Abstract
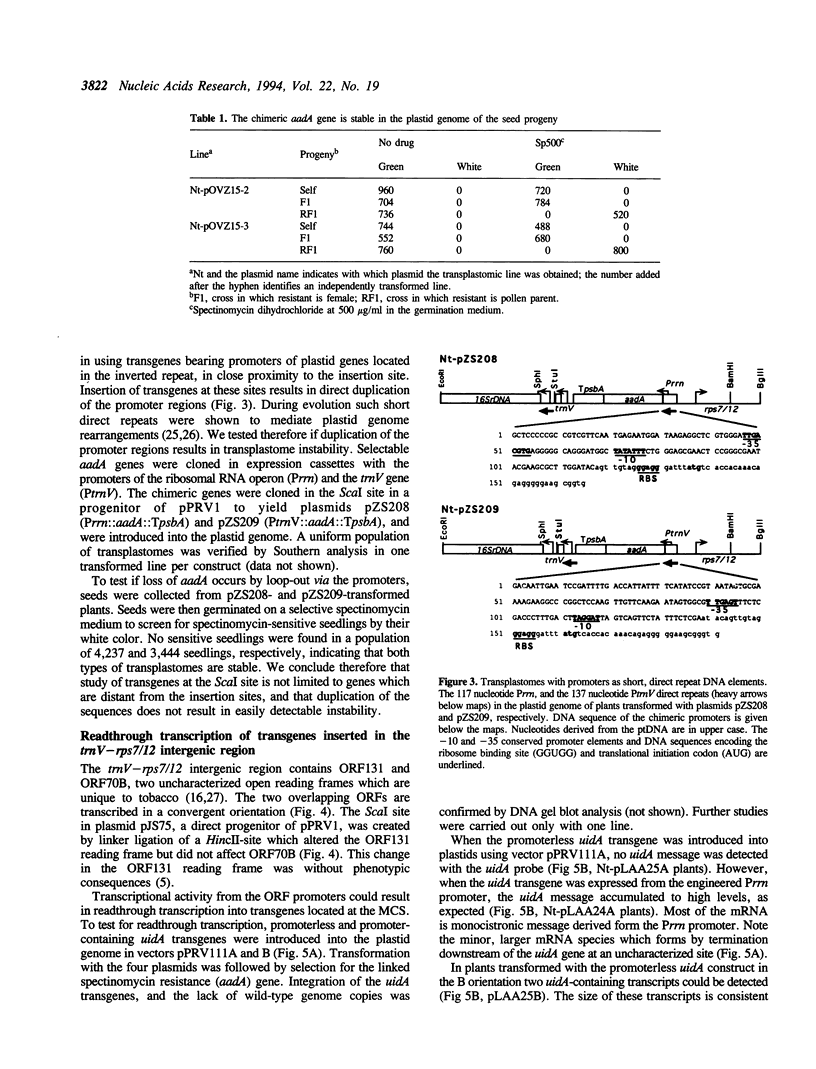
The pPRV plasmids are vectors for targeted insertion of foreign genes into the tobacco plastid genome (ptDNA). The vectors are based on the pUC119 plasmid which replicates in E. coli but not in plastids. The spectinomycin resistance (aadA) gene and a multiple cloning site (MCS) are flanked by 1.8-kb and 1.2-kb ptDNA sequences. Biolistic delivery of vector DNA, followed by spectinomycin selection, yields plastid transformants at a reproducible frequency, approximately 1 transplastomic line per bombarded sample. The selected aadA gene and linked non-selectable genes cloned into the MCS are incorporated into the ptDNA by two homologous recombination events via the flanking ptDNA sequences. The transplastomes thus generated are stable, and are maternally transmitted to the seed progeny. The pPRV vector series targets insertions between the divergently transcribed trnV gene and the rps12/7 operon. The lack of readthrough transcription of appropriately oriented transgenes makes the vectors an ideal choice for the study of transgene promoter activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich J., Cherney B. W., Merlin E., Christopherson L. The role of insertions/deletions in the evolution of the intergenic region between psbA and trnH in the chloroplast genome. Curr Genet. 1988 Aug;14(2):137–146. doi: 10.1007/BF00569337. [DOI] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W. Chloroplast transformation in Chlamydomonas. Methods Enzymol. 1993;217:510–536. doi: 10.1016/0076-6879(93)17087-l. [DOI] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Carrer H., Hockenberry T. N., Svab Z., Maliga P. Kanamycin resistance as a selectable marker for plastid transformation in tobacco. Mol Gen Genet. 1993 Oct;241(1-2):49–56. doi: 10.1007/BF00280200. [DOI] [PubMed] [Google Scholar]
- Hildebrand M., Hallick R. B., Passavant C. W., Bourque D. P. Trans-splicing in chloroplasts: the rps 12 loci of Nicotiana tabacum. Proc Natl Acad Sci U S A. 1988 Jan;85(2):372–376. doi: 10.1073/pnas.85.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanevski I., Maliga P. Relocation of the plastid rbcL gene to the nucleus yields functional ribulose-1,5-bisphosphate carboxylase in tobacco chloroplasts. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1969–1973. doi: 10.1073/pnas.91.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B., Fromm H., Galun E., Edelman M. Evidence for in vivo trans splicing of pre-mRNAs in tobacco chloroplasts. Cell. 1987 Jan 16;48(1):111–119. doi: 10.1016/0092-8674(87)90361-8. [DOI] [PubMed] [Google Scholar]
- O'Neill C., Horváth G. V., Horváth E., Dix P. J., Medgyesy P. Chloroplast transformation in plants: polyethylene glycol (PEG) treatment of protoplasts is an alternative to biolistic delivery systems. Plant J. 1993 May;3(5):729–738. [PubMed] [Google Scholar]
- Rochaix J. D. Post-transcriptional steps in the expression of chloroplast genes. Annu Rev Cell Biol. 1992;8:1–28. doi: 10.1146/annurev.cb.08.110192.000245. [DOI] [PubMed] [Google Scholar]
- Shimada H., Sugiura M. Fine structural features of the chloroplast genome: comparison of the sequenced chloroplast genomes. Nucleic Acids Res. 1991 Mar 11;19(5):983–995. doi: 10.1093/nar/19.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub J. M., Maliga P. Accumulation of D1 polypeptide in tobacco plastids is regulated via the untranslated region of the psbA mRNA. EMBO J. 1993 Feb;12(2):601–606. doi: 10.1002/j.1460-2075.1993.tb05692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub J. M., Maliga P. Long regions of homologous DNA are incorporated into the tobacco plastid genome by transformation. Plant Cell. 1992 Jan;4(1):39–45. doi: 10.1105/tpc.4.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. B., Gruissem W. Control of plastid gene expression: 3' inverted repeats act as mRNA processing and stabilizing elements, but do not terminate transcription. Cell. 1987 Dec 24;51(6):1145–1157. doi: 10.1016/0092-8674(87)90600-3. [DOI] [PubMed] [Google Scholar]
- Svab Z., Hajdukiewicz P., Maliga P. Stable transformation of plastids in higher plants. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8526–8530. doi: 10.1073/pnas.87.21.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z., Harper E. C., Jones J. D., Maliga P. Aminoglycoside-3''-adenyltransferase confers resistance to spectinomycin and streptomycin in Nicotiana tabacum. Plant Mol Biol. 1990 Feb;14(2):197–205. doi: 10.1007/BF00018560. [DOI] [PubMed] [Google Scholar]
- Svab Z., Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohdoh N., Shinozaki K., Sugiura M. Sequence of a putative promoter region for the rRNA genes of tobacco chloroplast DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5399–5406. doi: 10.1093/nar/9.20.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]