Abstract
A cross-sectional pilot study of hookworm infection was carried out among 292 subjects from 62 households in Kintampo North, Ghana. The overall prevalence of hookworm infection was 45%, peaking in those 11–20 years old (58.5%). In children, risk factors for hookworm infection included coinfection with malaria and increased serum immunoglobulin G reactivity to hookworm secretory antigens. Risk factors for infection in adults included poor nutritional status, not using a latrine, not wearing shoes, and occupation (farming). Although albendazole therapy was associated with an overall egg reduction rate of 82%, 37 subjects (39%) remained infected. Among those who failed therapy, treatment was not associated with a significant reduction in egg excretion, and nearly one-third had higher counts on repeat examination. These data confirm a high prevalence of low-intensity hookworm infection in central Ghana and its association with poor nutritional status. The high rate of albendazole failure raises concern about emerging resistance.
Introduction
It has been estimated that more than 600 million people worldwide, including 156 million children, are infected with hookworms, nematode parasites that attach to the intestinal mucosa and feed on blood.1,2 In sub-Saharan Africa, hookworm (Ancylostoma duodenale and Necator americanus) prevalence is approximately 30%,1 and in Northeastern Ghana, the prevalence has been reported to be as high as 50%.3 The effects of hookworm infection include growth delay, especially in children, and anemia, which, in pregnancy, is associated with poor birth outcomes.4 Infection is most prevalent in communities of rural poverty, because transmission occurs primarily through physical contact with soil contaminated by infective larvae.5,6 In these settings, coinfection with other endemic infectious diseases (e.g., malaria, human immunodeficiency virus [HIV], and tuberculosis) is also common, potentially compounding the negative sequelae of hookworm and other helminth infections.7,8
Hookworm control efforts have traditionally focused on community-based treatment of high-risk populations (for instance, school-age children) with benzimidazole anthelminthics. Periodic deworming has been shown to increase growth in some populations9 but not in others,10 whereas treatment of school children may decrease the risk of anemia.11,12 The 54th meeting of the World Health Assembly in 2001 called for ensuring access to anthelminthics “with the goal of attaining a minimum target of regular administration of chemotherapy to at least 75% and up to 100% of all school-age children at risk of morbidity by 2010.”13–15 However, repeated large-scale administration of benzimidazoles has raised concerns about the potential emergence of resistance, especially in light of evidence of reduced efficacy.16–18
We conducted a cross-sectional field study of hookworm infection in Kintampo North Municipality located in central Ghana. The primary purpose of this pilot study (292 subjects) was to define the epidemiology of hookworm infection, including associated risk factors and response to therapy, in an endemic population that had not previously been well-characterized. Evidence confirms a high prevalence of low-intensity hookworm infection in Kintampo North as well as significant rates of hookworm–malaria coinfection in children and young adults. We also report a high rate of treatment failure (39%) after administration of single-dose albendazole. Together, these data raise concerns about the potential for emerging benzimidazole resistance in Ghana, further highlighting the need for enhanced surveillance and monitoring of treatment efficacy in hookworm-endemic areas.
Materials and Methods
Sample selection and surveys.
This study was approved by the Yale Human Investigations Committee (protocol number 0705002669) and the Institutional Review Board of the Noguchi Memorial Institute for Medical Research (NMIMR) at the University of Ghana. In July 2007, four communities in Kintampo North Municipality of central Ghana were surveyed: Jato-Akuraa (JA), Cheranda (C), Kawampe (KA), and Gulumpe (GU). These communities were suspected to be endemic for hookworm infection based on anecdotal reports from providers at local health clinics. The two smallest and neighboring communities (JA and C) were analyzed as one community (JA/C). The three communities included approximately 1,200 households, 62 of which were sampled for this pilot study. The estimated sample size needed was determined based on the limited prevalence data for Kintampo North Municipality available from the Ghana Ministry of Health. Within each community, 20 households were selected at random based on government-assigned house numbers, with the intent to sample two adults and three children per house, yielding a total sample of approximately 300 participants. The final study population consisted of 292 participants from 62 households (Figure 1). More than one-half (51.2%) of the participants were 15 years old or younger; 258 individuals submitted a baseline stool sample and a blood sample for analysis. Four participants were pregnant and excluded from analyses that relied on measurement of body mass index (BMI).
Figure 1.
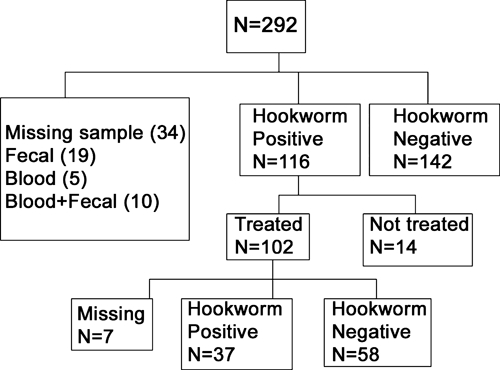
Characteristics of the study population.
The study team visited each selected house to invite participation. After completion of the consent process, the team administered a short demographic and health questionnaire (see below), measured weight and height, and distributed labeled stool-collection containers. Stool samples were collected the following day, and approximately 2 mL blood were taken by venipuncture from each participant at that time.
Survey instrument.
The study questionnaire was translated into Twi and back-translated into English by native speakers. Questions included information on age, gender, education, occupation, use of healthcare resources, previous use of anthelminthic drugs and other medications, perception of medical symptoms, shoe usage, latrine usage, daily activities, and other habits.
Specimen collection and processing.
At the time of sampling, two drops of whole blood were used to create thick and thin smears on a microscope slide, and 20 μL were placed in 5 mL Drabkins solution for measurement of hemoglobin concentration.19 The remaining blood sample was separated by centrifugation, and the serum was stored at −80°C. Thick and thin blood smears were analyzed for the presence of Plasmodium species using light microscopy. Fecal samples were analyzed for the presence of helminth eggs on the day of collection using the Kato–Katz method.20 Individuals who were positive for hookworm or other soil transmitted nematodes (STNs) were treated with a single observed dose of 400 mg albendazole (Remedica, Limassol, Cyprus) within 1 week of detection. At 14–21 days post-treatment, a follow-up stool sample was obtained from those who had received albendazole to determine the efficacy of treatment.
Measurement of serum immunoglobulin G against hookworm excretory–secretory proteins.
Total immunoglobulin G (IgG) antibody levels to A. ceylanicum excretory–secretory (ES) proteins were measured in serum samples from study subjects using a previously described enzyme-linked immunosorbent assay (ELISA).19,21 Serum samples (diluted 1:200) from study participants were incubated in ES-coated microtiter plate wells for 3 hours at room temperature. After washing, wells were incubated with horseradish peroxidase-conjugated goat antihuman IgG (diluted 1:5,000), and bound IgG was detected by adding ABTS (2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)) substrate solution (Sigma, St. Louis, MO). The absorbance was recorded using a SpectraMax 190 microplate reader (Molecular Devices Corporation), and the level of serum reactivity (optical density [OD] at 405nm) for the study population was categorized by quartile for statistical analysis.
Comparison of in vitro anthelminthic activity of albendazole preparations.
The anthelminthic activity of the albendazole used in the study was evaluated using an in vitro egg hatch assay.22 Stock solutions of albendazole were prepared in methanol and diluted in distilled H2O to 6.0 μg/mL. Approximately 100 A. ceylanicum hookworm eggs extracted from the feces of infected laboratory hamsters were placed in individual wells of a 96-well microtiter plate along with increasing concentrations of albendazole. Plates were incubated at 27°C, and at 48 hours, the number of hatched first-stage (L1) hookworm larvae was counted under light microscopy. The hatch rate (percent) was determined by using the equation:
Data were plotted to produce hatch rate curves comparing albendazole used in the field study (Remedica) with the identical drug purchased from Sigma.
Statistical analysis.
All data generated from this study were stored in Microsoft Excel (2003), and statistical analysis was carried out in SPSS version 15.0. Statistical tests included parametric and non-parametric comparison of means, χ2 tests, z tests for comparison of proportions, and logistic regression analyses. Separate logistic regression analysis was conducted for adults (age > 15 years) and children (age ≤ 15 years) for baseline hookworm infection status. Initial variables tested were derived from a theoretical framework of factors thought to affect exposure and susceptibility to hookworm infection. Adjusted odds ratios are reported for significant variables, controlling for age, gender, and community.
Results
Study subject characteristics.
Individuals from 62 households participated in the survey, with an average of 4.2 subjects per household and a range of 1 to 9 subjects from each. The final sample included 20 households each from two communities (KA and GU) and 22 households from the third community (JA/C). Household size ranged from 1 to 88 people, with a median of 9 people. The ages of subjects ranged from 1 to 80 years, and 51.6% were female; 46% of the subjects were aged 10 years or younger, and 21% were aged 41 or older. Of the children aged 5–14, 74% were enrolled in school. Of those over age 15, 33% reported some primary or secondary school, and 64% were farmers.
Nineteen percent of the participants reported using a latrine, and 16% of the households reported having a pit latrine, all of which were shared. Overall, 13% of the study households used well water exclusively, whereas others also used pond water.
The overall rate of wasting (low weight for height) among children 5 years of age and younger was 6.5%, which is similar to a previously reported rate of wasting among children in Ghana (7.1%).23 More than one-half of those ages 15 and under had consulted a healthcare worker in the last year (60%) compared with 40% of the adults surveyed. Adults and children reported similar rates of fever in the past month (37% and 38%, respectively) and diarrhea in the last 6 months (24% and 31%, respectively).
Prevalence of geohelminths and malaria.
The overall prevalence of hookworm in the study population was 45% (116 of 258 subjects), with 107 subjects (92.4%) classified as having light infection (< 2,000 eggs per gram [epg] of feces).24 Only five (4.3%) subjects were moderately infected (2,000–3,999 epg), and four (3.4%) were heavily infected (> 4,000 epg). Other intestinal parasites were present in fewer than 3% of the population, including Hymenolepis nana (3%), Taenia solium (1.1%), and Trichuris trichiura (1.2%). Ascaris lumbricoides was not detected. Figure 2A shows the age-related prevalence of hookworm infection, which was lowest in children under 6 years of age (20.5%) and highest in the 11–20 year age group (58.5%). The prevalence in children under age 6 was significantly different from the prevalence in the 6–10 and 11–20 year age groups (P < 0.05).
Figure 2.
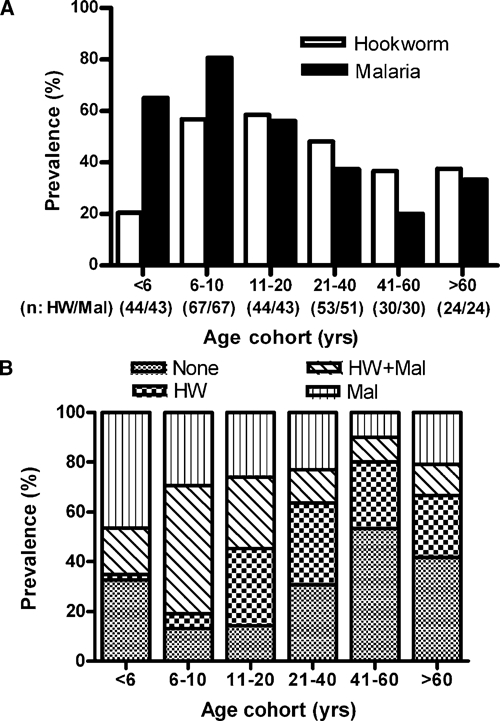
(A) Age-associated prevalence of hookworm and malaria infections. Numbers in parentheses represent the number of subjects screened for hookworm (HW) and malaria (Mal), respectively. (B) Age-associated prevalence of single infection (HW or Mal), coinfection (HW and Mal), and no infection.
The overall prevalence of a positive blood smear for malaria (Plasmodium falciparum) was 54% and was highest (81%) in children 6–10 years old (Figure 2A). Of note, all study participants with malaria were asymptomatic. The prevalence of malaria in children ages 6–10 was significantly different from all older age groups (P < 0.05). The prevalence of coinfection with both malaria and hookworm (Figure 2B) was highest in children ages 6–10 (51%) and lowest in adults ages 41–60 (10%). Of note, 86.8% of those 6–10 years and 85.7% of those 11–20 years were infected with hookworm, malaria, or both, indicative of a potentially high burden of disease in these age cohorts.
Factors associated with hookworm infection status at baseline.
Using univariate analysis, there was no statistically significant association of hookworm infection status with gender, community of residence, or history of anthelminthic treatment in the past year (Table 1). In children (ages ≤ 15 years), demographic factors associated with hookworm infection included level of education (P < 0.01), occupation (student versus non-student; P < 0.01), and malaria parasitemia (P = 0.015). There was no association between hookworm infection and shoe use or access to latrines. Among those 15 years and younger, the mean age of those infected with hookworm (8.37 ± 2.8 years) was significantly higher than those who were negative for hookworm (5.95 ± 3.37 years; P < 0.001).
Table 1.
Study sample characteristics as defined by age and hookworm infection status
| Characteristic | Age ≤ 15 years (N = 132) | Age > 15 years (N = 126) | ||
|---|---|---|---|---|
| Number (%) of hookworm-positive subjects | P value* | Number (%) of hookworm-positive subjects | P value | |
| Community | ||||
| Kawampe | 25 (56%) | 0.117 | 17 (53%) | 0.124 |
| Gulumpe | 18 (35%) | 19 (54%) | ||
| Jato-Akuraa/Cheranda | 16 (46%) | 21 (36%) | ||
| Gender | ||||
| Male | 37 (50%) | 0.166 | 21 (41%) | 0.405 |
| Female | 22 (38%) | 36 (48%) | ||
| Education | ||||
| None | 17 (30%) | < 0.01 | 39 (46%) | 0.431 |
| Primary | 41 (56%) | 11 (52%) | ||
| Secondary | 0 | 7 (33%) | ||
| Occupation | ||||
| Nothing | 17 (32%) | < 0.01 | 4 (36%) | < 0.001 |
| Student | 42 (55%) | 9 (82%) | ||
| Farmer | 0 | 42 (53%) | ||
| Trades and other occupations | 0 | 2 (9%) | ||
| Shoe usage | ||||
| Always or often | 11 (39%) | 0.473 | 28 (37%) | < 0.01 |
| All others | 46 (47%) | 26 (63%) | ||
| Latrine use | ||||
| Uses a latrine | 5 (31%) | 0.248 | 6 (18%) | < 0.001 |
| Does not use a latrine | 54 (47%) | 51 (55%) | ||
| Anthelminthic usage | ||||
| In the last year | 19 (51%) | 0.340 | 7 (39%) | 0.563 |
| Not within the last year | 37 (42%) | 49 (46%) | ||
| Malaria | ||||
| No parasitemia | 10 (28%) | 0.015 | 38 (46%) | 0.632 |
| Parasites present | 49 (52%) | 18 (42%) | ||
| BMI mean (standard deviation) | ||||
| Hookworm-positive at baseline | Not available | 20.5 (2.3) | < 0.01 | |
| Hookworm-negative at baseline | Not available | 22.0 (3.1) | ||
| Age | ||||
| Hookworm-positive at baseline | 8.37 (2.8) | < 0.001 | 37.3 (17.7) | 0.132 |
| Hookworm-negative at baseline | 5.95 (3.37) | 42.2 (18.2) | ||
Compared with hookworm-negative subjects from same age group.
In subjects older than 15 years, there was no association between hookworm infection status and level of education or malaria parasitemia (Table 1). However, in contrast with the younger cohort (≤ 15 years), hookworm infection status in this group was associated with occupation (< 0.001), shoe use (< 0.01), and access to a latrine (< 0.001). In addition, the average BMI of those older than 15 years who were hookworm-positive (20.5) was significantly lower than in those who were hookworm-negative (22.1; P < 0.01). Although only 15.6% of adults with a BMI > 23 were hookworm-positive, 54% of those with a BMI ≤ 23 were hookworm-positive at baseline, a difference that was statistically significant (P < 0.001). Figure 3 shows the distribution of BMI as a function of baseline hookworm infection.
Figure 3.
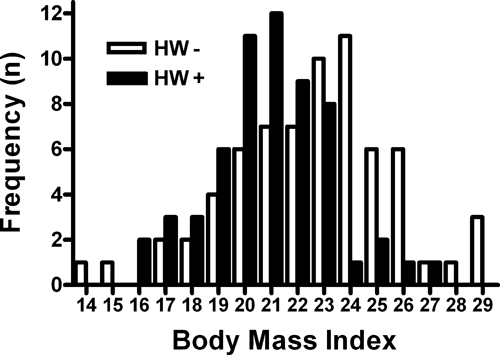
Hookworm infection is associated with poor nutritional status in adults. Mean body mass index (BMI) of study subjects older than 15 years who were infected with hookworm (HW+) at baseline was lower than in those who were not infected (HW−; P < 0.01; details in the text). Association was seen in both males and females (not shown).
Using multivariate analysis, hookworm infection in children was associated with a positive malaria smear when controlling for age, gender, and community (Table 2). The adjusted odds ratio (OR) for infection with hookworm in children with malaria was 2.84 (95% confidence interval [CI] = 1.11, 7.26) compared with those without malaria. Infection was also associated with serum reactivity to hookworm ES antigens (Table 2). Children in the second (OR = 5.43; CI = 1.59, 18.54), third (OR = 4.97; CI = 1.26, 19.68), or fourth (OR = 6.24; CI = 1.72, 22.65) highest quartile of serum reactivity to hookworm ES were significantly more likely to have hookworm infection than those in the lowest quartile (Table 2).
Table 2.
Risk factors for hookworm infection
| Adjusted odds ratio* | P value | 95% CI | |
|---|---|---|---|
| Children | |||
| Malaria | 2.84 | 0.03 | 1.11, 7.26 |
| ES IgG 2nd quartile | 5.43 | 0.007 | 1.59, 18.54 |
| ES IgG 3rd quartile | 4.97 | 0.02 | 1.26, 19.68 |
| ES IgG 4th quartile | 6.24 | 0.005 | 1.72, 22.65 |
| Adults | |||
| Body mass index | 0.71 | < 0.001 | 0.60, 0.85 |
| Does not wear shoes | 4.06 | 0.004 | 1.57, 10.53 |
| Does not use a latrine | 6.10 | 0.001 | 2.09, 17.54 |
| Farmer | 4.89 | 0.001 | 1.90, 12.61 |
Adjusted for age, gender, and community.
In those over 15 years of age, statistically significant risk factors for hookworm infection included farming as an occupation (OR = 4.89; CI = 1.90, 12.61), lack of latrine use (OR = 6.10; CI = 2.09, 17.54), and infrequent wearing of shoes (OR = 4.06; CI = 1.57, 10.53) (Table 2). Likewise, a higher BMI was associated with decreased prevalence of hookworm infection (OR = 0.71; CI = 0.60, 0.85) when adjusted for age, gender, and community (P < 0.01). In contrast to younger subjects, this group showed no statistically significant association between serum IgG levels against hookworm ES products and baseline infection status.
Prevalence of anemia.
The overall prevalence of anemia in the study population was 51%, and it varied by age and hookworm/malaria infection status (Figure 4). In children ages 6–15 years, a positive blood smear for malaria in the absence of concurrent hookworm infection was significantly associated with anemia (OR = 4.92; CI = 1.05, 23.09; P = 0.04) compared with those without either infection (Table 3). By contrast, in this age group, there was an approximately twofold increase in the risk of anemia with hookworm (but not malaria) infection (OR = 1.99; CI = 0.27, 14.89), a difference that was not statistically significant (P = 0.5). Despite the increased risk in children singly infected, the risk of anemia in children positive for both hookworm and malaria was comparable with that in subjects with hookworm alone (adjusted OR = 2.34; CI = 0.56, 9.84), and the difference was not statistically significant (P = 0.25). These data suggest that concurrent low-intensity hookworm infection is associated with a reduced risk of malarial anemia, at least in school-age children. Of note, in older age groups (> 15 years), anemia was not significantly correlated with hookworm or malaria infection status, when controlling for age, gender, and education.
Figure 4.
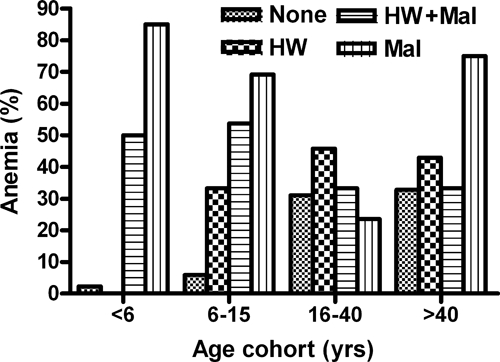
Age-associated prevalence of anemia in subjects with single infection (HW or malaria Mal), coinfection (HW + Mal), and no infection (none).
Table 3.
Infection status and risk of anemia in school-age children (6–15 years)
| Adjusted odds ratio* | 95% CI | P value | |
|---|---|---|---|
| Malaria | 4.92 | 1.05, 23.09 | 0.04 |
| Hookworm | 1.99 | 0.27, 14.89 | 0.5 |
| Hookworm + malaria | 2.34 | 0.56, 9.84 | 0.25 |
Adjusted for age, gender, and education.
Response to treatment with albendazole.
One hundred two individuals who were positive for hookworm infection at baseline were treated with a single 400-mg oral dose of albendazole; 95 individuals provided follow-up fecal samples. Based on follow-up fecal exams, the cure rate was estimated at 61.1%, with an overall egg reduction rate of 81.5% (Table 4). Of 37 individuals (39%) who failed albendazole therapy, 14 showed an increase in fecal egg excretion on follow-up exam. Within the group that failed therapy, the difference in fecal egg excretion pre- and post-treatment was not statistically significant (317 epg at baseline versus 187 epg at follow-up; P = 0.084). There was also no significant difference in baseline fecal egg excretion between those who were cured (215.9 epg) and those who failed treatment (314.7 epg; P = 0.25), evidence that intensity of infection was not a factor mediating response to albendazole.
Table 4.
Cure rate and egg reduction rate in albendazole-treated subjects
| Prevalence (%) | Value |
|---|---|
| Baseline* | 100 |
| Post-treatment | 38.9 |
| Cure rate (%) | 61.1 |
| Arithmetic mean egg count† | |
| Baseline | 739.3 ± 1,583.2 |
| Post-treatment | 136.8 ± 329.0 |
| Egg reduction rate (%) | 81.5% |
Only hookworm-positive subjects at baseline are included.
Table includes mean eggs per gram ± standard deviation.
To confirm that the albendazole used in the study was fully active against hookworm, an in vitro analysis of drug efficacy was carried out using a laboratory strain of A. ceylanicum.25–27 As shown in Figure 5, there was no detectable difference in anthelminthic activity between the study drug (Remedica) and newly purchased albendazole (Sigma).18,22 It is, therefore, unlikely that inadequate storage or poor-quality pharmaceutical manufacturing explain the high treatment failure rate in this population.
Figure 5.
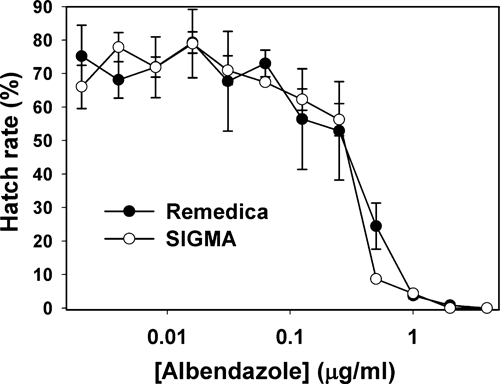
Comparison of anthelminthic activity of two preparations of albendazole using in vitro egg hatch assay. No difference in activity was observed between the Remedica and Sigma albendazole preparations against a laboratory strain of hookworm (A. ceylanicum).
Discussion
Hookworm infection is widespread throughout the tropics, with negative effects on the health and productivity of hundreds of millions of people.1,2 Across sub-Saharan Africa, the prevalence of hookworm is approximately 29%, corresponding to nearly 200 million people being infected.28 At present, there is no available vaccine to prevent hookworm infection, leaving periodic deworming and avoidance of risk behavior as the only effective measures of disease control.
Although prior studies of hookworm infection in Ghana have been published, most recent work has focused on populations in the northern part of the country. By contrast, little is known about the age-associated prevalence, intensity, social/demographic factors, and patterns of other infectious diseases in the Kintampo North Municipality located near the center of the country. To address this lack of baseline information, we conducted a cross-sectional pilot study to define the epidemiology of hookworm infection in this region of central Ghana. The data confirm a high prevalence (45%) of low-intensity hookworm infection that is comparable with published data from West Africa.29,30 In addition, we found a peak prevalence in school-age children, similar to previous findings from Africa31 but different from the steadily increasing prevalence with age reported from Asia.32–34
The risk of contracting hookworm correlates with behavioral, social, and economic factors that mediate potential exposure, including lack of access to a latrine, crowded households, and overall socioeconomic status.5,35–38 Risk factors identified in this population confirm these findings but also link infection with general health status (BMI and concurrent malaria infection) and occupational exposure (farmers), which may represent surrogate markers of socioeconomic status. For example, although lack of access to latrines and infrequent shoe use are more likely to be seen in poorer communities, providing latrines or shoes does not impact the prevalence of soil-transmitted helminth infections.39
School-age children with hookworm infection were nearly three times more likely (OR = 2.84; 95% CI = 1.11, 7.26) to have a positive malaria smear at baseline than those who were hookworm-negative (Table 2), and this age cohort also had the highest prevalence of coinfection (51%) (Figure 2B). Effects of concurrent helminth infection on the prevalence and clinical severity of malaria have been studied; however, the data are inconclusive, and mechanisms are poorly defined. For example, a cross-sectional study of school-age children in Tanzania found a higher prevalence of helminth infection (hookworm or Schistosoma mansoni) among children with P. falciparum,40 although a comparable study of younger children (6–23 months) found a lower prevalence.41 A deworming trial in Nigeria showed a significantly reduced rate of increase in malaria prevalence and intensity among children who were treated with albendazole.42 Two studies in Thailand found that Ascaris coinfection was associated with a decreased risk of cerebral malaria,43,44 although a prospective study by the same authors found a higher risk.45 A recent study by Pullan and others46 reported significant clustering (spatial and household) of hookworm and malaria infections in Uganda as well as evidence of an association between the two in pre–school-age children. Other studies have found no association between hookworm and malarial episodes,47,48 and the interpretation of existing data is complicated by differences in study populations, presence of multiple helminth species, variations in helminth infection intensities, and variations in the definition of malaria. Given the complexity of the relationship between these two highly endemic parasitic diseases, it is difficult to draw conclusions about the degree to which one may have influenced susceptibility to the other in the Kintampo North study population.
Both hookworm infection and malaria are known to cause anemia, especially in children and women of childbearing age.49,50 In this study population, the risk of anemia among children ages 6–15 years was highest in those who had isolated malaria infection (OR = 4.92), and this risk was greater than in those who were infected with both hookworm and malaria (OR = 2.34) (Table 3). Despite the small sample size, this pattern (greater risk of anemia with isolated malaria infection compared with coinfection) was observed across three of four age groups studied, the exception being adults ages 16–40 years old (data not shown). Our findings are consistent with previous reports, including a study in Zanzibar that reported a larger negative effect on blood hemoglobin levels in children with malaria infection alone than in those who were coinfected with helminths.41 Similarly, in a study of Peruvian school children, there was a significant drop in hemoglobin during a symptomatic infection with Plasmodium vivax among children who were not infected with intestinal helminths, whereas anemia was not exacerbated in those harboring worms.51 By contrast, others have reported varying increases in the risk of anemia with malaria and helminth coinfection across various age groups.46,52–56 Given that there are more than 400 million cases of P. falciparum each year,57 many occurring in areas endemic for helminths, a greater understanding of the interaction between these two highly prevalent and clinically important parasitic diseases could lead to the formulation of more effective control strategies for both.
Adults who tested positive for hookworm had a significantly lower mean BMI (20.5 versus 22.5; P < 0.01) than those who did not test positive (Table 1 and Figure 3), consistent with cross-sectional studies that have found an association between poor nutritional status and intestinal helminth infections.58–61 Treating intestinal helminth infections, particularly in areas with a high prevalence of hookworm, can improve children's nutritional status,9,62–65 supporting a causal role for helminth infections in the pathogenesis of malnutrition. However, although the lower BMI may be a consequence of hookworm, we propose that malnutrition could also mediate susceptibility to infection. There is a large body of evidence from animal studies of intestinal nematode infections, including hookworm, to suggest that micronutrient intake as well as underlying nutritional status can play a significant role in susceptibility and the host's capacity to control intensity of infection.66–71
Several recent studies have followed infected children after deworming to determine the influence of nutritional factors on reinfection. Hagel and others72 in Venezuela found that children aged 6–11 years with lower height for age z score (HAZ) and weight for age z score (WAZ) scores had significantly higher risks of reinfection with Ascaris 8 months after completing treatment. Similarly, rural schoolchildren in Malaysia who were stunted had significantly higher rates of reinfection with STNs,73 and a study in Brazil found a significantly higher rate of reinfection in undernourished children (38%) than in those who were well-nourished (25%).59 Ultimately, a greater understanding of the influence of nutritional status on susceptibility to helminth infections could inform future strategies aimed at integrating dietary supplementation with deworming programs.
We also observed an association in children between infection status and total IgG antibody levels against pooled adult hookworm ES proteins. It has previously been noted that infected individuals from endemic populations exhibit strong humoral response to various worm antigens and that certain immunoglobulin subtypes may be more closely associated with infection status.74–77 The serologic data presented here show that ES proteins from the human and animal hookworm A. ceylanicum25–27 can serve as useful reagents for measuring parasite-specific antibody responses in sub-Saharan Africa. This finding confirms significant cross-reactivity between ES proteins of A. ceylanicum and those from the two most common human hookworm species in Ghana, A. duodenale and N. americanus.78,79 Also, the observation that levels of IgG correlate with infection status, at least in children, validates the use of A. ceylanicum as a source of potentially relevant hookworm antigens that can be identified based on reactivity with human sera. Because the laboratory model of A. ceylanicum infection recapitulates the clinical features of human hookworm infection,19,21,80 specific ES antigens recognized by infected individuals may represent valuable targets for human drug and vaccine development.
It is interesting to note that, among the three most common STN infections (i.e., hookworm, ascariasis, and trichuriasis), only hookworm was present in significant numbers in the study population. The reason for this marked disparity in Kintampo North is not known, because central Ghana is known to be endemic for the three major STNs.56,81 Although we were unable to document evidence of prior albendazole exposure, this region of Kintampo North has participated in an intensive government sponsored onchocerciasis control program, which relies on periodic mass drug administration (MDA) with ivermectin. Ivermectin has modest efficacy against hookworm compared with other STNs,16,82 and MDA programs for onchocerciasis can alter the epidemiology of STN infections.82,83 For example, in the study by Moncaya and others,82 a reduction in T. trichiura prevalence because of ivermectin therapy was associated with a marked increase in the prevalence of hookworm.
Although administration of albendazole was associated with an overall egg reduction rate of 81% in the study population, treatment failed to eliminate fecal egg excretion in more than one-third (39%) of subjects on follow-up exam. For comparison, the efficacy of albendazole ranges from 45% to 100% in published studies, placing the cure rate in this study (61%) to the lower end of what has been reported. Outcome did not correlate with age, sex, occupation, community of residence, or pre-treatment intensity of infection, because nearly all of the subjects had light infections (< 2,000 epg). To investigate the quality of the medication, we compared the albendazole preparation used in the study (Remedica) with newly purchased drug (Sigma) using an in vitro egg hatch assay, which showed no difference in activity against a laboratory strain of hookworm (Figure 5). Compliance was also not an issue, because the administration of albendazole was directly observed by members of the study team.
In the absence of an alternative explanation, the prospect of benzimidazole resistance in Kintampo North must be considered, especially because, among those who were not cured, there was no statistically significant difference between pre- and post-treatment fecal egg counts (P = 0.084). Although resistance to benzimidazoles among veterinary nematodes is widespread, genetically mediated resistance of human hookworms to this class of anthelminthic has not been confirmed. However, increasing treatment failure rates with pyrantel pamoate and mebendazole have resulted in these drugs no longer being recommended for the treatment of human hookworm infection.16,84,85 Moreover, reduced in vitro activity of mebendazole has been observed in human hookworm isolates from Pemba Island, an endemic area known for high rates of treatment failure,86 compelling evidence of emerging resistance. Preliminary analysis of a 2010 follow-up study in Kintampo North points to an albendazole treatment failure rate of greater than 50% (Humphries D and others, unpublished observation), confirming the data reported here and raising further doubts about the effectiveness of this agent for the treatment of hookworm in Ghana.
In summary, results from a cross-sectional pilot study show a high prevalence of hookworm and malaria coinfection in Kintampo North Municipality of central Ghana. In addition to validating previously recognized risk factors, including nutritional status, we have also shown a novel association between hookworm infection and antibody levels to ES proteins from A. ceylanicum. We also observed that the risk of anemia was lower in children coinfected with malaria and hookworm than in those with malaria alone, suggesting that hookworm may modulate the pathogenesis of P. falciparum. Lastly, the high rate of albendazole treatment failure observed in this population raises concerns about benzimidazole resistance, a finding that warrants further investigation in Ghana and throughout sub-Saharan Africa.
ACKNOWLEDGMENTS
This work was presented in part at the 2009 annual meeting of the American Society of Tropical Medicine and Hygiene. The study was conducted under the auspices of the Ghana–Yale Partnership for Infectious Diseases Research. E.M. was supported by a Wilbur Downs International Health Research Fellowship from Yale University. B.B.-P. was supported by the Yale College Beckman Scholars Program. The authors would like to thank the people of Kintampo North Municipality for their participation in this study, the staff and administration of the Noguchi Memorial Institute for Medical Research, the Kintampo Rural Health Training School, and the University of Ghana. We would also like to thank Jon Vermeire and Dylan Davey for careful reading of the manuscript.
Footnotes
Authors' addresses: Debbie Humphries, Yale School of Public Health, New Haven, CT, E-mail: debbie.humphries@yale.edu. Emily Mosites, Tennessee Department of Health, Communicable and Environmental Disease Services, Nashville, TN, E-mail: Emily.Mosites@tn.gov. Joseph Otchere, Kwabena Bosompem, and Michael Wilson, Noguchi Memorial Institute for Medical Research, University of Ghana, Legon, Ghana, E-mails: JOtchere@noguchi.mimcom.org, KBosompem@noguchi.mimcom.org, and wilsonmi@who.int. Welbeck Amoani Twum, Rural Health Training School, Ministry of Health, Kintampo B/A, Ghana, E-mail: watbeckat@yahoo.com. Lauren Woo, Lisa M. Harrison, Blair Benham-Pyle, and Michael Cappello, Yale Child Health Research Center, New Haven, CT, E-mails: laurenwoo13@gmail.com, lisa.harrison@yale.edu, bbenhampyle2010@gmail.com, and michael.cappello@yale.edu. Hinckley Jones-Sanpei, School of Social Work, University of North Carolina, Chapel Hill, NC, E-mail: jonessan@email.unc.edu. Richard D. Bungiro, Department of Molecular Microbiology and Immunology, Providence, RI, E-mail: Richard_Bungiro@Brown.edu. Langbong Bimi and Dominic Edoh, Department of Animal Biology and Conservation Science, University of Ghana, Legon, Ghana, E-mails: lbimi@ug.edu.gh and dedoh@ug.edu.gh.
References
- 1.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 3.Yelifari L, Bloch P, Magnussen P, van Lieshout L, Dery G, Anemana S, Agongo E, Polderman AM. Distribution of human Oesophagostomum bifurcum, hookworm and Strongyloides stercoralis infections in northern Ghana. Trans R Soc Trop Med Hyg. 2005;99:32–38. doi: 10.1016/j.trstmh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Ndyomugyenyi R, Kabatereine N, Olsen A, Magnussen P. Malaria and hookworm infections in relation to haemoglobin and serum ferritin levels in pregnancy in Masindi district, western Uganda. Trans R Soc Trop Med Hyg. 2008;102:130–136. doi: 10.1016/j.trstmh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Hotez P. Hookworm and poverty. Ann N Y Acad Sci. 2008;1136:38–44. doi: 10.1196/annals.1425.000. [DOI] [PubMed] [Google Scholar]
- 6.Mabaso ML, Appleton CC, Hughes JC, Gouws E. The effect of soil type and climate on hookworm (Necator americanus) distribution in KwaZulu-Natal, South Africa. Trop Med Int Health. 2003;8:722–727. doi: 10.1046/j.1365-3156.2003.01086.x. [DOI] [PubMed] [Google Scholar]
- 7.Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, Sachs JD. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med. 2006;3:e102. doi: 10.1371/journal.pmed.0030102. doi:10.1371/journal.pmed.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bundy D, Sher A, Michael E. Good worms or bad worms: do worm infections affect the epidemiological patterns of other diseases? Parasitol Today. 2000;16:273–274. doi: 10.1016/s0169-4758(00)01689-6. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson LS, Latham MC, Kurz KM, Kinoti SN, Brigham H. Treatment with a single dose of albendazole improves growth of Kenyan schoolchildren with hookworm, Trichuris trichiura, and Ascaris lumbricoides infections. Am J Trop Med Hyg. 1989;41:78–87. [PubMed] [Google Scholar]
- 10.Dossa RA, Ategbo EA, de Koning FL, van Raaij JM, Hautvast JG. Impact of iron supplementation and deworming on growth performance in preschool Beninese children. Eur J Clin Nutr. 2001;55:223–228. doi: 10.1038/sj.ejcn.1601126. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava A, Jukes M, Lambo J, Kihamia CM, Lorri W, Nokes C, Drake L, Bundy D. Anthelmintic treatment improves the hemoglobin and serum ferritin concentrations of Tanzanian schoolchildren. Food Nutr Bull. 2003;24:332–342. doi: 10.1177/156482650302400403. [DOI] [PubMed] [Google Scholar]
- 12.Ndyomugyenyi R, Kabatereine N, Olsen A, Magnussen P. Efficacy of ivermectin and albendazole alone and in combination for treatment of soil-transmitted helminths in pregnancy and adverse events: a randomized open label controlled intervention trial in Masindi district, western Uganda. Am J Trop Med Hyg. 2008;79:856–863. [PubMed] [Google Scholar]
- 13.World Health Assembly Schistosomiasis and Soil-transmitted Helminths. 2001. http://www.who.int/wormcontrol/documents/wha/en/ Available at. Accessed June 15, 2009.
- 14.Bundy DA, Kremer M, Bleakley H, Jukes MC, Miguel E. Deworming and development: asking the right questions, asking the questions right. PLoS Negl Trop Dis. 2009;3:e362. doi: 10.1371/journal.pntd.0000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Bank School Deworming at a Glance. 2003. http://web.worldbank.org/WBSITE/EXTERNAL/TOPICS/EXTHEALTHNUTRITIONANDPOPULATION/EXTPHAAG/0,contentMDK:20785786~menuPK:1314819~pagePK:64229817~piPK:64229743~theSitePK:672263,00.html#PDF Available at. Accessed November 15, 2010.
- 16.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 17.Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Health Organ. 2003;81:343–352. [PMC free article] [PubMed] [Google Scholar]
- 18.Albonico M, Engels D, Savioli L. Monitoring drug efficacy and early detection of drug resistance in human soil-transmitted nematodes: a pressing public health agenda for helminth control. Int J Parasitol. 2004;34:1205–1210. doi: 10.1016/j.ijpara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Bungiro RD, Jr, Greene J, Kruglov E, Cappello M. Mitigation of hookworm disease by immunization with soluble extracts of Ancylostoma ceylanicum. J Infect Dis. 2001;183:1380–1387. doi: 10.1086/319867. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . Informal Consultation on Intestinal Parasite Infections. Geneva, Switzerland: World Health Organization; 1996. WHO Document No. WHO/CDC/PI/90.1. [Google Scholar]
- 21.Bungiro RD, Jr, Sun T, Harrison LM, Shoemaker CB, Cappello M. Mucosal antibody responses in experimental hookworm infection. Parasite Immunol. 2008;30:293–303. doi: 10.1111/j.1365-3024.2008.01023.x. [DOI] [PubMed] [Google Scholar]
- 22.Cappello M, Bungiro RD, Harrison LM, Bischof LJ, Griffitts JS, Barrows BD, Aroian RV. A purified Bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma ceylanicum. Proc Natl Acad Sci USA. 2006;103:15154–15159. doi: 10.1073/pnas.0607002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghana Statistical Service Ghana 2003 Demographic and Health Survey Key Findings: Demographic and Health Surveys (DHS) 2003.
- 24.World Health Organization . Report of the WHO Informal Consultation on Hookworm Infection and Anaemia in Girls and Women. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 25.Yoshida Y, Okamoto K, Matsuo K, Kwo EH, Retnasabapathy A. The occurrence of Ancylostoma braziliense (de Faria, 1910) and Ancylostoma ceylanicum (Looss, 1911) in Malaysia. Southeast Asian J Trop Med Public Health. 1973;4:498–503. [PubMed] [Google Scholar]
- 26.Chowdhury AB, Schad GA. Ancylostoma ceylanicum: a parasite of man in Calcutta and environs. Am J Trop Med Hyg. 1972;21:300–301. doi: 10.4269/ajtmh.1972.21.300. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida Y, Okamoto K, Chiu JK. Ancylostoma ceylanicum infection in dogs, cats, and man in Taiwan. Am J Trop Med Hyg. 1968;17:378–381. doi: 10.4269/ajtmh.1968.17.378. [DOI] [PubMed] [Google Scholar]
- 28.Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooker S, Clements AC, Hotez PJ, Hay SI, Tatem AJ, Bundy DA, Snow RW. The co-distribution of Plasmodium falciparum and hookworm among African schoolchildren. Malar J. 2006;5:99. doi: 10.1186/1475-2875-5-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raso G, Luginbuhl A, Adjoua CA, Tian-Bi NT, Silue KD, Matthys B, Vounatsou P, Wang Y, Dumas ME, Holmes E, Singer BH, Tanner M, N'Goran EK, Utzinger J. Multiple parasite infections and their relationship to self-reported morbidity in a community of rural Cote d'Ivoire. Int J Epidemiol. 2004;33:1092–1102. doi: 10.1093/ije/dyh241. [DOI] [PubMed] [Google Scholar]
- 31.Mwangi TW, Bethony JM, Brooker S. Malaria and helminth interactions in humans: an epidemiological viewpoint. Ann Trop Med Parasitol. 2006;100:551–570. doi: 10.1179/136485906X118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Changhua L, Xiaorong Z, Dongchuan Q, Shuhua X, Hotez PJ, Defu Z, Hulian Z, Mingden L, Hainan R, Bing Z, Haichou X, Hawdon J, Zheng F. Epidemiology of human hookworm infections among adult villagers in Hejiang and Santai Counties, Sichuan Province, China. Acta Trop. 1999;73:243–249. doi: 10.1016/s0001-706x(99)00035-2. [DOI] [PubMed] [Google Scholar]
- 33.Humphries DL, Stephenson LS, Pearce EJ, The PH, Dan HT, Khanh LT. The use of human faeces for fertilizer is associated with increased intensity of hookworm infection in Vietnamese women. Trans R Soc Trop Med Hyg. 1997;91:518–520. doi: 10.1016/s0035-9203(97)90007-9. [DOI] [PubMed] [Google Scholar]
- 34.Bethony J, Chen J, Lin S, Xiao S, Zhan B, Li S, Xue H, Xing F, Humphries D, Yan W, Chen G, Foster V, Hawdon JM, Hotez PJ. Emerging patterns of hookworm infection: influence of aging on the intensity of Necator infection in Hainan Province, People's Republic of China. Clin Infect Dis. 2002;35:1336–1344. doi: 10.1086/344268. [DOI] [PubMed] [Google Scholar]
- 35.Olsen A, Samuelsen H, Onyango-Ouma W. A study of risk factors for intestinal helminth infections using epidemiological and anthropological approaches. J Biosoc Sci. 2001;33:569–584. doi: 10.1017/s0021932001005697. [DOI] [PubMed] [Google Scholar]
- 36.Chongsuvivatwong V, Pas-Ong S, McNeil D, Geater A, Duerawee M. Predictors for the risk of hookworm infection: experience from endemic villages in southern Thailand. Trans R Soc Trop Med Hyg. 1996;90:630–633. doi: 10.1016/s0035-9203(96)90412-5. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen PH, Nguyen KC, Nguyen TD, Le MB, Bern C, Flores R, Martorell R. Intestinal helminth infections among reproductive age women in Vietnam: prevalence, co-infection and risk factors. Southeast Asian J Trop Med Public Health. 2006;37:865–874. [PubMed] [Google Scholar]
- 38.Al-Mekhlafi MS, Atiya AS, Lim YA, Mahdy AK, Ariffin WA, Abdullah HC, Surin J. An unceasing problem: soil-transmitted helminthiases in rural Malaysian communities. Southeast Asian J Trop Med Public Health. 2007;38:998–1007. [PubMed] [Google Scholar]
- 39.Yajima A, Jouquet P, Do TD, Dang TC, Tran CD, Orange D, Montresor A. High latrine coverage is not reducing the prevalence of soil-transmitted helminthiasis in Hoa Binh province, Vietnam. Trans R Soc Trop Med Hyg. 2009;103:237–241. doi: 10.1016/j.trstmh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Midzi N, Sangweme D, Zinyowera S, Mapingure MP, Brouwer KC, Munatsi A, Mutapi F, Mudzori J, Kumar N, Woelk G, Mduluza T. The burden of polyparasitism among primary schoolchildren in rural and farming areas in Zimbabwe. Trans R Soc Trop Med Hyg. 2008;102:1039–1045. doi: 10.1016/j.trstmh.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Kung'u JK, Goodman D, Haji HJ, Ramsan M, Wright VJ, Bickle QD, Tielsch JM, Raynes JG, Stoltzfus RJ. Early helminth infections are inversely related to anemia, malnutrition, and malaria and are not associated with inflammation in 6- to 23-month-old Zanzibari children. Am J Trop Med Hyg. 2009;81:1062–1070. doi: 10.4269/ajtmh.2009.09-0091. [DOI] [PubMed] [Google Scholar]
- 42.Kirwan P, Jackson AL, Asaolu SO, Molloy SF, Abiona TC, Bruce MC, Ranford-Cartwright L, O'Neill SM, Holland CV. Impact of repeated four-monthly anthelmintic treatment on Plasmodium infection in preschool children: a double-blind placebo-controlled randomized trial. BMC Infect Dis. 2009;10:277. doi: 10.1186/1471-2334-10-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nacher M, Singhasivanon P, Traore B, Vannaphan S, Gay F, Chindanond D, Franetich JF, Mazier D, Looareesuwan S. Helminth infections are associated with protection from cerebral malaria and increased nitrogen derivatives concentrations in Thailand. Am J Trop Med Hyg. 2002;66:304–309. doi: 10.4269/ajtmh.2002.66.304. [DOI] [PubMed] [Google Scholar]
- 44.Nacher M, Singhasivanon P, Treeprasertsuk S, Vannaphan S, Traore B, Looareesuwan S, Gay F. Intestinal helminths and malnutrition are independently associated with protection from cerebral malaria in Thailand. Ann Trop Med Parasitol. 2002;96:5–13. doi: 10.1179/000349802125000448. [DOI] [PubMed] [Google Scholar]
- 45.Nacher M, Singhasivanon P, Yimsamran S, Manibunyong W, Thanyavanich N, Wuthisen R, Looareesuwan S. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. J Parasitol. 2002;88:55–58. doi: 10.1645/0022-3395(2002)088[0055:IHIAAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 46.Pullan RL, Kabatereine NB, Bukirwa H, Staedke SG, Brooker S. Heterogeneities and consequences of Plasmodium species and hookworm coinfection: a population based study in Uganda. J Infect Dis. 2011;203:406–417. doi: 10.1093/infdis/jiq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapiro AE, Tukahebwa EM, Kasten J, Clarke SE, Magnussen P, Olsen A, Kabatereine NB, Ndyomugyenyi R, Brooker S. Epidemiology of helminth infections and their relationship to clinical malaria in southwest Uganda. Trans R Soc Trop Med Hyg. 2005;99:18–24. doi: 10.1016/j.trstmh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Bejon P, Mwangi TW, Lowe B, Peshu N, Hill AV, Marsh K. Helminth infection and eosinophilia and the risk of Plasmodium falciparum malaria in 1- to 6-year-old children in a malaria endemic area. PLoS Negl Trop Dis. 2008;2:e164. doi: 10.1371/journal.pntd.0000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brooker S, Hotez PJ, Bundy DA. Hookworm-related anaemia among pregnant women: a systematic review. PLoS Negl Trop Dis. 2008;2:e291. doi: 10.1371/journal.pntd.0000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, Snow RW, Hotez PJ. Epidemiology of Plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg. 2007;77:88–98. [PMC free article] [PubMed] [Google Scholar]
- 51.Melo GC, Reyes-Lecca RC, Vitor-Silva S, Monteiro WM, Martins M, Benzecry SG, Alecrim MG, Lacerda MV. Concurrent helminthic infection protects schoolchildren with Plasmodium vivax from anemia. PLoS One. 2010;5:e11206. doi: 10.1371/journal.pone.0011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demissie F, Kebede A, Shimels T, Beyene P. Assessment of public health implication of malaria-geohelminth co-infection with an emphasis on hookworm-malaria anemia among suspected malaria patients in asendabo, southwest Ethiopia. Ethiop Med J. 2009;47:153–158. [PubMed] [Google Scholar]
- 53.Midzi N, Mtapuri-Zinyowera S, Mapingure MP, Sangweme D, Chirehwa MT, Brouwer KC, Mudzori J, Hlerema G, Mutapi F, Kumar N, Mduluza T. Consequences of polyparasitism on anaemia among primary school children in Zimbabwe. Acta Trop. 2010;115:103–111. doi: 10.1016/j.actatropica.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Koukounari A, Estambale BB, Njagi JK, Cundill B, Ajanga A, Crudder C, Otido J, Jukes MC, Clarke SE, Brooker S. Relationships between anaemia and parasitic infections in Kenyan schoolchildren: a Bayesian hierarchical modelling approach. Int J Parasitol. 2008;38:1663–1671. doi: 10.1016/j.ijpara.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Degarege A, Animut A, Legesse M, Erko B. Malaria and helminth co-infections in outpatients of Alaba Kulito Health Center, southern Ethiopia: a cross sectional study. BMC Res Notes. 2010;3:143. doi: 10.1186/1756-0500-3-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yatich NJ, Jolly PE, Funkhouser E, Agbenyega T, Rayner JC, Ehiri JE, Turpin A, Stiles JK, Ellis WO, Jiang Y, Williams JH. The effect of malaria and intestinal helminth coinfection on birth outcomes in Kumasi, Ghana. Am J Trop Med Hyg. 2010;82:28–34. doi: 10.4269/ajtmh.2010.09-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, Snow RW. Estimating the global clinical burden of Plasmodium falciparum Malaria in 2007. PLoS Med. 2010;7:e1000290. doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tshikuka JG, Gray-Donald K, Scott M, Olela KN. Relationship of childhood protein-energy malnutrition and parasite infections in an urban African setting. Trop Med Int Health. 1997;2:374–382. doi: 10.1111/j.1365-3156.1997.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 59.Saldiva SR, Silveira AS, Philippi ST, Torres DM, Mangini AC, Dias RM, da Silva RM, Buratini MN, Massad E. Ascaris-Trichuris association and malnutrition in Brazilian children. Paediatr Perinat Epidemiol. 1999;13:89–98. doi: 10.1046/j.1365-3016.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- 60.Casapia M, Joseph SA, Nunez C, Rahme E, Gyorkos TW. Parasite and maternal risk factors for malnutrition in preschool-age children in Belen, Peru using the new WHO Child Growth Standards. Br J Nutr. 2007;98:1259–1266. doi: 10.1017/S0007114507795272. [DOI] [PubMed] [Google Scholar]
- 61.Hughes RG, Sharp DS, Hughes MC, Akau'ola S, Heinsbroek P, Velayudhan R, Schulz D, Palmer K, Cavalli-Sforza T, Galea G. Environmental influences on helminthiasis and nutritional status among Pacific schoolchildren. Int J Environ Health Res. 2004;14:163–177. doi: 10.1080/0960312042000218589. [DOI] [PubMed] [Google Scholar]
- 62.Stephenson LS, Latham MC, Adams EJ, Kinoti SN, Pertet A. Physical fitness, growth and appetite of Kenyan school boys with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved four months after a single dose of albendazole. J Nutr. 1993;123:1036–1046. doi: 10.1093/jn/123.6.1036. [DOI] [PubMed] [Google Scholar]
- 63.Stephenson LS, Latham MC, Kinoti SN, Kurz KM, Brigham H. Improvements in physical fitness of Kenyan schoolboys infected with hookworm, Trichuris trichiura and Ascaris lumbricoides following a single dose of albendazole. Trans R Soc Trop Med Hyg. 1990;84:277–282. doi: 10.1016/0035-9203(90)90286-n. [DOI] [PubMed] [Google Scholar]
- 64.Hlaing T, Saw T, Lwin M. Reinfection of people with Ascaris lumbricoides following single, 6-month and 12-month interval mass chemotherapy in Okpo village, rural Burma. Trans R Soc Trop Med Hyg. 1987;81:140–146. doi: 10.1016/0035-9203(87)90306-3. [DOI] [PubMed] [Google Scholar]
- 65.Stoltzfus RJ, Albonico M, Tielsch JM, Chwaya HM, Savioli L. School-based deworming program yields small improvement in growth of Zanzibari school children after one year. J Nutr. 1997;127:2187–2193. doi: 10.1093/jn/127.11.2187. [DOI] [PubMed] [Google Scholar]
- 66.Coop RL, Kyriazakis I. Influence of host nutrition on the development and consequences of nematode parasitism in ruminants. Trends Parasitol. 2001;17:325–330. doi: 10.1016/s1471-4922(01)01900-6. [DOI] [PubMed] [Google Scholar]
- 67.Hoste H, Torres-Acosta JF, Aguilar-Caballero AJ. Nutrition-parasite interactions in goats: is immunoregulation involved in the control of gastrointestinal nematodes? Parasite Immunol. 2008;30:79–88. doi: 10.1111/j.1365-3024.2007.00987.x. [DOI] [PubMed] [Google Scholar]
- 68.van Houtert MF, Barger IA, Steel JW, Windon RG, Emery DL. Effects of dietary protein intake on responses of young sheep to infection with Trichostrongylus colubriformis. Vet Parasitol. 1995;56:163–180. doi: 10.1016/0304-4017(94)00668-3. [DOI] [PubMed] [Google Scholar]
- 69.Houdijk JG, Jackson F, Kyriazakis I. Nutritional sensitivity of resistance to Trichostrongylus colubriformis in lactating ewes. Vet Parasitol. 2009;160:258–266. doi: 10.1016/j.vetpar.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 70.Ing R, Su Z, Scott ME, Koski KG. Suppressed T helper 2 immunity and prolonged survival of a nematode parasite in protein-malnourished mice. Proc Natl Acad Sci USA. 2000;97:7078–7083. doi: 10.1073/pnas.97.13.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Held MR, Bungiro RD, Harrison LM, Hamza I, Cappello M. Dietary iron content mediates hookworm pathogenesis in vivo. Infect Immun. 2006;74:289–295. doi: 10.1128/IAI.74.1.289-295.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hagel I, Lynch NR, Di Prisco MC, Perez M, Sanchez JE, Pereyra BN, Soto de Sanabria I. Helminthic infection and anthropometric indicators in children from a tropical slum: ascaris reinfection after anthelmintic treatment. J Trop Pediatr. 1999;45:215–220. doi: 10.1093/tropej/45.4.215. [DOI] [PubMed] [Google Scholar]
- 73.Al-Mekhlafi MH, Surin J, Atiya AS, Ariffin WA, Mahdy AKM, Abdullah HC. Pattern and predictors of soil-transmitted helminth reinfection among aboriginal schoolchildren in rural Peninsular Malaysia. Acta Trop. 2008;107:200–204. doi: 10.1016/j.actatropica.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 74.Haichou X, Yan W, Shuhua X, Sen L, Yong W, Guangjin S, Weituo W, Bin Z, Drake L, Zheng F, Hotez PJ. Epidemiology of human ancylostomiasis among rural villagers in Nanlin County (Zhongzhou Village), Anhui Province, China: II. Seroepidemiological studies of the age relationships of serum antibody levels and infection status. Southeast Asian J Trop Med Public Health. 2000;31:736–741. [PubMed] [Google Scholar]
- 75.Mahmoud MS, Abou Gamra MM, Elkhayat MM. Ancylostoma duodenale infection: a study of serum immunoglobulin G4 response to the excretory secretory antigen of adult worm. J Egypt Soc Parasitol. 2005;35:1–17. [PubMed] [Google Scholar]
- 76.Quinnell RJ, Woolhouse ME, Walsh EA, Pritchard DI. Immunoepidemiology of human necatoriasis: correlations between antibody responses and parasite burdens. Parasite Immunol. 1995;17:313–318. doi: 10.1111/j.1365-3024.1995.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 77.Quinnell RJ, Bethony J, Pritchard DI. The immunoepidemiology of human hookworm infection. Parasite Immunol. 2004;26:443–454. doi: 10.1111/j.0141-9838.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 78.Verweij JJ, Brienen EA, Ziem J, Yelifari L, Polderman AM, Van Lieshout L. Simultaneous detection and quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum bifurcum in fecal samples using multiplex real-time PCR. Am J Trop Med Hyg. 2007;77:685–690. [PubMed] [Google Scholar]
- 79.de Gruijter JM, van Lieshout L, Gasser RB, Verweij JJ, Brienen EA, Ziem JB, Yelifari L, Polderman AM. Polymerase chain reaction-based differential diagnosis of Ancylostoma duodenale and Necator americanus infections in humans in northern Ghana. Trop Med Int Health. 2005;10:574–580. doi: 10.1111/j.1365-3156.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- 80.Bungiro RD, Jr, Cappello M. Detection of excretory/secretory coproantigens in experimental hookworm infection. Am J Trop Med Hyg. 2005;73:915–920. [PubMed] [Google Scholar]
- 81.Addy PA, Antepim G, Frimpong EH. Prevalence of pathogenic Escherichia coli and parasites in infants with diarrhoea in Kumasi, Ghana. East Afr Med J. 2004;81:353–357. doi: 10.4314/eamj.v81i7.9190. [DOI] [PubMed] [Google Scholar]
- 82.Moncayo AL, Vaca M, Amorim L, Rodriguez A, Erazo S, Oviedo G, Quinzo I, Padilla M, Chico M, Lovato R, Gomez E, Barreto ML, Cooper PJ. Impact of long-term treatment with ivermectin on the prevalence and intensity of soil-transmitted helminth infections. PLoS Negl Trop Dis. 2008;2:e293. doi: 10.1371/journal.pntd.0000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beach MJ, Streit TG, Addiss DG, Prospere R, Roberts JM, Lammie PJ. Assessment of combined ivermectin and albendazole for treatment of intestinal helminth and Wuchereria bancrofti infections in Haitian schoolchildren. Am J Trop Med Hyg. 1999;60:479–486. doi: 10.4269/ajtmh.1999.60.479. [DOI] [PubMed] [Google Scholar]
- 84.Flohr C, Tuyen LN, Lewis S, Minh TT, Campbell J, Britton J, Williams H, Hien TT, Farrar J, Quinnell RJ. Low efficacy of mebendazole against hookworm in Vietnam: two randomized controlled trials. Am J Trop Med Hyg. 2007;76:732–736. [PubMed] [Google Scholar]
- 85.Albonico M, Ramsan M, Wright V, Jape K, Haji HJ, Taylor M, Savioli L, Bickle Q. Soil-transmitted nematode infections and mebendazole treatment in Mafia Island schoolchildren. Ann Trop Med Parasitol. 2002;96:717–726. doi: 10.1179/000349802125001942. [DOI] [PubMed] [Google Scholar]
- 86.Albonico M, Wright V, Ramsan M, Haji HJ, Taylor M, Savioli L, Bickle Q. Development of the egg hatch assay for detection of anthelminthic resistance in human hookworms. Int J Parasitol. 2005;35:803–811. doi: 10.1016/j.ijpara.2005.02.016. [DOI] [PubMed] [Google Scholar]


