Abstract
The therapeutic efficacy and effects of artemether-lumefantrine (AL) and artesunate-amodiaquine co-formulated (AAcf) or co-packaged (AAcp) on malaria-associated anemia (MAA) were evaluated in 285 children < 12 years of age with uncomplicated Plasmodium falciparum malaria randomized to receive one of the three drug combinations. Fever and parasite clearance times were similar in all treatment groups. Mean drug-attributable fall in hematocrit (DAFH), defined as difference between hematocrit values pre- and 3 d post- initiation of treatment, was low (< 4.5%) and rates of recovery from MAA were similar with all treatments. Mean areas under curve (AUCs) of the plot of deficit in hematocrit levels from 30% versus time in anemic children were similar in all groups. All regimens were well tolerated. AL, AAcf and AAcp cleared fever and parasitemia rapidly and had similar rates of resolution of MAA after treatment in malarious Nigerian children.
Introduction
Artemisinin-based combination therapies (ACTs) are now the recommended first-line treatment of falciparum malaria globally, because they rapidly decrease parasitemia, hasten recovery, and reduce chances of development of drug resistance.1,2 Of the available ACTs, artesunate-amodiaquine (AA) and artemether-lumefantrine (AL) seem to be the most widely used.3–7 Although there are many formulations of AA, including coformulated (AAcf) or fixed-dose and copackaged (AAcp), it is generally assumed that AAcf may improve compliance and treatment outcomes because of a simple dosing regimen and fewer tablets compared with AAcp. In addition, fixed combination prevents the use of monotherapy, which can contribute to antimalarial drug resistance, because only the artemisinin component of the coblistered packages is often taken. A recent study in five African countries has shown that fixed-dose AA is as efficacious as AL in children.8 However, there are few or no recent studies comparing fixed-dose and copackaged AA with AL in malarious African children. It is, therefore, necessary to evaluate whether AAcf or AAcp is biologically and therapeutically equivalent in malarious children in endemic areas, because cost and effectiveness are issues that may militate against reduction of morbidity and mortality in these areas. In addition, in vitro studies have shown that both amodiaquine and AL are associated with selection of Plasmodium falciparum multidrug resistance gene 1 (Pfmdr gene 1), which is associated with recrudescence to antimalarial drugs, in children after treatment.9,10
In this study, we report the tolerability, antimalarial treatment efficacy, and effects on malaria-associated anemia (MAA) of AAcp, AAcf, and AL in children ≤ 12 years of age with acute symptomatic, uncomplicated P. falciparum malaria.
Material and Methods
Study area.
The study was carried out in Ibadan in southwestern Nigeria from July 2009 to May 2010. In this area of hyperendemic malaria, transmission occurs all year round but is more intense during the rainy season from April to October.11 The prevalence of AL, AA, and amodiaquine resistance in the area has been reported to be < 2%, 2%, and 4%, respectively.7,9,12
Patients, treatment, and follow-up.
Patients were eligible to participate in the study if they were ≤ 12 years of age, had symptoms compatible with acute uncomplicated malaria such as anorexia, vomiting, or abdominal discomfort with or without diarrhea, had P. falciparum parasitemia > 2,000 asexual forms/μL, had a body (axillary) temperature > 37.4°C or history of fever in the 24–48 hours preceding presentation, had absence of other concomitant illness, had no history of antimalarial drug use in the 2 weeks preceding presentation, and had written informed consent given by parents or guardians. Patients with severe malaria,13 severe malnutrition, serious underlying diseases (renal, cardiac, or hepatic), and known allergy to study drugs were excluded from the study. The study protocol was reviewed and approved by the Ethics Committee of the Ministry of Health, Ibadan. The disease history, taken by the attending physician, was recorded by asking patients or their parents or guardians when the present symptomatic period started, and this was followed by a full physical examination by the same physician.
Enrolled patients were randomly assigned to receive AL, AAcf, or AAcp in a ratio of 2:3:3. AL (Coartem; Novartis, Basel, Switzerland) was given according to body weight: patients weighing 5–14 kg received one tablet, those weighing 14–24 kg received two tablets, those weighing 25–34 kg received three tablets, and those weighing more than 34 kg received four tablets at presentation (0 hours), 8 hours later, and 24, 36, 48, and 60 hours after the first dose. Each tablet of AL contains 20 mg artemether and 120 mg lumefantrine. AAcf (Coarsucam; Sanofi Aventis, Casablanca, Morocco) was given as follows: children weighing ≥ 9 to < 18 kg or aged 1–5 years received 0.5 tablets, children weighing ≥ 18–36 kg or aged 6–13 years received one tablet, and children weighing ≥ 36 kg or aged ≥ 14 years received two tablets. Each tablet of AAcf contains 270 mg amodiaquine base and 100 mg artesunate coformulated in a bilayer. AAcp (Dart; Swiss Pharma, Lagos, Nigeria) was given according to age or body weight as follow: 1–5 years or 5–15 kg received one tablet each of amodiaquine and artesunate, 6–10 years or 16–24 kg received two tablets each of amodiaquine and artesunate, and 11–15 years or 25–34 kg received three tablets each of amodiaquine and artesunate. Each tablet of amodiaquine contains 153 mg base, and each tablet of artesunate contains 50 mg in the copacked unit.
All drugs were given orally, and all patients waited for at least 3 hours to ensure that the drugs were not vomited. If they were, the patient was excluded from the study. If necessary, patients were provided with antipyretics (paracetamol tablets, 10–15 mg/kg every 8 hours for 24 hours). The randomization was computer generated, and treatment codes were sealed in individual envelopes. Patient evaluation at enrolment and follow-up after drug administration was performed by another physician blinded to the drug treatment. The study nurse obtained thick and thin blood films from each child as soon as they came to the clinic. The slides were carefully labeled with the patients' codes and were air-dried before staining.
Follow-up with clinical and parasitological evaluation was carried out at 0, 1, 2, 4, 8, 16, and 24 hours after treatment daily on days 2–7, 14, 21, 28, 35, and 42. These consisted of enquiry about the patient's well-being, presence or absence of initial presenting symptoms, and presence of additional symptoms, measurement of body temperature, heart rate, and respiratory rate, and taking a blood smear for the quantification of parasitemia.
Side effects were defined as symptoms and signs that first occurred or became worse after treatment was started. Any new events occurring during treatment were also considered as side effects.
Thick and thin blood films prepared from a finger prick were stained with Geimsa and examined by light microscopy under an oil-immersion objective at 1,000× magnification by two independent assessors who did not know the drug treatment of the patient. A senior member of the study team reviewed the slides if there was any disagreement between the microscopists. In addition, the slides of every third child enrolled in the study were reviewed by this senior member. Parasitemia (asexual or sexual) in thick films was estimated by counting asexual or sexual parasites relative to 1,000 leukocytes or 500 asexual or sexual forms, whichever occurred first. From this figure, the parasite density was calculated assuming a leukocyte count of 6,000/1 μL blood.
Capillary blood collected before and during follow-up was used to measure packed cell volume (PCV) or hematocrit. Hematocrits were measured using a microhematocrit tube and microcentrifuge (Hawksley, Lancing, UK). Routine hematocrit was done on days 0–7, 14, 21, 28, 35, and 42. Drug-attributable fall in hematocrit (DAFH) during treatment was defined as the difference between the patient's hematocrit on day 0 and day 3 after starting treatment.14 Anemia resolution time (AnRT) was defined as the time for hematocrit value to return to 30% in those that were anemic at presentation. Area under the curve of deficit in hematocrit (AUCdef) was calculated as previously described.15 Briefly, in all anemic patients (PCV < 30%), hematocrit values below 30% (the lower threshold of normal) at enrollment and follow-up were subtracted from 30% at each time of measurement until hematocrit rose to 30%, and the resulting values were plotted against time. The deficit in hematocrit when anemia resolved was, therefore, zero in all patients. The AUCs of deficit in hematocrit (from 30%) versus time were obtained by the trapezoidal rule using the computer program Turbo Ken (designed by Clinical Pharmacology Group, University of Southampton, Southampton, UK). If there was no resolution of anemia during follow-up, AUC was calculated until 1,008 hours (day 42). AUC was also obtained manually by calculating the average hematocrit values between two consecutive time measurements, multiplying this number by the time interval between the measurements, and summing up all the values in a manner similar to that for the numeric estimation of area under a drug concentration–time curve.16 Both measurements by digital computer and manual methods gave the same values. The unit of quantification would be %h if hematocrit values were used or g.h/dL if hemoglobin values were used. Hematocrit values may be converted to hemoglobin values by dividing by three.
Blood was spotted on filter papers on days 0, 3, 7, 14, 21, 28, 35, and 42 and at the time of treatment failure for parasite genotyping. Paired primary and post-treatment parasites were analyzed using parasite loci that exhibit repeated numbers of polymorphisms to distinguish true treatment failures from new infections. Briefly, block 2 of merozoite surface protein-1 (MSP-1), block 3 of MSP-2, and region II of glutamine-rich protein (GLURP) were amplified by two rounds of polymerase chain reaction (PCR) using primers and amplification conditions described previously.10 Ten microliters of nested PCR products were resolved by electrophoresis on a 2% agarose gel and sized against a 100-bp molecular weight marker (New England Biolabs, Beverly, MA). The banding pattern of the post-treatment parasites was compared with matched primary parasites in each of the patients who had parasitemia after treatment with either AL or AA. Post-treatment and primary infection parasites showing identical bands were considered true treatment failure, whereas non-identity in banding patterns was considered newly acquired infection.
Response to drug treatment was assessed using World Health Organization (WHO) 1973 criteria17 as follows: S = sensitive, clearance of parasitemia without recurrence; RI (mild resistance) = parasitemia disappears but reappears within 7–14 days; RII (moderate resistance) = decrease of parasitemia but no complete clearance from peripheral blood; RIII (severe resistance) = no pronounced decrease or increase in parasitemia at 48 hours after treatment. In those with sensitive or mild resistance, parasite clearance time was defined as the time elapsing between drug administration and absence of detectable parasitemia for at least 48 hours. Times taken to clear 50% and 90% parasitemia were calculated from the plot of decline in parasitemia versus time. Fever clearance time was defined as the time from drug administration until the body temperature fell to or below 37.4°C and remained so for 48 hours. Response to drug treatment was also classified according to a modified version of the WHO 14-day in vivo clinical classification system18; because all patients were not febrile at enrolment, a temperature < 37.5°C was not an exclusion criterion for enrolment. The modification also involved a follow-up for 42 days in this area of intense transmission. The clinical classification system consisted of the following categories of response: adequate clinical and parasitological response (ACPR), late parasitological failure (LPF), late clinical failure (LCF), and early treatment failure (ETF).
Cure rates were defined as the percentages of patients whose asexual parasitemia cleared from peripheral blood and who were free of patent asexual parasitemia on days 14, 21, 28, 35, and 42 of follow-up. The cure rates on days 21–42 were adjusted on the basis of the PCR genotyping results of paired samples for patients with recurrent parasitemia after day 14 of commencing treatment.
Retreatment of drug treatment failures.
All patients failing treatment (within 35 days) with AL, AAcf, or AAcp were retreated with these drug combinations and were followed for 42 days. Patients were retreated whenever they became symptomatic (usually between 23 and 42 days after initial enrolment). Patients with profound clinical (hyperpyrexia or oral fluid intolerance) and parasitological deterioration (> 20% increase in baseline parasitemia) during follow-up were treated with parenteral quinine and were regarded as treatment failures.
Data analysis.
Sample size was calculated so that the study would be able to detect a 14% absolute difference in parasitologic failure rate between AL on one hand and AAcf and AAcp groups on the other hand with 80% power and a 5% significance level. The expected treatment success rates were 99% for AL and 85% for AAcf and AAcp on days 28–42. At a randomization ratio of 1:1.5:1.5 for AL, AAcf, and AAcp, respectively, the minimum sample size in each treatment arm was 63, 95, and 95, respectively, for AL, AAcf, and AAcp. Data were analyzed using version 6 of the Epi-Info software19 and the statistical program SPSS for Windows version 10.01.20 Variables considered in the analysis were related to the densities of P. falciparum gametocytes and trophozoites. Proportions were compared by calculating χ2 with Yates' correction or using Fisher exact tests. Normally distributed, continuous data were compared by Student t test and analysis of variance (ANOVA). Data not conforming to a normal distribution were compared by the Mann–Whitney U tests, the Kruskal–Wallis tests, or the Wilcoxon ranked sum tests. All tests of significance were two-tailed. P values ≤ 0.05 were taken to indicate significant differences. Data were double-entered serially using the patients' codes and were only analyzed at the end of the study.
Results
Patient characteristics.
Between July 2009 and May 2010, 285 children were recruited: 81 in the AL group, 100 in the AAcf group, and 104 in the AAcp group. There were 67 children less than 5 years old: 20 (24.6%) in the AL group, 20 (20%) in the AAcf group, and 27 (26%) in the AAcp group. Table 1 shows the characteristics of children enrolled in the study. The characteristics of the children in the three groups were similar at enrolment. Overall, 267 children completed at least 28 days of follow-up (Figure 1). Of the remaining 18 children, 12 children completed 21 days, 5 children completed 14 days, and 1 child completed 7 days of follow-up; 265 children completed 42 days of follow-up. Virtually all children who did not complete 42 days of follow-up relocated from the area of study or had recurrence of parasitemia on or before day 42.
Table 1.
Demographic and clinical characteristics of patients and immediate therapeutic responses
| AL | AAcf | AAcp | |
|---|---|---|---|
| No. of patients | 81 | 100 | 104 |
| Male/female | 43/37 | 55/45 | 57/45 |
| Age (years) | |||
| Mean (SD) | 7.2 (3.0) | 7.4 (2.8) | 7.2 (3.4) |
| Range | 1.17–12 | 0.67–12 | 1.5–12 |
| No. < 5 years | 20 | 20 | 27 |
| Weight (kg) | |||
| Mean (SD) | 19.9 (7.4) | 19.6 (6.3) | 19.5 (7.1) |
| Range | 7–59 | 6–39 | 7–42 |
| Height (cm) | |||
| Mean (SD) | 115.2 (17.3) | 116.7 (17.9) | 115.3 (19.8) |
| Range | 70–150 | 65–157 | 62–151 |
| Hematocrit (%) | |||
| Mean (SD) | 32.6 (4.4) | 31.7 (4.4) | 32.3(4.5) |
| Range | 22–40 | 17–40 | 20–44 |
| Temperature (°C) | |||
| Mean (SD) | 38.4 (1.2) | 38.4 (1.1) | 38.4 (1.2) |
| Range | 36–40.6 | 36–41.1 | 35.9–40.5 |
| Duration of illness (days) | |||
| Mean (SD) | 2.8 (1.2) | 2.7 (1.4) | 2.7 (1.3) |
| Range | 1–7 | 1–7 | 1–7 |
| Pulse rate per minute | |||
| Mean (SD) | 123.2 (20.7) | 119.3 (20.1) | 122 (22.9) |
| Range | 62–180 | 72–184 | 68–192 |
| Respiration rate per minute | |||
| Mean (SD) | 30.4 (6.8) | 30.6 (7.3) | 30.6 (8.0) |
| Range | 20–60 | 20–56 | 22–80 |
| GMPD per microliter blood | 71,794 | 56,102 | 64,145 |
| Range | 2,024–459,000 | 2,504–346,153 | 3,749–1,096,636 |
| > 250,000 | 5 | 4 | 4 |
| GMGD per microliter blood | 88 | 18 | 127 |
| Range | 72–108 | 6–54 | 36–450 |
| N | 2 | 4 | 2 |
| Hepatomegaly | 25 | 21 | 24 |
| Splenomegaly | 29 | 23 | 25 |
| Hepatosplenomegaly | 6 | 9 | 7 |
AL = artemether-lumefantrine; AAcf = artesunate-amodiaaquine coformulated; AAcp = artesunate-amodiaquine copackaged; SD = standard deviation; GMGD = geometric mean gametocyte density; GMPD = geometric mean parasite density.
Figure 1.
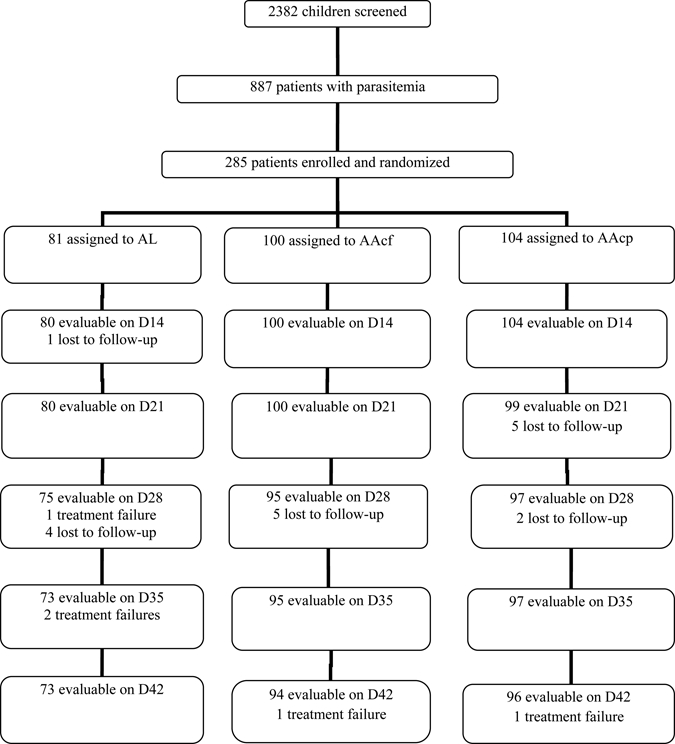
Trial profile for the study.
Fever and parasite clearance.
Two hundred thirty-two children were febrile at enrolment: 61 in the AL group, 82 in the AAcf group, and 89 in the AAcp group. By day 1, fever cleared in 213 (92%) of the children: 53, 77, and 83 children in AL, AAcf, and AAcp groups, respectively. These proportions were similar in the three groups (χ2 = 2.69, degree of freedom [df] = 2, P = 0.26). By day 2, fever had cleared in all children. Overall, fever clearance time was similar in the three groups (Table 2). Similarly, parasitemia cleared in 262 (92%) of the children by day 1: 73, 92, and 97 children in AL, AAcf, and AAcp groups, respectively. Overall parasite clearance times in the three treatment arms were similar: 21.4 ± 9.1, 20.9 ± 9.3, and 19.1 ± 8.3 hours in AL, AAcf, and AAcp groups, respectively (P = 0.17) (Table 2). Times to clear 50% and 90% parasitemia were also similar. Response to the three treatment regimens was not related to age. Fever clearance was similar in children < 5 and ≥ 5 years (1.1 ± 0.3 versus 1.1 ± 0.3 days, respectively; P = 0.73). Similarly, parasite clearance was similar in children < 5 and ≥ 5 years (18.1 ± 6.6 versus 20.3 ± 9.0 hours, respectively; P = 0.28). Overall, 1 of 67 and 4 of 196 children < 5 and ≥ 5 years old, respectively, had reappearance of parasitemia by day 42 (χ2 = 0.055, P = 0.82). All 284 children treated with AL, AAcf, and AAcp had ACP up to day 14.
Table 2.
Therapeutic responses to artemether-lumefantrine (AL), artesunate-amodiaquine coformulated (AAcf), or artesunate-amodiaquine copackaged (AAcp)
| AL | AAcf | AAcp | P value | |
|---|---|---|---|---|
| No. of patients | 81 | 100 | 104 | – |
| Fever clearance time (days) | N = 61 | N = 82 | N = 89 | |
| Mean ± SD | 1.1 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.3 | 0.26 |
| Range | 1–2 | 1–2 | 1–2 | |
| 95% CI | 1.04–1.2 | 1.01–1.1 | 1.01–1.12 | |
| No. of patients with parasitemia on day 1 | 4 | 6 | 6 | 0.35 |
| Time to clear 50% parasitemia (hours) | ||||
| Mean ± SD | 10.7 ± 4.6 | 10.4 ± 4.6 | 9.6 ± 4.2 | 0.18 |
| Range | 2–24 | 2–24 | 2–24 | |
| 95% CI | 9.7–11.7 | 9.5–11.4 | 8.8–10.4 | |
| Time to clear 90% parasitemia (hours) | ||||
| Mean ± SD | 19.3 ± 8.2 | 18.8 ± 8.3 | 17.2 ± 7.5 | 0.18 |
| Range | 3.6–43.2 | 3.6–43.2 | 3.6–43.2 | |
| 95% CI | 17.5–21.1 | 17.1–20.5 | 15.6–18.7 | |
| Parasite clearance time (hours) | ||||
| Mean ± SD | 21.4 ± 9.1 | 20.9 ± 9.3 | 19.1 ± 8.3 | 0.18 |
| Range | 4–48 | 4–48 | 4–48 | |
| 95% CI | 19.4–23.4 | 19.0–22.7 | 17.5–20.8 | |
| Day and responses (S/RI/RII) | ||||
| 14 | 81/0/0 | 100//0 | 104/0/0 | – |
| 21 | 81/0/ | 100/0/ | 104/0/ | – |
| 28 | 80/1/ | 99/1/ | 104/0/ | 0.55 |
| 35 | 79/2/ | 99/1/ | 103/1/ | 0.63 |
| 42 | 78/3/ | 99/1/ | 103/1/ | 0.29 |
| ACPR (%) | 78 (96.3) | 99 (99) | 103 (99) | 0.29 |
| LPF (%) | 3 (3.7) | 1 (1) | 1 (1) | |
| LCF | 0 | 0 | 0 | |
| ETF | 0 | 0 | 0 | |
| PCR-corrected cure rate (%) | 96.3 | 100 | 99 | 0.09 |
AL = artemether-lumefantrine; AAcf = artesunate-amodiaaquine coformulated; AAcp = artesunate-amodiaquine copackaged; SD = standard deviation.
Gametocyte carriage.
At enrolment, gametocytes were detected in the peripheral blood of eight patients: two in the AL group, four in the AAcf group, and two in the AAcp group. One week after treatment, gametocytes were found in peripheral blood of five children: one in the AL group, two in the AAcf group, and two in the AAcp group. These five patients did not have gametocytes at enrolment.
Rate of reappearance of parasitemia.
Five of two hundred sixty-eight children who completed at least 28 days follow-up had reappearance of parasitemia (three in the AL group, one in the AAcf group, and one in the AAcp group) after initial clearance of parasitemia between days 21 and 42. There was no significant difference in the proportions of patients with reappearance of parasitemia in the three treatment arms (χ2 = 2.5, P = 0.29) (Table 2). Kaplan–Meier survival analysis showed that there was no significant difference in the rate of reappearance of parasitemia during follow-up between the three treatment arms (log rank statistic = −1.83, P = 0.18).
PCR findings.
All five patients who were parasitemic during follow-up had their samples analyzed at enrolment and post-treatment. Parasite genotypes in the pre- and post-treatment samples were non-identical in one patient treated with AAcf and were considered as newly acquired infections. In three patients treated with AL and one patient treated with AAcp, parasite genotypes in the pre- and post-treatment samples were identical and considered as recrudescence.
Adverse events.
Overall, 66 children (19 in the AL group, 24 in the AAcf group, and 23 in the AAcp group) reported at least one adverse event within the first week of starting treatment from a list of solicited adverse events: anorexia, vomiting, headache, cough, abdominal pain, diarrhea, fever, rash, itching, excessive salivation, puffy face, and herpetic eruptions. The most frequent (> 20%) solicited adverse event was cough (Table 3). There was no child reporting more than three adverse events. All adverse events were reversible and did not require hospitalization.
Table 3.
Adverse events reported within the first week of the study
| AL | AAcf | AAcp | P value | |
|---|---|---|---|---|
| No. of children | 81 | 100 | 104 | – |
| Total reporting adverse event | 19 | 24 | 23 | 0.95 |
| Cough | 9 | 7 | 7 | 0.40 |
| Pruritus | 0 | 3 | 3 | 0.26 |
| Vomiting | 1 | 2 | 2 | 0.90 |
| Abdominal pain | 0 | 3 | 7 | 0.02 |
| Weakness | 2 | 5 | 4 | 0.66 |
| Insomnia | 0 | 0 | 3 | 0.053 |
| Diarrhea | 3 | 3 | 2 | 0.78 |
| Herpes labialis | 0 | 2 | 1 | 0.43 |
| Excessive salivation | 1 | 4 | 1 | 0.27 |
| Rashes | 2 | 1 | 2 | 0.71 |
| Puffy face | 1 | 0 | 1 | 0.55 |
| Dizziness | 0 | 1 | 1 | 0.66 |
| Headache | 0 | 1 | 0 | 0.41 |
AL = artemether-lumefantrine; AAcf = artesunate-amodiaaquine coformulated; AAcp = artesunate-amodiaquine copackaged.
DAFH and resolution of anemia after treatment.
Hematocrit data were available in 270 children: 77, 96, and 97 in AL, AAcf, and AAcp groups, respectively. Hematocrit values were available in 260 patients both on day 0 and 3; these were used for the assessment of DAFH. Of these children, 45, 56, and 57 had a fall in hematocrit on day 3 in AL, AAcf, and AAcp groups, respectively. The proportions of children with a fall in hematocrit on day 3 were similar in all treatment groups (χ2 = 0.04, P = 0.98). DAFH values were also similar in the three treatment groups (4.4 ± 3.3%, 95% confidence interval [CI] = 2–4.4%; 4.0 ± 2.4%, 95% CI = 3.0–4.9%; 3.4 ± 2.0%, 95% CI = 2.7–4.9%, respectively; P = 0.17) (Figure 2). In general, the rates of rise in hematocrit in the three treatment groups were also similar (Figure 2) such that, by day 28, there were no more increases in hematocrit values in all treatment groups. Overall, 66 children were considered anemic (PCV < 30%) at enrolment: 17 in the AL group, 28 in the AAcf group, and 21 in the AAcp group. Table 4 shows the rates of resolution of anemia after treatment in the 66 children in whom complete data were available from days 0 to 42. These rates were similar. Anemia resolution times were also similar: 11.4 ± 6.1, 11.0 ± 4.5, and 13.0 ± 4.1 days in the AL, AAcf, and AAcp groups, respectively (P = 0.42) (Table 4).
Figure 2.
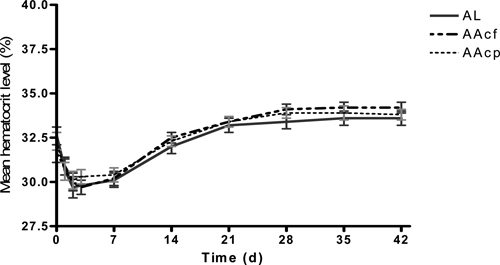
Changes in hematocrit before, during, and after treatment with AL (solid line), AAcf (broken line), or AAcp (dotted line) in malarious children.
Table 4.
Resolution of malaria-associated anemia after treatment
| AL | AAcf | AAcp | P | |
|---|---|---|---|---|
| No. with PCV < 30% | 17 (N = 81) | 28 (N = 100) | 21 (N = 104) | 0.36 |
| Mean PCV (%) and range | 23.8% (22–29) | 24% (17–29) | 22.4% (20–29) | 0.35 |
| No. of males (%) | 8 (47.5) | 18 (64.3) | 14 (66.7) | 0.41 |
| Age (years) | ||||
| Mean ± SD | 4.8 ± 2.5 | 6.6 ± 2.9 | 5.4 ± 3.8 | 0.49 |
| Range | 1.17–12 | 2–10 | 1.83–12 | |
| No. < 5 years | 6 | 7 | 8 | 0.59 |
| Parasite count (per microliter) | ||||
| Geometric mean | 113,716 | 73,221 | 75,458 | 0.30 |
| Range | 2,024–288,461 | 16,393–346,156 | 8,159–1,096,636 | |
| No. with gametocytemia at presentation (%) | 0 | 3 | 0 | 0.08 |
| No. with PCV < 30 (%) on that specific day | ||||
| Day 7 | 10 (N = 14) | 15 (N = 27) | 14 (N = 19) | 0.38 |
| Day 14 | 3 (N = 12) | 2 (N = 20) | 3 (N = 16) | 0.53 |
| Day 21 | 2 (N = 10) | 1 (N = 19) | 2 (N = 17) | 0.48 |
| Day 28 | 2 (N = 9) | 0 | 1 (N = 10) | 0.58 |
| Day 35 | 2 (N = 9) | 0 | 1 (N = 9) | 1.0 |
| Day 42 | 1 (N = 9) | 0 | 0 | – |
| Anemia resolution time (day) | 11.4 ± 6.1 | 11.0 ±4.5 | 13.0 ± 4.1 | 0.42 |
| DAFH (%) range | 4.4 ± 3.3 | 4.0 ± 2.4 | 3.4 ± 2.0 | 0.17 |
| 1–10 | 1–10 | 1–9 | ||
| N | 44 | 56 | 57 | |
AL = artemether-lumefantrine; AAcf = artesunate-amodiaaquine coformulated; AAcp = artesunate-amodiaquine copackaged; SD = standard deviation; DAFH = drug-attributable fall in hematocrit.
Comparison of AUC of deficit in hematocrit.
Figure 3A shows the distribution of individual AUCdef in the three treatment groups. Mean AUCdef was similar in the three groups (35.7%.d in the AL group, 24.8%.d in the AAcf group, and 40%.d in the AAcp group; P = 0.27) (Figure 3B). The proportion of children with AUCdef > 30%.d was also similar (6 of 16, 10 of 28, and 10 of 19 in the AL, AAcf, and AAcp groups, respectively; P = 0.48).
Figure 3.
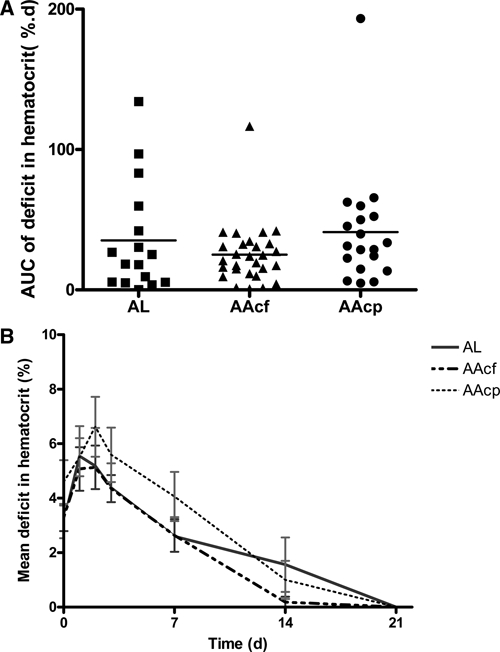
Individual (A) and group mean (B) AUCs of deficit in hematocrit in patients treated with AL (solid line), AAcf (broken line), and AAcp (dotted line). In A, horizontal bars indicate group mean values.
Discussion
Of the ACTs, AL and AA are the most frequently used in Africa.3–7,14 However, very few studies have evaluated the safety, tolerability, effects on gametocyte carriage, and recovery from malaria-associated anemia of both fixed-dose and copackaged combination of AA and compared these with AL in malarious children.14 We documented the tolerability, safety, and efficacy of AL, AAcf, and AAcp in the treatment of uncomplicated P. falciparum malaria, and the study was based on a 42-day follow-up. In the children studied, parasite clearance was similar in all treatment regimens. In addition, clearance rates were similar in younger (< 5 years) and older children, suggesting that clearance of parasites in these children is independent of immunity.21 It is particularly noteworthy that clearance was similar whether it was copackaged or coformulated; fever clearance was similar in all treatment groups.
Amodiquine has antipyretic propertyies.22 It is, therefore, surprising that, in patients treated with AL where the lumefantrine component has little or no antipyretic property, fever clearance was similar to that of the children treated with AA. This would suggest that, in this cohort of children, fever clearance paralleled parasite clearance. This finding is in contrast to a finding from the same area where the amodiaquine component of amodiaquine sulfalene-pyrimethamine produced a similar fever clearance with AL, despite a slower clearance of parasitemia.12 Prompt fever and parasite clearance times by both AL and the formulations of AA are in agreement with findings from Africa and elsewhere.5,6,8,12,23
Gametocyte carriage at enrolment in the cohort of children evaluated (3%) was lower than in previous studies from the same area during the use of monotherapies (10–12%).24 ACTs have been used consistently in children from the study community in the last 5 years and may have been responsible for the relatively low gametocyte carriage compared with the pre-ACT use period. The low gametocyte carriage rate made it impossible to accurately assess the effects of ACTs on gametocyte carriage, emergence, and clearance as was previously reported in a recent study from this endemic endemic.25 However, it is possible that gametocyte carriage rates are underestimates, because submicroscopic gametocytemia is common in this and other endemic areas.9,26,27 This would explain the non-reduction in transmission intensity, which was evidenced by the high parasite rate of 43% in the present study (Figure 1) despite the low gametocyte carriage after consistent ACT. However, in the few patients that carried gametocytes at enrolment, gametocytemia cleared within 3 days.
Recovery from malaria-associated anemia was similar in the three treatment groups and took approximately 1.5–2 weeks in patients who were anemic on presentation. In addition, recovery from the total malaria-attributable fall in hematocrit28,29 (defined as the time interval between hematocrit levels on days 3 and 28 in this study, a time when no further increases in hematocrit levels were seen in the absence of malaria infection) seems to be shorter than that reported in the same area when monotherapies were used (approximately 5 weeks).29 This would suggest that, compared with non-ACTs, ACTs may hasten recovery in malaria-associated anemic children from this endemic area.
Further evaluation of the effects of the three treatments on recovery from malaria-associated anemia was also done using the area inscribed by plotting the deficit in hematocrit versus time. This method seems to be more sensitive than the conventional estimation of anemia recovery time.15 Using this method, we showed that the areas under curve of the deficit in hematocrit versus time were similar, suggesting that, in this cohort of children, the use of AL or AAcf has no advantage over AAcp. This indicates that the artemisinin components of these ACTs are the major contributors to the observed responses. This should predicted, because artemisinin drugs are known to cause lesser degrees of fall in hematocrit, particularly in patients with heavy parasitemia.30,31
All three treatments were well-tolerated; adverse drugs reactions reported during the study were indistinguishable from those of malaria. No patient treated with AL had pruritus. Because lumefantrine is closely related to halofantrine, which has been reported to induce chloroquine-like pruritus,32 it is likely that AL-induced pruritus will be encountered as use of AL increases in the future. In conclusion, AL, AAcf, and AAcp administered over 3 days produced similar clinical and parasitological efficacy and similar effects on the recovery from the malaria-associated anemia. Use of AL or AAcf has no advantage in terms of efficacy or recovery from P. falciparum-associated malaria over AAcp. These antimalarial combinations offer effective treatment of uncomplicated falciparum malaria in children.
Footnotes
Authors' addresses: Grace O. Gbotosho, Akintunde Sowunmi, Titilope M. Okuboyejo, Christian T. Happi, Onikepe A. Folarin, and Obaro S. Michael, Department of Pharmacology and Therapeutics and Institute for Medical Research and Training, University of Ibadan, Ibadan, Nigeria, E-mails: solagbotosho@yahoo.co.uk, akinsowunmi@hotmail.com, attitte@yahoo.com, christianhappi@hotmail.com, onikepefolarin@yahoo.com, and micobaro@yahoo.com. Elsie O. Adewoye, Department of Physiology, University of Ibadan, Ibadan, Nigeria, E-mail: elolade@yahoo.com.
References
- 1.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Report of a WHO Technical Consultation. Geneva, Switzerland: World Health Organization; 2001. (Antimalarial drug combination therapy). [Google Scholar]
- 3.Omari AA, Gamble C, Garner P. Artemether-lumefantrine for uncomplicated malaria: a systematic review. Trop Med Int Health. 2004;9:192–199. doi: 10.1046/j.1365-3156.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- 4.Kamya MR, Yeka A, Burkiwa H, Lugemwa M, Rwankimari JB, Staedke SG, Talisuna AO, Greenhouse B, Nosten F, Rosenthal PJ, Wabwire-Mangen F, Dorsey G. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trial. PLoS Clin Trials. 2007;2:e20. doi: 10.1371/journal.pctr.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falade C, Makanga M, Premji Z, Ortmann CE, Stockmeyer M, de Palcios PI. Efficacy and safety of artemether-lumefantrine (Coartem) tablets (six-dose regimen) in African infants and children with acute, uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 2005;99:459–467. doi: 10.1016/j.trstmh.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Adjuik M, Agnamey P, Babiker A, Borrmann S, Brasseur P, Cisse M, Cobelens F, Diallo S, Faucher JF, Garner P, Gikunda S, Kremsner PG, Krishna S, Lell B, Loolpapit M, Matsiegui PB, Missinou MA, Mwanza J, Ntoumi F, Olliaro P, Osimbo P, Rezbach P, Some E, Taylor WRJ. Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomized, multicentre trial. Lancet. 2002;359:1365–1372. doi: 10.1016/s0140-6736(02)08348-4. [DOI] [PubMed] [Google Scholar]
- 7.Sowunmi A, Balogun T, Gbotosho GO, Happi CT, Adedeji AA, Fehintola FA. Activities of amodiaquine, artesunate, and artesunate-amodiaquine against asexual- and sexual-stage parasites in falciparum malaria in children. Antimicrob Agents Chemother. 2007;51:1694–1699. doi: 10.1128/AAC.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ndiaye JL, Milijaona R, Sagara I, Brasseur P, Ndiaye I, Faye B, Randrianasolo L, Ratsimbasoa A, Forlemu D, Moor VA, Traore A, Dicko Y, Dara N, Lameyre V, Diallo M, Djimde A, Same-Ekobo A, Gaye O. Randomized multicentre assessment of the efficacy and safety of artesunate-amodiaquine-a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria. Malar J. 2008;8:125. doi: 10.1186/1475-2875-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Happi CT, Gbotosho GO, Folarin OA, Sowunmi A, Hudson T, O' Neil M, Milhous W, Wirth DF, Oduola AMJ. Selection of Plasmodium falciparum multi-drug resistance gene 1 alleles in asexual stages and gametocytes by artemether-lumefantrine in Nigerian children with uncomplicated falciparum malaria. Antimicrob Agents Chemother. 2009;53:888–895. doi: 10.1128/AAC.00968-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Happi CT, Gbotosho GO, Folarin OA, Bolaji OM, Sowunmi A, Kyle DE, Milhous W, Wirth DF, Oduola AMJ. Association between mutations in Plasmodium falciparum chloroquine resistance transporter and P. falciparum multidrug resistance 1 genes and in vivo amodiaquine resistance in P. falciparum malaria-infected children in Nigeria. Am J Trop Med Hyg. 2006;75:155–161. [PubMed] [Google Scholar]
- 11.Salako LA, Ajayi FO, Sowunmi A, Walker O. Malaria in Nigeria: a revisit. Ann Trop Med Parasitol. 1990;84:435–445. doi: 10.1080/00034983.1990.11812493. [DOI] [PubMed] [Google Scholar]
- 12.Sowunmi A, Gbotosho GO, Happi CT, Adedeji AA, Fehintola FA, Folarin OA, Tambo E, Fateye BA. Therapeutic efficacy and effects of artemether-lumefantrine and amodiaquine-sulfalene-pyrimethamine on gametocyte carriage in children with uncomplicated Plasmodium falciparum malaria in southwestern Nigeria. Am J Trop Med Hyg. 2007;77:235–241. [PubMed] [Google Scholar]
- 13.World Health Organization Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94((Suppl 1)):1–90. [PubMed] [Google Scholar]
- 14.Sowunmi A, Balogun ST, Gbotosho GO, Happi CT. Effects of amoqiauine, artesunate, and artesunate-amodiaquine on Plasmodium falciparum malaria-associated anaemia in children. Acta Trop. 2009;109:55–60. doi: 10.1016/j.actatropica.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Sowunmi A, Gbotosho GO, Happi CT, Folarin O, Okuboyejo T, Micheal O, Fatunmbi B. Use of area under the curve to evaluate the effects of antimalarial drugs on malaria-associated anemia after treatment. Am J Ther. 2010 doi: 10.1097/MJT.0b013e3181d169c9. July 30 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowland M, Towzer TN. Clinical Pharmacokinetics: Concepts and Applications. Philadelphia, PA: Lea & Febiger; 1980. [Google Scholar]
- 17.World Health Organization . Chemotherapy of Malaria and Resistance to Antimalarials. Geneva: World Health Organization; 1973. [PubMed] [Google Scholar]
- 18.World Health Organization . Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated Falciparum Malaria. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 19.Anonymous . Epi Info Version 6. A Word Processing Data Base and Statistics Program for Public Health on IBM-Compatible Microcomputers. Atlanta, GA: Centers for Disease Control and Prevention; 1994. [Google Scholar]
- 20.Anonymous . SPSS for Windows Release 10.01 (Standard Version) Chicago, IL: SPSS Inc; 1999. [Google Scholar]
- 21.Djimde AA, Doumbo OK, Traore O, Guindo AB, Kayentao K, Diourte Y, Niare-Dumbo S, Coulibaly D, Kone AK, Cissoko Y, Tekete M, Diallo DA, Wellems TE, Kwiatowski D, Plowe CV. Clearance of drug-resistant parasites as a model for protective immunity in Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:558–563. [PubMed] [Google Scholar]
- 22.Olliaro P, Mussano P. Amodiaquine for treating malaria. Cochrane Database Syst Rev. 2003;2:CD000016. doi: 10.1002/14651858.CD000016. [DOI] [PubMed] [Google Scholar]
- 23.Price R, van Vugt M, Nosten F, Luxemburger C, Brockman A, Phaipun L, Chongsuphajaisiddhi T, White NJ. Artesunate versus artemether for the treatment of recrudescent multi-drug resistant falciparum malaria. Am J Trop Med Hyg. 1998;59:883–888. doi: 10.4269/ajtmh.1998.59.883. [DOI] [PubMed] [Google Scholar]
- 24.Sowunmi A, Balogun ST, Gbotosho GO, Happi CT. Plasmodium falciparum gametocyte sex ratios in symptomatic children treated with antimalarial drugs. Acta Trop. 2009;109:108–117. doi: 10.1016/j.actatropica.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Sowunmi A, Nkogho OO, Okuboyejo TM, Gbotosho GO, Happi CT, Adewoye EO. Effects of mefloquine and artesunate mefloquine on the emergence, clearance and sex ratio of Plasmodium falciparum gametocytes in malarious children. Malar J. 2009;8:297. doi: 10.1186/1475-2875-8-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouédraogo AL, Bousema T, Schneider P, de Vlas SJ, Iboudo-Sanogo E, Cuzin-Ouattara N, Néblé I, Roeffen W, Verhave JP, Luty AJ, Sauerwein R. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS One. 2009;4:e8140. doi: 10.1371/journal.pone.0008410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bousema JT, Schneider P, Gougna LC, Drakeley CJ, Tostmann A, Houben R, Githure JI, Ord R, Sutherland CJ, Omar SA, Sauerwein RW. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis. 2006;193:1151–1159. doi: 10.1086/503051. [DOI] [PubMed] [Google Scholar]
- 28.Price R, Simpson JA, Nosten F, Luxemburger C, Hkirjaroen I, ter Kuile F, Chongsuphajaisiddhi T, White NJ. Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg. 2001;65:614–622. doi: 10.4269/ajtmh.2001.65.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sowunmi A, Gbotosho GO, Happi CT, Fateye BA. Factors contributing to anaemia after uncomplicated falciparum malaria in children. Acta Trop. 2010;113:155–161. doi: 10.1016/j.actatropica.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Chotivanich K, Udomsangpetch R, Dondorp A, Williams T, Angus B, Simpson JA, Pukrittayakamee S, Looaresuwan S, Newbold CI, White NJ. The mechanism of parasite clearance after antimalarial treatment of Plasmodium falciparum malaria. J Infect Dis. 2000;182:629–633. doi: 10.1086/315718. [DOI] [PubMed] [Google Scholar]
- 31.Newton PN, Chotivanich K, Cheirakul W, Ruangveerayuth R, Teerapong P, Silamut K, Looareesuwan S, White NJ. A comparison of the in vivo kinetics of Plasmodium falciparum ring-infected erythrocyte surface antigen-positive and-negative erythrocytes. Blood. 2001;15:450–457. doi: 10.1182/blood.v98.2.450. [DOI] [PubMed] [Google Scholar]
- 32.Sowunmi A, Walker O, Salako LA. Pruritus and antimalarial drugs in Africans. Lancet. 1989;2:213. doi: 10.1016/s0140-6736(89)90391-7. [DOI] [PubMed] [Google Scholar]


