Abstract
The objective of this study was to compare quality of life measures in patients with neurocysticercosis (NCC) to those of a matched control group. The NCC outpatients and their controls were recruited from two neurology referral hospitals in Mexico City, Mexico during 2007–2008. The quality of life of 224 NCC patients was compared with 224 age-sex-hospital-day matched controls using the short form 12 v2 (SF-12 v2) quality of life survey. Medical chart reviews were also conducted for the NCC outpatients to evaluate presenting clinical manifestations. Compared with the controls, NCC patients had a significantly lower score for each of the eight domains of health evaluated and significantly lower Physical and Mental Component Summary scores. Chart reviews indicated that hydrocephalus (48%), severe headaches (47%), and epilepsy (31%) were the most common clinical manifestations in these NCC outpatients.
Introduction
Neurocysticercosis (NCC) is a major public health problem in many countries and is caused by the larva of the zoonotic cestode Taenia solium. This disease is predominantly found and considered endemic in Latin American, Asian, and African countries where sanitation is poor.1–3 However, it is now being seen in non-endemic countries such as the United States because of an increasing flow of immigrants from endemic areas who may already have NCC at entry into the country or who may have taeniasis and infect people who had never traveled to endemic areas.4,5 Neurocysticercosis is considered endemic in Mexico, although true prevalence estimates are not available.6
Neurocysticercosis occurs when immature T. solium larvae migrate to the central nervous system. When NCC manifests, it is often in the form of acute seizures, epilepsy, severe progressively worsening headaches, or focal deficits. Other clinical manifestations include cranial hypertension, hydrocephalus, stroke, and dementia.7,8 Various studies performed with tools such as the short form 12 (SF-12) quality of life survey, the Comprehensive Quality of Life Scale, and the EuroQol Group Index have shown that, regardless of etiology, epilepsy, stroke, and migraine reduce the quality of life of affected individuals.9–11 However, no previous study has compared the quality of life of NCC patients with a control population.
Quality of life assessment is used to measure changes in physical, mental, and social health caused by conditions and interventions that are influenced by a person's experiences and perceptions.12 The short form 12 v2 health survey (SF-12 v2) (Quality Metric Inc., Lincoln, RI) is a short and easy to use tool to assess the quality of life of an individual at a given point in time. The SF-12 v2 survey is considered a generic health survey and, therefore, can be used across age, health conditions, and treatment groups. The survey is specifically designed for adults 18 years of age and older and is available in multiple languages. It uses 12 questions to measure eight domains of functional health and well being. The eight domains of health assessed are physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. A brief description of each domain is found in Table 1.13 The objective of this study was to assess the quality of life of NCC patients in Mexico using the SF-12 v2 and compare quality of life of these patients to that of a matched control group. In addition, medical chart reviews were conducted for the NCC outpatients to evaluate presenting clinical manifestations.
Table 1.
Description of the health domains used in the SF-12 v2 quality of life survey14
| Domain | Description |
|---|---|
| Physical functioning | Degree to which health limits everyday physical activities |
| Role physical | Degree to which physical problems interfere with usual daily activities such as work or school |
| Bodily pain | Degree of pain to the body |
| General health | Ratings of current health in general |
| Vitality | Ratings of energy level |
| Social functioning | Degree to which health interferes with social activities |
| Role emotional | Degree to which emotional problems interfere with usual daily activities such as school or work |
| Mental health | Degree to which health limits emotional well being, including depression, anxiety and well being |
Materials and Methods
Source and study population.
The source population included adults (> 18 years of age) in Mexico who have access to formal health care. This study was conducted in the two major referral hospitals for adult neurological cases in Mexico City, Mexico, the Instituto Nacional de Neurologia y Neurocirugia (INNN), and the Hospital de Especialidades of the Instituto Mexicano del Seguro Social (HE-IMSS).
Study design.
A cross-sectional study was conducted to compare SF-12 v2 health scores of NCC outpatients coming to either the INNN or the HE-IMSS to age-sex-hospital-date of visit matched controls selected from individuals accompanying patients with neurological disorders other than NCC. In addition, medical chart reviews were conducted for the NCC outpatients to evaluate presenting clinical manifestations.
Definition and selection of NCC cases and non-NCC cases (controls).
The NCC patients were identified by reviewing daily outpatient appointment books for individuals with a confirmed diagnosis of NCC. The diagnosis of NCC was made by the patient's physician on the basis of the presence of cerebral lesions compatible with NCC on a computed tomography scan or magnetic resonance imaging.14 All NCC patients enrolled in the study had at least one computed tomography scan of the brain and/or magnetic resonance imaging performed. Patients who had epilepsy, recurrent symptomatic seizures, hydrocephalus, dementia, vasculitis, stroke, and/or severe headaches for more than three continuous days were eligible for participation in the study. All NCC cases with the symptoms mentioned previously and who presented themselves for an outpatient appointment to the INNN from July 17, 2007 to December 7, 2007 or to the HE-IMSS from June 2, 2008 to August 12, 2008 were asked for their consent to participate in the study. All patients who provided written consent were interviewed by a trained member of the research team (i.e., a Mexican intern, resident, or social worker) at the time of their appointment.
An individual who accompanied a non-NCC outpatient to either the INNN or the HE-IMSS was considered as a possible control. Eligibility for acting as a control was further narrowed by selecting only individuals who accompanied a non-related patient to reduce the potential impact of living with a person suffering from a neurological disease on quality of life. Controls were paired with an NCC patient seen at the same hospital on the same day by sex and age (±5 years) to reduce any possible confounding effect from these variables. This 1:1 matched control group was selected and interviewed in the hospital's waiting area by a member of the research team after obtaining written consent.
Quality of life.
The SF-12 v2 survey (Mexican Spanish version) was completed by all consenting participants. Twelve questions were used to assess eight domains of health in the survey: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. All domains were checked for missing data for each patient-control pair. If more than four health domains were missing for any case or control, that case/control pair was not included in the analysis. Data were rescored for uniformity (i.e., positive health responses received higher scores) according to Quality Metric guidelines.15 Raw scores were then transformed to a 0–100 scale in accordance with standard procedures.15 This transformation converts the lowest and highest possible scores to zero and 100, with scores between these values representing the percentage of the total possible score achieved.
Physical Component Summary and Mental Component Summary scores were determined using norm-based methods. In short, Physical and Mental Component Summary scores were calculated by aggregating the eight domains of the SF-12 v2, transforming to z-scores and multiplying by factor score coefficients, and standardizing as t scores with a mean of 50 and a standard deviation of 10 according to Quality Metric guidelines.15 Because of a lack of information on factor score coefficients for Mexico, means and standard deviations used in scoring came from the 1998 general United States population and the factor score coefficients from the 1990 general United States population. Therefore, scores above 50 were above the United States population mean and scores below 50 were below the United States population mean. The Physical Component Summary score evaluates the physical health of a person and weighs more heavily physical functioning, role physical, and bodily pain than to the other scales. On the other hand, the Mental Component Summary score evaluates the mental health of a person and gives greater weight to mental health, social functioning, and role emotional than to the other scales.16
Description of manifestations associated with NCC cases.
Intake forms were completed by reviewing the medical charts of all consenting NCC cases who participated in this study. The intake forms were used to obtain information on the clinical manifestations of NCC that caused the patient to be referred to the neurology referral hospital. Chart reviews took place between July 17, 2007 and December 7, 2007 at the INNN and between June 2, 2008 and August 12, 2008 at the HE-IMSS. The forms were prepared in English, translated into Spanish, and back translated into English independently by two persons. All forms were pilot tested locally before use.
Data management and statistical analysis.
Information obtained from the SF-12 v2 survey and chart reviews was captured in paper form. Later, the information was entered into the Select Survey program (ClassApps Com., Overland Park, KS) and then exported into Excel (Microsoft Corp., Redmond, WA) spreadsheets for analysis. Paper copies of the completed SF-12 v2 survey and chart review forms were kept in a locked filing cabinet at Texas A&M University, with access provided only to members of the research team.
Descriptive analyses of the data were performed using means, medians, and proportions. The distribution of the scores for the eight SF-12 v2 domains for NCC patients and controls were visually compared by drawing box plot graphs. Normality of the scores for each of the eight domains was analyzed by the Shapiro–Wilk method, with the data found to be non-Gaussian. Therefore, the Wilcoxon matched pairs test was used to compare the ranks of the scores of the eight individual domains for the case and control populations. Norm-based Physical and Mental Component Summary scores for cases and controls were compared using paired sample t tests. A P value of 0.05 or less was considered statistically significant.
All eight health scores and the two summary scores for the NCC outpatient and control groups were compared overall and stratified by sex, age, time since diagnosis (< 6 and ≥ 6 years prior) and the clinical manifestations of epilepsy, severe headaches, and hydrocephalus. Patients were divided into two age groups (≤ 45 years and > 45 years of age). The cut-off points were based on the median age (46 years) and median time since diagnosis (5 years) among NCC outpatients.
Ethical approval.
This study received Institutional Review Board approval from Texas A&M University, the INNN, and the HE-IMSS.
Results
Chart reviews were conducted for 123 patients from the INNN and 101 patients from the HE-IMSS. There were slightly more female patients (117) than male patients (107). Patient ages ranged from 19 to 88 years, with a mean age of 47 years. One hundred one patients were 45 years of age or younger and the remaining 123 patients were older than 45 years of age.
Clinical manifestations associated with NCC cases.
The NCC outpatients reported numerous clinical manifestations (Figure 1), with hydrocephalus (48%), severe headaches (47%), and epilepsy (31%) being the most common. In 46% of patients, more than one clinical manifestation was present such as epilepsy and severe headaches; epilepsy, hydrocephalus, and severe headaches; or hydrocephalus and epilepsy. The most common combination was hydrocephalus and severe headaches (15%).
Figure 1.
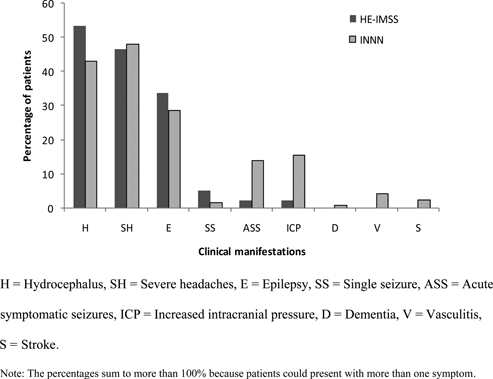
Neurocysticercosis (NCC)-related clinical manifestations of patients seeking treatment at the Instituto Nacional de Neurologia y Neurocirugia (INNN) or the Hospital de Especialidades of the Instituto Mexicano del Seguro Social (HE-IMSS).
Comparison of the eight domains' scores for quality of life.
All outpatients consented to complete the SF-12 v2 quality of life survey. Four pairs of cases and controls were not used for the analysis because of missing data. Individuals with NCC had a significantly lower score in each of the eight domains of health (physical functioning, role physical, bodily pain, vitality, general health, social functioning, role emotional, and mental health) compared with their matched controls (P < 0.05) (N = 220 pairs) (Figure 2).
Figure 2.
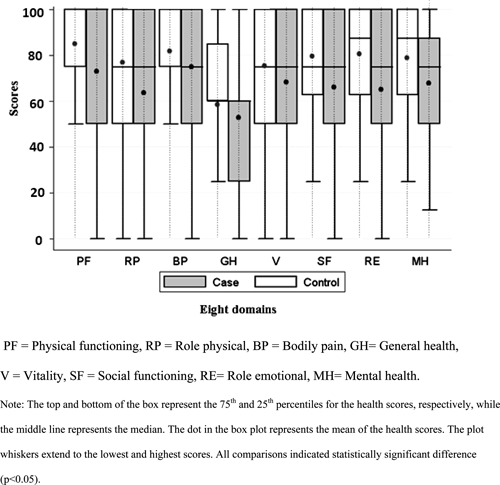
Health scores from the SF-12 v2 health survey for neurocysticercosis (NCC) patients vs. their matched control group (overall population).
Male NCC outpatients had significantly lower scores in role physical, bodily pain, vitality, social functioning, role emotional, and mental health compared with their matched controls (P < 0.05) (N = 104 pairs). Except for general health, female NCC outpatients had significantly lower scores in all domains compared with their matched controls (P < 0.05) (N = 116 pairs). Younger individuals (≤ 45 years) with NCC had lower health scores in all eight domains compared with their matched controls (N = 100 pairs). However, for the older individuals (> 45 years), three domains (bodily pain, general health, and vitality) were not significantly different between the two groups (N = 120 pairs).
The NCC cases diagnosed < 6 years ago had lower health scores in all eight domains compared with their matched controls (N = 102 pairs). However, two domains (bodily pain and vitality) were not significantly different for NCC cases diagnosed 6 or more years prior (N = 82 pairs). Thirty-six NCC cases did not have time of diagnosis documented in their medical charts and these individuals and their corresponding controls were, therefore, not included in this comparison.
The NCC outpatients with epilepsy had significantly lower scores in all domains compared with their matched controls, except for physical functioning and bodily pain (N = 66 pairs). The NCC outpatients with severe headaches had significantly lower scores in all domains compared with their matched controls, except for general health (N = 103 pairs). The NCC outpatients with hydrocephalus had significantly lower scores in all domains except for general health and vitality (N = 104). Patients with more than one clinical manifestation (epilepsy, severe headaches, or hydrocephalus) could be included in multiple categories because relatively few patients presented with only one of the three evaluated clinical manifestations. Patients without epilepsy, severe headaches, or hydrocephalus were not included in this comparison.
Comparison of the Physical and Mental Component Summary scores.
Both the Physical and Mental Component Summary scores for all NCC outpatients assessed were significantly lower than the corresponding scores for the matched control group (Table 2). Physical and Mental Component Summary scores for different sub-groups of NCC outpatients and for their respective matched controls are shown in Table 2.
Table 2.
Norm-based mean physical component summary (PCS) and mental component summary (MCS) scores for neurocysticercosis (NCC) patients and their matched controls overall and stratified by demographic and clinical characteristics
| Characteristic | Mean PCS | P value* | Mean MCS | P value* | |||
|---|---|---|---|---|---|---|---|
| NCC | Control | NCC | Control | ||||
| All | 45.9 | 48.5 | < 0.01† | 45.8 | 51.8 | < 0.01† | |
| Sex | Male | 46.1 | 48.2 | 0.1 | 47.2 | 53.5 | < 0.01† |
| Female | 45.7 | 48.7 | 0.01† | 44.7 | 50.3 | < 0.01† | |
| Age | Younger (≤ 45 years) | 47.8 | 51.8 | < 0.01† | 42.9 | 53.1 | < 0.01† |
| Older (> 45 years) | 44.4 | 45.8 | 0.29 | 48.2 | 50.8 | 0.08 | |
| Manifestation | Epilepsy | 46.4 | 48.1 | 0.3 | 43.2 | 52.0 | < 0.01† |
| Severe headaches | 45.8 | 48.5 | 0.02† | 46.7 | 52.2 | < 0.01† | |
| Hydrocephalus | 45.8 | 48.2 | 0.06 | 47.1 | 52.3 | < 0.01† | |
| Time since diagnosis | Less than 6 years ago | 45.3 | 48.6 | 0.02† | 45.4 | 51.2 | < 0.01† |
| 6 or more years ago | 45.8 | 48.0 | 0.1 | 45.7 | 52.4 | < 0.01† | |
P value calculated using paired t tests.
Represents significant difference at P ≤ 0.05.
Discussion
This study is the first to report on the quality of life of patients suffering from NCC compared with a matched control group. Overall, NCC patients scored significantly lower in all eight domains of health compared with their matched controls. Therefore, findings from this study suggest that NCC is associated with both poorer physical health and mental health. These findings are consistent with those of previous studies using the SF-12, the Comprehensive Quality of Life Scale, and the EuroQol Group Index, which showed that clinical manifestations such as epilepsy, stroke, and migraine reduced the quality of life of affected individuals.9–11
The difference in quality of life between NCC outpatients and their controls was more marked among females than among males, suggesting that females with NCC may be more impaired mentally and physically than males. This may be caused by differences in the types of jobs that males and females perform in their daily lives. Females tend to perform more unpaid and socially vital work in the home and in the informal sector, which may make them more unable (because of monetary constraints) or unwilling (because of family commitments) to seek care unless they become severely ill.17
Similarly, the difference in quality of life between NCC outpatients and their controls was more marked among younger individuals (aged 45 years or less) as compared with the older group. This finding was especially pronounced in terms of mental health, which could result because the general population of older individuals generally has a lower quality of life, making them more similar to the older patients with NCC. Another explanation could have been that older NCC patients had been sick for a longer period of time, and had acclimatized more to the symptoms compared with the younger group. However, this was not the case because the mean time since diagnosis of NCC patients was similar in both the younger and older categories (5.7 and 4.9 years, respectively). The difference in scores between NCC cases and their controls was larger among those diagnosed < 6 than among those diagnosed 6 or more years prior. This may be caused by individuals who were diagnosed longer ago having somewhat acclimated to their situation or that their quality of life has improved because of the treatment they received.
The results of this study suggest that NCC patients with epilepsy may be particularly affected in the mental health domains. These findings are consistent with the literature, which indicates that epilepsy affects psychosocial variables caused by depression, stress, anxiety, and social isolation.18,19 In contrast, scores for both physical and mental domains were lower in NCC outpatients with severe headaches and hydrocephalus compared with controls. These differences were statistically significant for all but the physical domain associated with hydrocephalus, which had a P value of 0.06. This too is consistent with the literature that has shown headaches affect both physical and emotional dimensions of health.20,21
When the norm-based Physical and Mental Component Summary scores from this study were compared with a study evaluating severe mental illness in patients within the public mental health systems of the United States, the mean Physical Component Summary scores for NCC-associated epilepsy, hydrocephalus, and severe headaches (46.4, 45.8, and 45.8, respectively) were found to be similar to those of bipolar patients in the United States study (46.1). However, Physical Component Summary scores for the Mexican NCC outpatients with epilepsy, hydrocephalus, and severe headaches were lower than Physical Component Summary scores for United States schizophrenia patients (48.2). Mean Mental Component Summary scores for Mexican outpatients with NCC-associated epilepsy, hydrocephalus, and severe headaches (43.2, 47.1, and 46.7, respectively) were higher compared with United States patients with bipolar disorder (39.6), but were similar to United States patients with schizophrenia (42.4).22 However, these comparisons need to be made with care because patients from two different countries were being compared and most of the Mexican patients had multiple clinical manifestations and, therefore, the effect may be caused by the combinations of symptoms. In addition, United States norms were used to calculate the Physical and Mental Component Summary scores for Mexican NCC outpatients, which may not be optimal for this population.
This study was conducted in two neurology reference hospitals in Mexico, and most likely only represents a fraction of the total regional population with NCC, with an over-representation of more severe cases. Indeed, hydrocephalus (48%) and severe headaches (47%) were the most common clinical manifestations presented among the participants with NCC. However, all known NCC manifestations were not included in this study because of their low frequency in the study population. Therefore, the distribution of clinical manifestations differs somewhat from other available literature. For example, a recent systematic review of the literature found that epilepsy was the most common clinical manifestation of NCC (mean: 79%, 95% confidence interval [CI]: 65–90%) followed by severe headaches (mean: 38%, CI: 23–54%) in neurology clinics in endemic regions (Carabin H and others, unpublished data). Nevertheless, the fact that the NCC outpatients in this study with epilepsy, hydrocephalus, and severe headaches had reduced quality of life scores compared with their matched controls suggests that these results may be somewhat generalizable to NCC cases with those three clinical manifestations. The overall results may, however, overestimate the impact of NCC on quality of life.
The Mexican Spanish version of the SF-12 v2 was administered to NCC outpatients by a native Spanish speaking interviewer. However, persons administering the questionnaire were not blinded, which may have resulted in information bias. Confounding by age, sex, hospital, and time was limited through matching. However, the controls were taken from individuals accompanying someone with a neurological disorder being seen at a tertiary reference hospital and do not necessarily represent the general population of Mexico. The fact that the controls were in contact with a person with a neurological condition may have affected their own quality of life, which could have underestimated the difference in quality of life scores between the NCC and control group.
In conclusion, individuals with NCC had a significantly lower score in each of the eight domains of health and significantly lower Physical Component Summary and Mental Component Summary scores measured by the SF-12 v2 health survey compared with a matched control group. Additional studies are needed to determine whether the differences observed in this cross-sectional study represent a causal link between NCC and an individual's decline in function. This information can then be used to better define and estimate the total disability associated with NCC.
ACKNOWLEDGMENTS
We thank all of the participants of the study and persons involved in collection of data in Mexico.
Footnotes
Financial support: This work was funded by the Texas A&M University-CONACYT Collaborative Research Grant Program.
Authors' addresses: Rachana Bhattarai, Christine M. Budke, and Renata Ivanek, Department of Veterinary Integrative Biosciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX, E-mails: rdhungel@cvm.tamu.edu, cbudke@cvm.tamu.edu, and rivanek@cvm.tamu.edu. Hélène Carabin and Linda D. Cowan, Department of Biostatistics and Epidemiology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, E-mails: helene-carabin@ouhsc.edu and Linda-Cowan@ouhsc.edu. Jefferson V. Proaño, Unidad de Investigación Médica en Enfermedades Neurológicas Hospital de Especialidades, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, México DF, Mexico, E-mail: proanio_00@yahoo.com. Jose Flores-Rivera and Teresa Corona, Laboratorio Clínico de Enfermedades Neurodegenerativas, Instituto Nacional de Neurología y Neurocirugía, México DF, Mexico, E-mails: jflores.rivera@gmail.com and coronav@servidor.unam.mx. Karen F. Snowden, Department of Veterinary Pathobiology, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX, E-mail: ksnowden@cvm.tamu.edu. Ana Flisser, Facultad de Medicina, Universidad Nacional Autónoma de México, UNAM, México DF, Mexico, E-mail: flisser@servidor.unam.mx.
References
- 1.Rajshekhar V, Joshi DD, Doanh NQ, van De N, Xiaonong Z. Taenia solium taeniosis/cysticercosis in Asia: epidemiology, impact and issues. Acta Trop. 2003;87:53–60. doi: 10.1016/s0001-706x(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 2.Zoli A, Shey-Njila O, Assana E, Nguekam JP, Dorny P, Brandt J, Geerts S. Regional status, epidemiology and impact of Taenia solium cysticercosis in Western and Central Africa. Acta Trop. 2003;87:35–42. doi: 10.1016/s0001-706x(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 3.Flisser A, Sarti E, Lightowlers M, Schantz P. Neurocysticercosis: regional status, epidemiology, impact and control measures in the Americas. Acta Trop. 2003;87:43–51. doi: 10.1016/s0001-706x(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 4.Sorvillo FJ, DeGiorgio C, Waterman SH. Deaths from cysticercosis, United States. Emerg Infect Dis. 2007;13:230–235. doi: 10.3201/eid1302.060527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del la Garza Y, Graviss EA, Daver NG, Gambarin KJ, Shandera WX, Schantz PM, White AC. Epidemiology of neurocysticercosis in Houston, Texas. Am J Trop Med Hyg. 2005;73:766–770. [PubMed] [Google Scholar]
- 6.Ramirez-Zamora A, Alarcón T. Management of neurocysticercosis. Neurol Res. 2010;32:229–237. doi: 10.1179/016164110X12644252260592. [DOI] [PubMed] [Google Scholar]
- 7.McCormick GF, Zee CS, Heiden J. Cysticercosis cerebri. Review of 127 cases. Arch Neurol. 1982;39:534–539. doi: 10.1001/archneur.1982.00510210004002. [DOI] [PubMed] [Google Scholar]
- 8.Sotelo J, Del Brutto OH. Brain cysticercosis. Arch Med Res. 2000;31:3–14. doi: 10.1016/s0188-4409(99)00073-9. [DOI] [PubMed] [Google Scholar]
- 9.Allotey P, Reidpath D. Epilepsy, culture, identity and well-being: a study of the social, cultural and environmental context of epilepsy in Cameroon. J Health Psychol. 2007;12:431–443. doi: 10.1177/1359105307076231. [DOI] [PubMed] [Google Scholar]
- 10.Lipton RB, Hamelsky SW, Kolodner KB, Steiner TJ, Stewart WF. Migraine, quality of life, and depression: a population-based case-control study. Neurology. 2000;55:629–635. doi: 10.1212/wnl.55.5.629. [DOI] [PubMed] [Google Scholar]
- 11.Xie J, Wu EQ, Zheng ZJ, Croft JB, Greenlund KJ, Mensah GA, Labarthe DR. Impact of stroke on health-related quality of life in the noninstitutionalized population in the United States. Stroke. 2006;37:2567–2572. doi: 10.1161/01.STR.0000240506.34616.10. [DOI] [PubMed] [Google Scholar]
- 12.Dennis RE, Williams W, Giangreco MF, Cloninger CJ. Quality of life as context for planning and evaluation of services for people with disabilities. Except Child. 1993;59:499–512. doi: 10.1177/001440299305900603. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993. (New England Medical Center Hospital, Health I). [Google Scholar]
- 14.Del Brutto OH, Rajshekhar V, White AC, Jr, Tsang VC, Nash TE, Takayanagui OM, Schantz PM, Evans CA, Flisser A, Correa D, Botero D, Allan JC, Sarti E, Gonzalez AE, Gilman RH, García HH. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–183. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12 Health Survey (With a Supplement Documenting Version 1) Lincoln, RI: QualityMetric Inc; 2002. [Google Scholar]
- 16.Ware JE, Kosinski M. SF-36 Physical and Mental Health Summary Scales: A Manual for Users of Version 1. Lincoln, RI: QualityMetric Inc; 2002. [Google Scholar]
- 17.Sethuraman SV. Gender, Informality and Poverty: A Global Review: Gender Bias in Female Informal Employment and Incomes in Developing Countries. Geneva: World Bank: WIEGO; 1998. World Bank, Women in Informal Employment and Organizing. [Google Scholar]
- 18.Suurmeijer TP, Reuvekamp MF, Aldenkamp BP. Social functioning, psychological functioning, and quality of life in epilepsy. Epilepsia. 2001;42:1160–1168. doi: 10.1046/j.1528-1157.2001.37000.x. [DOI] [PubMed] [Google Scholar]
- 19.Jacoby A. Epilepsy and the quality of everyday life. Findings from a study of people with well-controlled epilepsy. Soc Sci Med. 1992;34:657–666. doi: 10.1016/0277-9536(92)90193-t. [DOI] [PubMed] [Google Scholar]
- 20.Guitera V, Muñoz P, Castillo J, Pascual J. Quality of life in chronic daily headache: a study in a general population. Neurology. 2002;58:1062–1065. doi: 10.1212/wnl.58.7.1062. [DOI] [PubMed] [Google Scholar]
- 21.Wang SJ, Fuh JL, Lu SR, Juang KD. Quality of life differs among headache diagnoses: analysis of SF-36 survey in 901 headache patients. Pain. 2001;89:285–292. doi: 10.1016/s0304-3959(00)00380-8. [DOI] [PubMed] [Google Scholar]
- 22.Salyers MP, Bosworth HB, Swanson JW, Lamb-Pagone J, Osher FC. Reliability and validity of the SF-12 health survey among people with severe mental illness. Med Care. 2000;38:1141–1150. doi: 10.1097/00005650-200011000-00008. [DOI] [PubMed] [Google Scholar]


