 and DF. In the final model, we examined whether there was a significant association between DF and El Niño after controlling for weather. This model is presented in Table 6 and includes SST0,1 and Tmin1,2 as explanatory variables. It indicates that, even after controlling for weather, there is still a significant association between SST and DF. The results show that monthly increases in the Ln-CIR result after every increase by 1°C in SST (B = 0.311; P < 0.001), indicating an influence of SST above and below its effects on temperature. The association is stronger (B = 0.714; P < 0.001) during the El Niño events. There were no associations between SST0,1 and DF during non-El Niño periods (B = 0.058; P = 0.558). The overall association between SST and the incidence of DF persisted after the removal of the 1997–1998 El Niño. However, after the series was stratified by the El Niño months, the association during El Niño months disappeared.
Table 6.
Regression coefficients of the Ln-CIR versus SST0,1
| Model | N | SST0,1 | Adjusted R2 | |
|---|---|---|---|---|
| B (P < |t|) | 95% CI | |||
| Including 1997–1998 El Niño | ||||
| Whole year | 275 | 0.31 (0.00) | 0.20–0.42 | 0.62 |
| Stratified by El Niño | ||||
| Present | 75 | 0.71 (0.00) | 0.31–1.12 | 0.70 |
| Absent | 200 | 0.06 (0.56) | −0.13–0.27 | 0.63 |
| Not including 1997–1998 El Niño | ||||
| Whole year | 262 | 0.15 (0.01) | 0.04–0.27 | 0.68 |
| Stratified by El Niño | ||||
| Present | 62 | −0.09 (0.72) | −0.60–0.42 | 0.73 |
| Absent | 200 | 0.05 (0.60) | −0.14–0.25 | 0.61 |
Models were controlled for time and season and adjusted for Tmin1,2. CI = confidence interval.
Discussion
Associations with El Niño.
The results show that, in the presence of an El Niño event, the risk of DF infection is 2.77 times higher in the warm and humid region of Mexico compared with the neutral El Niño phase. This is corroborated in the multiple regression analysis, indicating a statistically significant and positive association between DF incidence and the strength of El Niño. However, there is some uncertainty as to the validity of this result, because when the exceptionally strong 1997–1998 El Niño is removed from the analyses, the strength of the El Niño becomes marginally insignificant. This suggests that the impacts of an El Niño are only apparent above a threshold exceeded by the strongest events.
When some of the variability in the series is controlled for by including temperature as a covariate, the strength of the El Niño becomes significant in the models with and without the 1997–1998 El Niño. We, therefore, conclude that El Niño has an association with DF in the warm and humid region of Mexico, and this corroborates previous studies.17,27,28 This also indicates that El Niño influences the dynamics of the disease through mechanisms that are not fully explained by its influence on weather. These mechanisms may be related to changes in environmental factors (e.g., vegetation coverage), human behavior, or cultural artifacts (e.g., water-storage practices).
The concurrent introduction of the DEN-3 serotype and the exceptionally strong 1997–1998 El Niño makes it difficult to separate these two effects and questions the linking of the unusual increases in the levels of DF in 1997, which have been previously attributed to weather conditions related to the 1997–98 El Niño.29 The importance of the introduction of the DEN-3 serotype is strengthened by the observation that, throughout Mexico, 88% of DF cases in 1997 were DEN-3.30 It could, therefore, be argued that the introduction of the DEN-3 serotype was entirely responsible for the peak in DF cases seen in 1997. However, we have shown that El Niño was positively associated with DF during other time periods, and therefore, it is likely that both the introduction of DEN-3 and the El Niño were responsible for the unusually large increase in DF observed in 1997.
Disentangling extrinsic from intrinsic factors in a quantitative way requires serotype-specific data. Separating the DF cases into different serotypes was impossible because of the lack of such information on SINAVE.
Associations with temperature.
The results also showed that increases in Tmin1,2 are positively associated with DF incidence. Additionally, when we removed the 1997–1998 El Niño, Tmin1,2 remained significant. This corroborates previous studies conducted in other regions of Mexico and the world.12,31 When the model was subdivided by rainy season, Tmin1,2 was significant during the cool and dry season, which is when temperatures in the region are at their lowest. This could indicate that low temperatures in the cool and dry season hamper the biology of the vector or the virus, diminishing the likelihood of DF transmission.
Low temperatures have been previously associated with increased development time and larval mortality, which results in decreased transmission.32 At temperatures below the 16°C threshold, the length of the larval stages of the vector increases.30 Besides, the vector stops feeding in ambient temperatures lower than 17°C,33 resulting in lower transmission rates. Additionally, the virus cannot amplify within the vector at temperatures below 18°C,34 and low temperatures increase the time of the extrinsic incubation period (EIP) of the virus, increasing its likelihood of exceeding the time span of the vector.1
Conversely, rising temperatures shorten the EIP and the development rate of the vector and increase the biting and contact rates.35 This increases the percentage of infected mosquitoes and the likelihood of successful transmission.1,33 High temperatures generate reductions in the larval sizes, resulting in smaller adults13 that feed more often than the larger ones.36 Additionally, mosquitoes digest blood faster at higher temperatures, increasing their persistence of feeding.32
Temperature-influenced human behavior may also play a key role in the dynamics of the disease. During the warm and wet season, individuals spend more time indoors sheltering from the rain, high humidity, and warm temperatures. This increase in the time spent indoors interacts with the lack of air conditioning, increasing the vector–host contact rate and the risk of transmission.1 During the cool and dry season, temperatures are cooler, the relative humidity lower, and people spend more time outdoors. This may lead to lower levels of DF and is corroborated by the fact that warmer minimum temperatures in the cool and dry season are associated with elevated DF.
Tmax is not associated with DF incidence in the region. However, in the time series, Tmax0,1 is less variable (σ = 1.6) than Tmin1,2 (σ = 2.7), making it statistically less likely for an effect to be apparent throughout the analyses.
Associations with precipitation.
The risk of infection is higher during the warm and wet season, corroborating previous studies conducted in Mexico11,12,16,17 and some other countries.22,37 However, after the long-term time trend and seasonality are controlled for in the model, precipitation is not statistically associated with DF. This could indicate that, although water is required for mosquito breeding, there is enough rainfall in the region all year to create breeding sites, and therefore, variations in monthly precipitation do not affect DF incidence. It could also indicate that rainfall does not influence the survival of adult vectors directly38 because of their indoor activity or that water-holding containers used as breeding sites in the region may be mainly man-filled containers.39
Increases in human population in urban areas, uncontrolled urbanization, and lack of adequate public services are common in Mexico.7 High levels of urbanization increase the risk of DF.40 Inadequate or inefficient water supply and sewage and solid waste disposal services increase the likelihood of water stagnation and offer potential breeding sites for the vector.40 Inefficient or intermittent water supplies lead people to store water for domestic usage.32 In other cases, people store water just in case.41 These situations create potential breeding sites for mosquitoes independent of precipitation.
The lack of association of precipitation with DF incidence may, therefore, be intrinsically linked to the presence of competing factors (e.g., social, political, and cultural features) in the region. Understanding the role of each of these factors in the dynamics of the disease requires detailed entomological, epidemiological, and socioeconomic data and more advanced statistical and mathematical methods, and it is beyond the scope of this study.
Strengths and limitations.
Our study explores a larger time series and geographical area than previous studies conducted in the region. This allowed us to reduce problems of small numbers in DF incidence observed at the provincial level and explore associations between DF and a large number of El Niño events. The stratification of models into El Niño, non-El Niño, warm and wet season, and cool and dry season also allowed us to explore differential associations between DF and climate variability at different periods. The use of such a geographically large study area averages out variations in all the variables, making them less likely to show associations with one another.
Final remarks.
In this study, we used multiple linear regression analysis to assess the associations between DF and climate variability in the warm and humid region of Mexico. We found that the incidence of DF was positively associated with the strength of El Niño. This association, however, is hugely influenced by the exceptionally strong 1997–1998 El Niño, suggesting that the impacts of an El Niño are only apparent above a specific threshold exceeded by the strongest events. We also found a concurrence between the same 1997–1998 El Niño and the introduction of the DEN-3 serotype, which would have increased DF incidence because of low herd immunity. Such concurrence questions previous attributions of the 1997–1998 outbreak to climatic conditions and could indicate that previous studies on the associations between DF incidence and El Niño may have overestimated its influence on the disease. It is likely that the introduction of the DEN-3 serotype and the El Nino were responsible for the unusual increase in DF incidence in 1997.
The mechanisms by which El Niño influences the dynamics of DF are still not clear, because El Niño shows a statistical association above and below its influence on the local weather. We provide robust evidence that El Niño exerts an influence on DF incidence in accordance with previous studies.17,27,28
We also found that increases in minimum temperature, especially during the cool and dry season, were associated with elevated levels of DF. There are a number of biologically plausible reasons for this association, such as (1) increased development time and larval mortality of the vector at low temperatures, (2) alterations in the feeding behavior of the vector, (3) amplification problems and increased EIP of the virus, and (4) reduction in the time spent indoors in the cool and dry season.
Precipitation does not show a statistical association, suggesting that there are suitable places for mosquitoes to breed all year. Socio-cultural, biological, or epidemiological conditions may be of great importance in ensuring that there are enough breeding sites all year.
The effects of climate variability on the transmission dynamics of DF remain controversial. However, our study complements the understanding of DF dynamics in the region and may help in the development of early warning systems based on climatic factors.
There is no consistent evidence of likely changes in the amplitude or frequency of El Niño because of climate change in the 21st century.42 However, climate change is likely to increase temperatures in the region,43 increasing the spatial and geographical distribution of the disease as well as the length of the transmission period.44 Our results suggest that this will worsen DF incidence in the region, especially during the cool and dry season when incidence is currently low.
Long-term surveillance and research will play a key role in the study of changes in DF dynamics and distribution. Our results can be used to determine future incidence trends in the region, giving the opportunity to improve the control measures for the disease and strengthen the adaptive capacity of the population.
ACKNOWLEDGMENTS
The authors thank Dr. Richard B. Darlington from Cornell University for his thoughtful comments and two anonymous referees for helpful suggestions.
Footnotes
Financial support: Felipe J. Colón-González received a scholarship from the National Council of Science and Technology of Mexico (CONACYT).
Authors' addresses: Felipe J. Colón-González, Tyndall Centre for Climate Change Research (Headquarters), School of Environmental Sciences, University of East Anglia, Norwich, Norfolk, United Kingdom, E-mail: F.Colon@uea.ac.uk. Iain R. Lake and Graham Bentham, School of Environmental Sciences, University of East Anglia, Norwich, Norfolk, United Kingdom, E-mails: I.Lake@uea.ac.uk and G.Bentham@uea.ac.uk.
References
- 1.Reiter P. Climate change and mosquito-borne disease. Environ Health Perspect. 2001;109((Suppl 1)):141–161. doi: 10.1289/ehp.01109s1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh YH, Chen WS. Turning points, reproduction number, and impact of climatological events for multi-wave dengue outbreaks. Trop Med Int Health. 2009;14:628–638. doi: 10.1111/j.1365-3156.2009.02277.x. [DOI] [PubMed] [Google Scholar]
- 3.Günther J, Ramírez-Palacio LR, Pérez-Ishiwara DG, Salas-Benito JS. Distribution of dengue cases in the state of Oaxaca, Mexico, during the period 2004–2006. J Clin Virol. 2009;45:218–222. doi: 10.1016/j.jcv.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Suaya J, Shepard D, Siqueira J, Martelli C, Lum L, Tan L, Kongsin S, Jiamton S, Garrido F, Montoya R, Armien B, Rekol H, Castillo L, Caram M, Sah B, Sughayyar R, Tyo K, Halstead S. Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. Am J Trop Med Hyg. 2009;80:846–855. [PubMed] [Google Scholar]
- 5.Clark D, Mammen M, Jr, Nisalak A, Puthimethee V, Endy T. Economic impact of dengue fever/dengue hemorrhagic fever in Thailand at the family and population levels. Am J Trop Med Hyg. 2005;72:786–791. [PubMed] [Google Scholar]
- 6.INEGI Instituto Nacional de Estadística, Geografía e Informática. Población rural y urbana. 2005. http://cuentame.inegi.gob.mx/poblacion/rur_urb.aspx?tema= Available at. Accessed May 2, 2010.
- 7.CONAPO Consejo Nacional de Población. Índice de marginación a nivel localidad 2005. 2005. http://www.conapo.gob.mx/index.php Available at. Accessed February 14, 2010.
- 8.Cazelles B, Chavez M, De Magny C, Guégan J, Hales S. Time-dependent spectral analysis of epidemiological time-series with wavelets. J R Soc Interface. 2007;4:625–636. doi: 10.1098/rsif.2007.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wearing HJ, Rohani P. Ecological and immunological determinants of dengue epidemics. Proc Natl Acad Sci USA. 2006;103:11802–11807. doi: 10.1073/pnas.0602960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nisalak A, Endy TP, Nimmannitya S, Kalayanarooj S, Thisayakorn U, Scott RM, Burke DS, Hoke CH, Innis BL, Vaughn DW. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- 11.Koopman JS, Prevots DR, Vaca-Mann MA, Gómez-Dantés H, Zárate-Aquino ML, Longini IM, Jr, Sepúlveda-Amor J. Determinants and predictors of dengue infection in Mexico. Am J Epidemiol. 1991;133:1168–1178. doi: 10.1093/oxfordjournals.aje.a115829. [DOI] [PubMed] [Google Scholar]
- 12.Brunkard JM, Cifuentes E, Rothenberg SJ. Assessing the roles of temperature, precipitation, and ENSO in dengue re-emergence on the Texas-Mexico border region. Salud Publica Mex. 2008;50:227–234. doi: 10.1590/s0036-36342008000300006. [DOI] [PubMed] [Google Scholar]
- 13.Focks D, Brenner R, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg. 2000;62:11–18. [PubMed] [Google Scholar]
- 14.Chadee D, Shivnauth B, Rawlins S, Chen A. Climate mosquito indices and the epidemiology of dengue fever in Trinidad. Ann Trop Med Parasitol. 2007;101:69–77. doi: 10.1179/136485907X157059. [DOI] [PubMed] [Google Scholar]
- 15.Chowell G, Sanchez F. Climate-based descriptive models of dengue fever: the 2002 epidemic in Colima, Mexico. J Environ Health. 2006;68:40–44. [PubMed] [Google Scholar]
- 16.García J, Loroño M, Farfan JA, Flores L, Rosado E, Rivero N, Najera R, Gomez S, Lira V, Gonzalez P, Lozano S, Elizondo D, Beaty BJ, Eisen L. Dengue virus-infected Aedes aegypti in the home environment. Am J Trop Med Hyg. 2008;79:940–950. [PubMed] [Google Scholar]
- 17.Hurtado-Díaz M, Riojas-Rodríguez H, Rothenberg SJ, Gómez-Dantés H, Cifuentes E. Impact of climate variability on the incidence of dengue in Mexico. Trop Med Int Health. 2007;2:1327–1337. doi: 10.1111/j.1365-3156.2007.01930.x. [DOI] [PubMed] [Google Scholar]
- 18.CENAVECE Centro Nacional de Vigilancia Epidemiológica y Control de Enfermedades (Mexican Ministry of Health). Lineamientos para la Vigilancia Epidemiológica de Fiebre por Dengue y Fiebre Hemorrágica por Dengue. 2008. http://www.dgepi.salud.gob.mx/denguepano/Lineamientos.pdf Available at. Accessed May 2, 2010.
- 19.NOAA National Oceanic and Atmospheric Administration (United States Department of Commerce). Monthly Atmospheric and SST Indices. 2009. http://www.cpc.noaa.gov/data/indices/sstoi.indices Available at. Accessed January 22, 2010.
- 20.Hanley DE, Bourassa MA, O'Brien JJ, Smith SR, Spade ER. A quantitative evaluation of ENSO indices. J Clim. 2003;16:1249–1258. [Google Scholar]
- 21.Trenberth KE. The definition of El Niño. Bull Am Meteorol Soc. 1997;78:2771–2777. [Google Scholar]
- 22.Cazelles B, Chavez M, McMichael A, Hales S. Nonstationary influence of El Niño on the synchronous dengue epidemics in Thailand. PLoS Med. 2005;2:e106. doi: 10.1371/journal.pmed.0020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johanson MA, Cummings DA, Glass GE. Multiyear climate variability and dengue—El Niño southern oscillation, weather, and dengue incidence in Puerto Rico, Mexico, and Thailand: a longitudinal data analysis. PLoS Med. 2009;6:e1000168. doi: 10.1371/journal.pmed.1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lake IR, Gillespie IA, Bentham CG, Nichols G, Lane C, Adak GK, Threlfall EJ. A re-evaluation of the impact of temperature and climate change on foodborne illness. Epidemiol Infect. 2009;137:1538–1547. doi: 10.1017/S0950268809002477. [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 26.Magaña V, Pérez J, Vázquez-Aguirre JL, Carrisoza E, Pérez J. In: Los impactos del Niño en México. Magaña V, editor. México City, Mexico: Universidad Nacional Autónoma de México and Secretaría de Gobernación; 2004. pp. 23–68. (El Niño y el clima). [Google Scholar]
- 27.Hales S, Weinstein P, Souares Y, Woodward A. El Niño and the dynamics of vectorborne disease transmission. Environ Health Perspect. 1999;107:99–102. doi: 10.1289/ehp.9910799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagnon AS, Bush ABG, Smoyer-Tomic KE. Dengue epidemics and the El Niño Southern Oscillation. Clim Res. 2001;19:35–43. [Google Scholar]
- 29.Kovats RS. El Niño and human health. Bull World Health Organ. 2000;78:1127–1135. [PMC free article] [PubMed] [Google Scholar]
- 30.CENAVECE Centro Nacional de Vigilancia Epidemiológica y Control de Enfermedades (Mexican Ministry of Health). Manual para la Vigilancia, Diagnóstico, Prevención y Control del Dengue. 2003. http://www.cenave.gob.mx/Dengue/ Available at. Accessed March 3, 2010.
- 31.Sriprom M, Chalvet-Monfray K, Chaimane T, Vongsawat K, Bicout D. Monthly district level risk of dengue occurrences in Sakon Nakhon Province, Thailand. Sci Total Environ. 2010;408:5521–5528. doi: 10.1016/j.scitotenv.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Hemme R, Tank JL, Chadee DD, Severson DW. Environmental conditions in water storage drums and influences on Aedes aegypti in Trinidad, West Indies. Acta Trop. 2009;112:59–66. doi: 10.1016/j.actatropica.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu PC, Lay JG, Guo HR, Lin CY, Lung SC, Su HJ. Higher temperature and urbanization affect the spatial patterns of dengue fever transmission in subtropical Taiwan. Sci Total Environ. 2009;407:2224–2233. doi: 10.1016/j.scitotenv.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 34.Watts D, Burke D, Harrison B, Whitmire R, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg. 1987;36:143–152. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- 35.Keating J. An investigation into the cyclical incidence of dengue fever. Soc Sci Med. 2001;53:1587–1597. doi: 10.1016/s0277-9536(00)00443-3. [DOI] [PubMed] [Google Scholar]
- 36.Conde-Osorio M. Estudio de la longevidad y el ciclo gronotrófico del Aedes (Stegomyia) aegypti (Linnaeus, 1762), cepa Girardot (Cundinamarca) en condiciones de laboratorio. Facultad de Ciencias, Pontificia Universidad Javeriana; Bogotá, Colombia: 2003. BSc thesis. [Google Scholar]
- 37.Focks DA, Barrera R. Dengue Transmission Dynamics: Assessment and Implications for Control. Report on the Scientific Working Group on Dengue, 2006. Geneva, Switzerland: World Health Organization; 2006. pp. 92–109. [Google Scholar]
- 38.Patz JA, Martens WJM, Focks DA, Jetten TH. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environ Health Perspect. 1998;106:147–153. doi: 10.1289/ehp.98106147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuzuki A, Duoc VT, Higa Y, Yen NT, Takagi M. High potential risk of dengue transmission during the hot-dry season in NhaTrang City, Vietnam. Acta Trop. 2009;111:325–329. doi: 10.1016/j.actatropica.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Gómez-Dantés H. Elementos económicos y políticos que impactan en el control del dengue en México. Salud Publica Mex. 2007;49:117–119. [Google Scholar]
- 41.Padmanabha H, Soto E, Mosquera M, Lord C, Lounibos L. ecological links between water storage behaviors and Aedes aegypti production: implications for dengue vector control in variable climates. EcoHealth. 2010;7:78–90. doi: 10.1007/s10393-010-0301-6. [DOI] [PubMed] [Google Scholar]
- 42.Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, Kitoh A, Knutti R, Murphy JM, Noda A, Raper SCB, Watterson IG, Weaver AJ, Zhao Z. In: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt K, Tignor M, Miller H, editors. Cambridge, UK: Cambridge University Press; 2007. pp. 747–845. (Global climate projections). [Google Scholar]
- 43.Christensen J, Hewitson B, Busuioc A, Chen A, Gao X, Held I, Jones R, Kolli R, Kwon W, Laprise R, Magaña-Rueda V, Mearns L, Menéndez C, Räisänen J, Rinke A, Sarr A, Whetton P. In: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt K, Tignor M, Miller H, editors. Cambridge, UK: Cambridge University Press; 2007. pp. 847–940. (Regional climate projections). [Google Scholar]
- 44.Confalonieri U, Menne B, Akhtar R, Ebi K, Hauengue M, Kovats R, Revich B, Woodward A. In: Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Parry M, Canziani O, Palutikof J, van der Linden P, Hanson C, editors. Cambridge, UK: Cambridge University Press; 2007. pp. 391–431. (Human health). [Google Scholar]


