Abstract
A sensitive biomarker of malaria infection would obviate the need for placebo control arms in clinical trials of malaria prophylactic drugs. Antibodies to the 42-kDa fragment of merozoite surface protein-1 (MSP142) have been identified as a potential marker of malaria exposure in individuals receiving prophylaxis with mefloquine. We conducted an open-label trial to determine the sensitivity of seroconversion to MSP142, defined as a fourfold rise in enzyme-linked immunosorbant assay (ELISA) titer, among 23 malaria naïve volunteers receiving mefloquine prophylaxis and 6 controls after Plasmodium falciparum sporozoite challenge. All members of the control cohort but none of the mefloquine cohort developed patent parasitemia. Four of six controls but zero of the mefloquine cohort seroconverted to MSP142. We conclude that malaria infection during suppressive prophylaxis does not induce antibody response to the blood-stage antigen MSP142 in a malaria-naïve study population.
Introduction
Despite the need for new antimalarials for chemoprophylaxis, there have been no new drugs approved for this indication by the US Food and Drug Administration (FDA) since 2000, and there has been little interest in the development of new agents by large pharmaceutical companies. Although there are multiple reasons for this, a contributing factor is that the traditional approach to showing prophylactic efficacy in clinical trials involves placebo-controlled studies conducted in malaria-endemic countries in semiimmune individuals. This approach has become problematic because of ethical considerations and the possibility that prophylactic efficacy might be overestimated in populations with background immunity.1 The ability to conduct efficacy studies using an active comparator drug would greatly facilitate the drug development process.
Conducting studies using an active comparator in place of a placebo arm requires a biomarker of infection to identify and confirm exposure; without this biomarker, a calculation of protective efficacy is impossible. Antibodies to the 42-kDa fragment of the Plasmodium falciparum blood-stage antigen merozoite surface protein-1 (MSP142) were selected for qualification as a biomarker after retrospective analysis of serum from individuals taking mefloquine prophylaxis as part of a field study showed adequate rates of seroconversion in the absence of detectable parasitemia (Ohrt C and others, unpublished data).
MSP142 is the major protein expressed on the surface of blood-stage Plasmodium parasites, and it is composed of four subunits. Both the 42-kDa fragment (MSP142) and its 19-kDa subfragment (MSP119) have been shown to elicit immune responses.2,3 Antibodies directed at MSP119 have been shown to correlate with malaria transmission intensity in endemic areas.4
Mefloquine is an FDA approved drug for the prevention and treatment of P. falciparum malaria.5 Mefloquine has no effect on the developing malaria parasite in the liver but does inhibit replication of blood-stage parasites.6,7 Therefore, patients are expected to be exposed to blood-stage antigens, such as MSP142, even during successful prophylaxis.
To qualify this biomarker for use as an endpoint in pivotal efficacy studies of novel prophylactic drugs, we sought to determine its sensitivity in individuals exposed to P. falciparum malaria while taking suppressive doses of mefloquine.
Materials and Methods
Ethics.
This study was conducted according to Good Clinical Practices under a protocol reviewed and approved by the Walter Reed Army Institute of Research (WRAIR) Institutional Review Board (IRB) as well as by the US Army Medical Research and Materiel Command Human Subjects Protection Office (USAMRMC-HRPO), and at its inception, it was registered with ClinicalTrials.gov (NCT00761020). Written informed consent was obtained from all potential participants before screening and enrollment.
Study design.
The study was a single-center, open-label, non-randomized challenge study conducted entirely on an outpatient basis. This study was conducted from September 2008 to April 2009 at the WRAIR Clinical Trials Center, Silver Spring, MD. Twenty-nine subjects were recruited and enrolled by volunteer preference into either a mefloquine chemoprophylaxis cohort (N = 23) or an infectivity control cohort (N = 6). Members of the mefloquine cohort received 250 mg of the drug (Lariam; F. Hoffman-La Roche Ltd., Basel, Switzerland) orally daily for 3 days beginning 2 days before malaria challenge and then weekly for 4 weeks post-challenge. All subjects were challenged on the same day (day 0) and thereafter, were followed for a total of 6 months. A flow diagram for study volunteers is provided in Figure 1.
Figure 1.
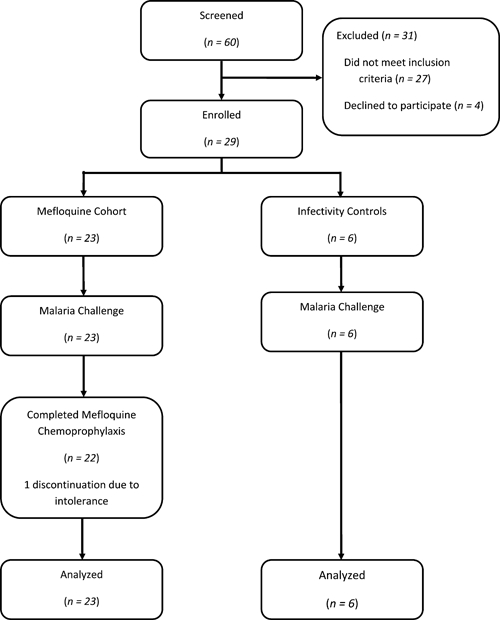
Study flow diagram. The numbers of subjects completing each phase of the study are shown.
Sample size justification.
Mefloquine cohort size was based on the exact test for a single proportion, assuming a true biomarker sensitivity of 60%, a target power of 90% (minimum acceptable power is 80%) to rule out a sensitivity of 30% or less (null hypothesis), and a one-sided type I error of 5%. Under these assumptions, it was calculated that a sample size between 19 and 25 would provide an actual power between 81% and 92% (nQuery Advisor, version 6.0; Statistical Solutions Ltd., Boston, MA).
Study participants.
Potential volunteers were recruited from the Washington DC metropolitan area. Recruitment was through IRB-approved advertisements in multiple media formats as well as by word of mouth. Healthy malaria-naïve males and non-pregnant, non-lactating females were eligible for inclusion if they were 18–55 (inclusive) years of age, available and willing to participate for the planned duration of the study, able to score at least 80% on a written study comprehension test, and able to give written informed consent. In addition, female participants had to either have no childbearing potential (i.e., surgically sterilized or at least 1-year post-menopausal) or be willing to practice abstinence or use adequate contraceptive precautions for the first 4 months after malaria challenge. Exclusion criteria included a history of malaria, receipt of any investigational malaria vaccines or significant malaria exposure, recent or chronic usage of agents with known antiplasmodial or immunomodulating activity, history of autoimmune or immunosuppressive conditions, splenectomy, psoriasis, clinically significant neuropsychiatric or physical abnormality on medical history, physical exam, or laboratory exam, and allergy or intolerance to mefloquine or mosquito bites.
Chemoprophylaxis.
A loading dose of mefloquine was given at 250 mg every day for 3 days beginning 2 days before challenge to reduce the medication burden on volunteers, shorten the study duration, and fully ensure an effective blood level before the emergence of schizonts post-challenge. Although not an FDA-approved regimen, the effectiveness, safety, and tolerability of this regimen has been established by others.8,9 After challenge, the cohort received 250 mg mefloquine weekly for 4 weeks per standard post-exposure prophylaxis recommendations.
Malaria sporozoite challenge.
On day 0 of the study, all participants underwent a malaria sporozoite challenge with P. falciparum parasites (strain NF54/clone 3D7) after a standardized challenge model that has been well-described previously.10 In vitro susceptibility to standard antimalarial drugs was verified for this isolate before challenge. The mean 50% inhibitory concentrations (IC50) for chloroquine, mefloquine, and atovaquone were 10.5 ng/mL (susceptible), 29.7 ng/mL (resistant), and 0.40 ng/mL (susceptible), respectively (WRAIR, unpublished data). Parasites were thawed and expanded from a master seed lot. They were then used to infect laboratory-born and reared Anopheles stephensi mosquitoes in a time frame (approximately 17–19 days) that would allow for an appropriate mean sporozoite density in the salivary glands of each mosquito on the day of challenge. During the actual challenge, groups of five mosquitoes were allowed to feed for 5 minutes on the forearm of each volunteer; then, they were dissected, and their salivary glands were scored to ensure the presence of a sufficient density of sporozoites. If required, additional mosquitoes were allowed to feed until a total of five mosquitoes with an appropriate sporozoite density had fed on a particular volunteer.
Management of Infected Human Volunteers
Infectivity control cohort.
Beginning on the fifth day after challenge, volunteers in the infectivity control cohort were evaluated daily for malaria symptoms, and daily Giemsa-stained thick blood films (hereafter referred to as smears) were obtained and examined for the presence of malaria parasites. At least 200 high-power (oil immersion) fields on each smear were scanned for the presence of malaria parasites in asymptomatic volunteers; at least 1,000 fields in symptomatic volunteers were scanned. Smears were obtained more frequently (up to every 6–8 hours) in symptomatic volunteers. Volunteers who developed patent parasitemia were treated with a standard oral regimen of chloroquine (a total of 1,500 mg chloroquine base [2,500 mg salt] in divided doses: 600 mg [two 500-mg chloroquine salt tablets] initially followed by 300 mg [one 500-mg chloroquine salt tablet] given approximately 6, 24, and 48 hours later) under direct observation. After initiation of treatment, daily blood smears continued to be obtained until three consecutive smears were negative.
Mefloquine cohort.
Members of the mefloquine cohort received chemoprophylaxis with the regimen described above. Mefloquine cohort volunteers were assessed regularly for the presence of malaria symptoms after challenge as well as possible side effects or intolerance to their chemoprophylaxis. Any volunteer showing malaria symptoms was evaluated (to include smear as described above) and treated, if indicated, with a standard oral regimen of atovaquone/proguanil (1,000 mg atovaquone/400 mg proguanil tablets; four tablets a day for 3 consecutive days). Likewise, any volunteer reporting or deemed by the investigators to be experiencing intolerable side effects related to mefloquine use was discontinued from further prophylaxis and presumptively treated for malaria with atovaquone/proguanil. In addition to any symptom-related assessments, each volunteer had four one-time weekly smears collected during the first month post-challenge that were read at the conclusion of that month.
Safety.
Mefloquine cohort volunteers were assessed for adverse events at every scheduled encounter from the time of the administration of the first dose of chemoprophylaxis until 60 days after the final dose. Infectivity cohort volunteers were assessed for adverse events at every scheduled encounter from the time of challenge and for 60 days thereafter. Solicited adverse events included fever (≥ 37.5°C), nausea, vomiting, diarrhea, headache, malaise, myalgia, fatigue, joint pain, flushing, hyper/hypotension, syncope, dizziness, paresthesia, tremor, ataxia, convulsions, seizures, sleep disturbances, anxiety, depression, paranoia, mood changes, confusion, forgetfulness, hallucinations, and suicidal ideation. Fever was graded on an intensity scale related to recorded temperature as follows: 0 (< 37.5°C), 1 (37.5–38.0°C), 2 (> 38–39°C), or 3 (> 39°C). Other symptoms were graded as follows: 0 (normal), 1 (easily tolerated), 2 (interferes with normal activity), or 3 (prevents normal activity). Serious adverse events, as defined by the FDA, were captured throughout the trial.11
Laboratories
Serology.
Anti–MSP-142 antibody titers were measured using a validated enzyme-linked immunosorbant assay (ELISA) using antigen derived from P. falciparum (strain NF54/clone 3D7), with a lower limit of detection of 0.015 µg antigen-specific antibody per mL (House B and others, unpublished data). ELISAs were performed on the day of screening and days −2, 0, 7, 12, 21, 28, 42, 56, 70, 84, 98, 112, and 168 for the mefloquine cohort and on the day of screening and days −2, 0, 7, 14, 21, 28, 35, 42, 56, 70, 84, 98, 112, and 168 for the infectivity control cohort.12 Seroconversion was defined as a fourfold rise in titer.
Mefloquine levels.
Whole-blood concentrations of mefloquine were measured by high-performance liquid chromatography (HPLC) by the Centers for Disease Control and Prevention (CDC; Atlanta, GA) from mefloquine cohort volunteers on days −2, 0, 7, 12, 21, 28, 42, and 56.13
Statistical analysis.
All statistical analysis was performed using Statistical Analysis Software (SAS) version 9.2 (Cary, NC).
Results
Volunteer characteristics.
Twenty-three volunteers were enrolled into the mefloquine cohort, and 6 volunteers were enrolled into the control cohort. The two cohorts were similar with respect to demographic characteristics (Table 1), with approximately equal numbers of males and females enrolled, with the majority of subjects being white and between 20 and 29 years of age.
Table 1.
Demographic and baseline characteristics
| Characteristic | Cohort 1 (mefloquine; N = 23) | Cohort 2 (infectivity control; N = 6) |
|---|---|---|
| Gender | ||
| Male (n) | 11 (47.8%) | 3 (50%) |
| Female (n) | 12 (52.2%) | 3 (50%) |
| Age (years) | ||
| Mean (SD) | 29.7 (6.8) | 34.3 (9.9) |
| Median | 28 | 35 |
| Range | 18–43 | 23–49 |
| Body mass index | ||
| Mean (SD) | 25.1 (5.3) | 28.3 (5.3) |
| Median | 24 | 27 |
| Range | 20–46 | 23–38 |
| Race (n) | ||
| American Indian or Alaskan Native | 1 (4.3%) | 0 (0%) |
| Asian | 1 (4.3%) | 0 (0%) |
| Black or African American | 7 (30.4%) | 2 (33.3%) |
| White/Caucasian | 13 (56.5%) | 4 (66.7%) |
| Other | 1 (4.3%) | 0 (0%) |
| Ethnicity (n) | ||
| Non-Hispanic or Latino | 22 (95.7%) | 5 (83.3%) |
| Hispanic or Latino | 1 (4.3%) | 1 (16.7%) |
SD = standard deviation.
Challenge.
All 29 enrolled volunteers underwent mosquito challenge, which was well-tolerated by all. Patent parasitemia was detected by examination of thick blood films in six of six members of the control cohort. Parasitemia was first detected on the same study day (day 11 after challenge) for all six members of this cohort. Three of six members (50%) of the control cohort showed malaria-related symptoms before detection of parasitemia, and all members of the cohort showed at least one malaria-related symptom during the week after diagnosis. Parasitemia was not detected at any time in any member of the mefloquine cohort.
Safety.
A total of 134 adverse events (AEs) were reported in this study, of which 112 (84%) occurred in the mefloquine cohort. The majority of AEs reported were grade 1 (Table 2). The most commonly reported AEs of all reported grades in the mefloquine cohort were diarrhea (N = 6), nausea (N = 9), vomiting (N = 4), fatigue (N = 5), upper respiratory infection (N = 6), gastroenteritis (N = 5), abnormal dreams (N = 7), headache (N = 9), insomnia (N = 7), and nasal congestion (N = 4). The most commonly reported AEs of all reported grades in the control cohort were upper respiratory infection (N = 2), musculoskeletal pain (N = 2), and headache (N = 2). Only one unexpected adverse event was reported during the study. A volunteer within the control cohort was evaluated and diagnosed with hyperthyroidism (Grave's disease) after reporting new onset agitation and tremors of the hands in the period after confirmation of infection and initiation of standard chloroquine treatment. This event was determined to be unrelated to study participation after testing of the subject's pre-challenge blood samples revealed that this condition was present at the time of the subject's entry into the study.
Table 2.
Overall number of adverse events
| Population | Cohort 1 (mefloquine; N = 23) | Cohort 2 (infectivity control; N = 6) |
|---|---|---|
| Total number of AEs | 112 | 22 |
| Total number of subjects with any AE | 20 (87.0%) | 6 (100.0%) |
| Total number of subjects with grade 1 AE | 19 (82.6%) | 6 (100.0%) |
| Total number of subjects with grade 2 AE | 13 (56.5%) | 4 (66.7%) |
| Total number of subjects with grade 3 AE | 2 (8.7%) | 0 (0.0%) |
Mefloquine.
Despite the open-label nature of this study and considerable volunteer education about potential side effects of mefloquine, the drug was generally well-tolerated in this study; 22 of 23 (96%) mefloquine cohort subjects completed all seven planned doses of medication. A single individual discontinued mefloquine after the fourth dose because of intolerance (insomnia) and was treated per protocol with a standard atovaquone/proguanil (Malarone; GlaxoSmithKline, Research Triangle Park, NC) treatment regimen. One volunteer vomited after receipt of the first loading dose and required redosing, and 13 of 23 (57%) volunteers experienced a mefloquine-related neuropsychiatric AE, predominantly some form of sleep disturbance and/or vivid dreams. Five of these AEs were grade 1, whereas eight were grade 2.
Pharmacokinetics.
Mefloquine trough levels were highly variable among individual subjects receiving chemoprophylaxis. As expected, no subject had a detectable mefloquine level before the administration of the first dose; 17 of 23 mefloquine cohort subjects (74%) showed drug levels greater than 620 ng/mL by day 12 of the study, and all cohort subjects exceeded this threshold at least one time by day 28 of the study. The mean whole-blood levels for mefloquine are shown in Figure 2.
Figure 2.
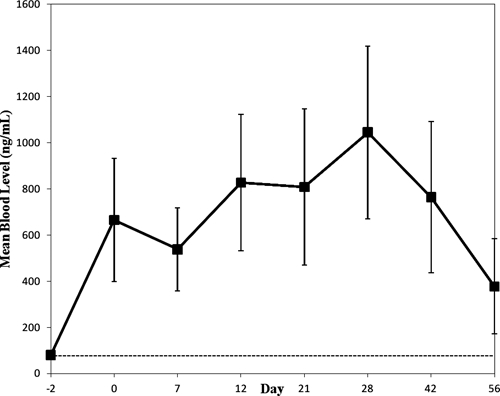
The mean whole-blood concentration of mefloquine is presented ±1 standard deviation (SD) in ng/mL over time for the mefloquine cohort. All samples on study day −2 were below the limit of detection (dashed line) for the assay used (80 ng/mL).
Serology.
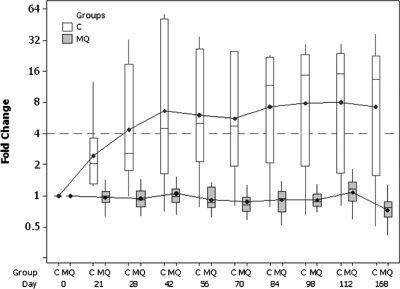
No subjects in the mefloquine cohort seroconverted to MSP-142 at any time during the course of the study. Four of six volunteers (67%) in the control cohort did develop an antibody response to this antigen. Seroconversions occurred as early as 21 days and as late as 84 days after challenge, with peak antibody levels occurring around day 42 with considerable individual variability. Antibody response persisted throughout the study period of 6 months. A box plot of MSP ELISA titers is provided in Figure 3.
Figure 3.
A box plot of fold changes in anti–PfMSP-1(42) ELISA titer by time (days) and treatment group is presented. Control cohort (C) and mefloquine cohort (MQ) mean (•), median (horizontal bar in box), interquartile (box), minimum, and maximum (vertical lines) titer values are plotted.
Discussion
In this study, through use of a human malaria challenge model, it was shown that antibodies to MSP142 are not elicited in non-immune individuals receiving protective prophylactic doses of mefloquine. As a result, this biomarker, when detected by the ELISA used in this study, does not have adequate sensitivity to serve as a surrogate endpoint of malaria infection in malaria-naïve study populations taking mefloquine as a suppressive prophylactic agent.
These results conflict with those obtained by previous researchers, who noted a sensitivity of 45–64% (defined as in our study) in a retrospective analysis of field exposures to malaria in Indonesian soldiers taking mefloquine chemoprophylaxis (Ohrt C and others, unpublished data). It is possible that the difference in MSP antibody detection between these two studies stems from prior malaria exposure (61% of the Indonesian soldiers reported prior clinical malaria) and possibly larger or repeated malaria field exposures than provided under the controlled conditions of our study. It is also difficult to exclude the possibility that seroconverting individuals in the field study may have had episodes of undetected patent parasitemia as the cause of their seroconversion.
In our study, seroconversion to MSP142 had moderate sensitivity (66%) in individuals with patent parasitemia. This is also lower than has been noted previously (Ohrt C and others, unpublished data). We speculate that early case detection by serial thick-film microscopy, as evidenced by 50% of control cohort subjects being asymptomatic at the time of diagnosis, and prompt treatment may have limited antigen exposure such that some individuals failed to generate a detectable immune response to this antigen. Interestingly, among individuals who did seroconvert, antibody response was long-lasting (at least 6 months), raising the possibility that this assay may have use in retrospective diagnosis of cases of suspected malaria.
In our study, mefloquine prophylaxis was generally well-tolerated and universally effective in preventing symptomatic malarial infection. Although therapeutic levels of mefloquine for prophylaxis have not been rigorously established, studies in the mid-1990s estimated a 95% rate of prophylactic success with serum levels above 620 ng/mL.14 That we observed universal protection in non-immune volunteers, despite failing to achieve these levels in 6 of 23 individuals (26%) during the first 12 days of infection, indicates that, for susceptible parasite strains, drug levels < 620 ng/mL can still result in prophylactic efficacy. Furthermore, the success of prophylaxis in our study supports the results of previous researchers regarding the efficacy of the 3-day loading regimen for mefloquine chemoprophylaxis.8,9
In conclusion, the lack of sensitivity (0%) shown in this trial makes antibodies to MSP-142 a poor candidate for a biomarker of malaria exposure in active comparator trials of drug efficacy in completely malaria-naive study subjects.
ACKNOWLEDGMENTS
The authors gratefully acknowledge and thank Carola Salas, MSc, for her production and reading of the study malaria smears, Michael Green, PhD, for his assistance in performing HPLC analysis of blood mefloquine levels, Michelle Spring, MD, for her time and effort as the Medical Monitor, and Jackie Williams, PhD, and the staffs of the WRAIR Departments of Entomology and Clinical Trials for their malaria challenge support.
Disclaimer: The opinions expressed herein are the private views of the authors and are not to be construed as official viewpoints of the Departments of State, Army, or Defense.
Footnotes
Financial support: This project was supported by the United States Army Medical Materiel Development Activity.
Authors' addresses: James E. Moon, Gregory A. Deye, Lori Miller, Susan Fracisco, R. Scott Miller, Donna Tosh, James F. Cummings, Colin Ohrt, and Alan J. Magill, Walter Reed Army Institute of Research, Silver Spring, MD, E-mails: james.e.moon@us.army.mil, gregory.deye@us.army.mil, robert.s.miller@us.army.mil, susan.fracisco@us.army.mil, robert.s.miller@us.army.mil, donna.tosh@amedd.army.mil, james.cummings@us.army.mil, colin.ohrt@amedd.army.mil, and alan.magill@us.army.mil.
References
- 1.Dow GS, Magill AJ, Ohrt C. Clinical development of new prophylactic antimalarial drugs after the 5th Amendment to the Declaration of Helsinki. Ther Clin Risk Manag. 2008;4:803–819. doi: 10.2147/tcrm.s1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyon JA, Angov E, Fay MP, Sullivan JS, Girourd AS, Robinson SJ, Bermann-Leitner ES, Duncan EH, Darko CA, Collins WE, Long CA, Barnwell JW. Protection induced by Plasmodium falciparum MSP142 is strain-specific, antigen and adjuvant dependent, and correlates with antibody responses. PLoS One. 2008;3:e2830. doi: 10.1371/journal.pone.0002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui G, Hashimoto C. Plasmodium falciparum anti-MSP1-19 antibodies induced by MSP1-42 and MSP1-19 based vaccines differed in specificity and parasite growth inhibition in terms of recognition of conserved versus variant epitopes. Vaccine. 2006;25:948–956. doi: 10.1016/j.vaccine.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 4.Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SLR, Carneiro I, Malima R, Lusingu J, Manjurano A, Nkya WMMM, Lemnge MM, Cox J, Reyburn H, Riley EM. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roche USA Lariam (Mefloquine Hydrochloride) Tablets Product Information. 2009. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm Available at. Accessed November 23, 2010.
- 6.Clyde DF, McCarthy VC, Miller RM, Hornick RB. Suppressive activity of mefloquine in sporozoite-induced human malaria. Antimicrob Agents Chemother. 1976;9:384–386. doi: 10.1128/aac.9.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bwire R, Slootman EJH, Verhave JP, Bruins J, Docters van Leeuwen WM. Malaria anticircumsporozoite antibodies in Dutch soldiers returning from sub-Saharan Africa. Trop Med Int Health. 1998;3:66–69. doi: 10.1046/j.1365-3156.1998.00165.x. [DOI] [PubMed] [Google Scholar]
- 8.Boudreau E, Schuster B, Sanchez J, Novakowski W, Johnson R, Redmond D, Hanson R, Dausel L. Tolerability of prophylactic Lariam regimens. Trop Med Parasitol. 1993;44:257–265. [PubMed] [Google Scholar]
- 9.Hopperus Buma APCC, van Thiel PPAM, Lobel HO, Ohrt C, van Ameijden EJC, Veltnik RL, Tendeloo DCH, van Gool T, Green MD, Todd GD, Kyle DE, Kager PA. Long-term malaria chemoprophylaxis with mefloquine in Dutch marines in Cambodia. J Infect Dis. 1996;173:1506–1509. doi: 10.1093/infdis/173.6.1506. [DOI] [PubMed] [Google Scholar]
- 10.Chulay JD, Schneider I, Cosgriff TM, Hoffman SL, Ballou WR, Quakyi IA, Carter R, Trosper JH, Hockmeyer WT. Malaria transmitted to humans by mosquitoes infected from cultured Plasmodium falciparum. Am J Trop Med Hyg. 1986;35:66–68. doi: 10.4269/ajtmh.1986.35.66. [DOI] [PubMed] [Google Scholar]
- 11.Federal Regulations 21 CFR 312.32: IND Safety Reports. Text from Code of Federal Regulations. 2010. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm Available at. Accessed November 23, 2010.
- 12.Yoon I, Angov E, Larson D, Heppner DG, Cummings JF, Stewart VA. Characterization of a human reference standard for antibody to Plasmodium falciparum merozoite surface protein 142. Am J Trop Med Hyg. 2005;72:714–718. [PubMed] [Google Scholar]
- 13.Green MD, Yngve B, Mount DL, Corbett S, D'Souza MJ. Improved validated assay for the determination of mefloquine and its carboxy metabolite in plasma, serum and whole blood using solid-phase extraction and high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 1999;727:159–165. doi: 10.1016/s0378-4347(99)00080-8. [DOI] [PubMed] [Google Scholar]
- 14.Lobel HO, Miani M, Eng T, Bernard KW, Hightower AW, Campbell CC. Long-term malaria prophylaxis with weekly mefloquine. Lancet. 1993;34:848–851. doi: 10.1016/0140-6736(93)93058-9. [DOI] [PubMed] [Google Scholar]