Abstract
Recent reports of reductions in malaria transmission in several African countries have resulted in optimism that malaria can be eliminated in parts of Africa where it is currently endemic. It is not known whether these trends are global or whether they are also present in areas where political instability has hindered effective malaria control. We determined malaria parasite carriage and age-dependent antibody responses to Plasmodium falciparum antigens in cross-sectional surveys in Apac, northern Uganda that was affected by political unrest. Under-five parasite prevalence was 55.8% (115/206) by microscopy and 71.9% (41/57) by polymerase chain reaction. Plasmodium ovale alone, or as a co-infection, was detected in 8.6% (12/139) and Plasmodium malariae in 4.3% (6/139) of the infections. Age seroprevalence curves gave no indication of recent changes in malaria transmission intensity. Malaria control remains a tremendous challenge in areas that have not benefited from large-scale interventions, illustrated here by the district of Apac.
Introduction
Despite reported reductions in malaria transmission intensity in several African regions,1–8 malaria remains one of the most important public health problems in sub-Saharan Africa with an estimated 863,000 deaths annually.9 Widespread use of insecticide-treated nets (ITNs),1–3,5,6 effective vector control,1,3,6 increased urbanization,10 and treatment of uncomplicated falciparum malaria with artemisinin-based combination therapies (ACT)1,2,7 have all been assumed to contribute to the reported reductions in malaria incidence, although in some areas these reductions were observed before control measures were scaled up.8 This widespread decline in the burden of malaria has resulted in optimism that malaria can be eliminated in parts of Africa where malaria is currently endemic.11 However, reports of reductions in malaria transmission mostly originate from areas that have been involved in intensive and effective malaria control programs1–5 and sustained implementation of health care, with some valuable findings from less well-controlled settings.6–8 It is uncertain if trends of declining transmission intensity are evident across Africa8 or whether they are apparent in areas where political instability and economic arrest have hindered effective control and surveillance of infectious diseases.12,13 Conflict and human insecurity pose considerable challenges by causing a breakdown in health delivery systems and a loss of human and financial resources for health programs.13–15 In those areas, malaria control is likely to have been less efficiently implemented and maintained and, as a result, malaria transmission intensity may have remained unaltered, or malaria may have re-emerged in areas where it was previously under control.15,16
In this study, we determined the current level of malaria transmission intensity in the Apac district in northern Uganda, a remote region that was previously described as holoendemic for malaria.17–19 Northern Uganda has been involved in conflict since the early 1980s with the Lord's Resistance Army as the main rebel group that continues to be a threat to the region up to the present day. This conflict hindered economic development in northern Uganda and resulted in a lower access to healthcare compared with other regions in Uganda.20 The area of Apac was affected by political unrest in the early 1990s.21 Although health facilities remained functioning throughout the conflict, serious supply shortages affected the quality of care.
The aim of this study was to determine the current prevalence of Plasmodium falciparum parasite carriage by microscopy and polymerase chain reaction (PCR) and to use age-dependent antibody responses to P. falciparum circumsporozoite (CSP) antigen and blood-stage antigens apical membrane antigen-1 (AMA-1) and merozoite surface protein-119 (MSP-119) to look for evidence of recent changes in transmission intensity.22–24
Methods
The study was conducted in Apac Sub-County, a rural district in Northern Uganda located between Kwania Lake and the Victoria Nile (latitude 1.985; longitude 32.535). Apac District covers an area of 6,684 square kilometers and ranges in altitude between 1,350 and 1,500 meters above sea level. The rainfall pattern is bimodal with a dry season from November to February and two short rainy seasons from April to May and from September to October. According to surveys conducted in 2001–2002, this area experiences perennial holoendemic malaria17 with parasite prevalence rates of 70–90% in children < 10 years of age.17–19 The entomological inoculation rate was estimated at > 1,500 infective bites per person per year and the major vector responsible for transmission is Anopheles funestus.17 Plasmodium falciparum is the dominant parasite species, Plasmodium malariae being responsible for ~3% of the infections and Plasmodium ovale was previously not observed.19 Ethical approval was obtained from the ethical review committee of the London School of Hygiene and Tropical Medicine (no. 5539), the ethical committee of the Medical Biotech Laboratory, and the national ethical committee of Uganda.
Data collection.
Subjects were recruited in October 2009 in four parishes. Sampling was done in Apac District Hospital, two health facilities in the parishes of Abedi and Akere, and a primary school in the parish of Atopi. Before the sampling days, community meetings were organized to explain the purpose of the study and to invite people to attend sampling points. At the health facilities and the hospital, all individuals attending the facilities for clinical care, antenatal visits, or who came specifically to benefit from the screening offered by this study were selected for enrollment together with accompanying family members or guardians. This approach was previously shown to provide an estimate of malaria-specific antibody prevalence that is comparable to that obtained through community surveys.22 Before sampling at the school in Atopi, a community meeting was organized and parents were informed of the survey through the school's pupils. All inhabitants of Atopi who attended the sampling point, including pupils and their parents or guardians, were eligible for enrollment and people were sequentially enrolled until the sample size was reached. We aimed to recruit 200–300 individuals per parish of whom half were < 15 years of age. This sample size was based on a previous study where this number of participants was found to be sufficient for a reliable determination of transmission intensity by serological markers of malaria exposure.23 To ensure a balanced representation of all age groups, essential for determining the age-dependent seroconversion rates (SCRs),25 seven age categories were defined per parish (1–2 years, N = 45; 3–5 years, N = 45; 6–10 years, N = 40; 11–15 years, N = 40; 16–25 years, N = 40; 26–55 years, N = 40; and > 30 years, N = 50) and questionnaires were printed in pre-defined quantities in different colors for each age group. Questionnaires contained clinical information, demographic data, information on the use of antimalarial drugs, and protective antimosquito measures. As soon as the sample size for an age category was reached, no further individuals were enrolled for this category but enrollment continued for other age categories.
Written informed consent or, in case of illiteracy, consent by thumb print, was obtained from each participant ≥ 15 years of age and from parents or guardians of younger individuals. Each individual enrolled in the study underwent a clinical examination, during which axillary temperature was measured twice using a digital thermometer and the higher of the two values was recorded. A single blood sample was obtained by finger prick (~0.3 mL) for thick and thin blood films, for filter paper blood collection (Whatman 3 mm, Maidstone, UK) and for Rapid Diagnostic Tests (RDT; Paracheck Orchid Biomedical Systems, Goa, India) for malaria. This RDT has an estimated detection rate of 97.5% for parasite densities > 2,000 parasites/μL and 54.4% for parasite densities of 200/μL.26 Filter papers were air-dried and stored in plastic bags with silica desiccant gel type III (Sigma, Dorset, UK), stored at −20°C in the field, transported at room temperature, and again stored at −20°C in the laboratory until further processing. Thick blood films were Giemsa-stained in the field and read after completion of the study. Clinical diagnosis was based on the result of the RDT; RDT-positive individuals with (reported) fever were treated with artemether-lumefantrine (Lonart; Bliss Gvs Pharma Ltd., India) according to national guidelines. The first dose was given under supervision; the remaining five doses were given to the participant/guardian for treatment at home.
Parasite detection by microscopy and PCR.
Microscopic slides were examined for the presence of parasites in 100 high-power fields by two experienced microscopists; the average parasite density of the two readings was recorded and a third microscopist consulted in case of disagreement. Asexual parasites were counted against 200 white blood cells and converted to parasites/μL by assuming a density of 8,000 white blood cells/μL blood. We explored the value of PCR for parasite detection in a single parish, Abedi. DNA was extracted from all filter paper blood spots from Abedi, using the chelex method27 and tested for the presence of P. falciparum, P. vivax, P. malariae, and P. ovale in the nested PCR approach originally described by Snounou and others28 and Padley and others.29 Samples that were negative by PCR were rescreened by PCR using as a template DNA extracted using QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany).
Enzyme-linked immunosorbant assay (ELISA).
Antibodies were eluted from filter paper blood spots and assayed by ELISA, as described in the online protocol by Corran and others.30 Briefly, a 3.5-mm circle was cut from the spot and placed into 300 μL of phosphate buffered saline with 0.5% Tween 20 (PBS-T) and 0.05% sodium azide, approximately equivalent to a 1/200 serum dilution. Immunoglobulin G (IgG) antibodies against circumsporozoite protein (CSP), apical membrane antigen (AMA-1), and merozoite surface protein 1 (MSP-119) were detected by ELISA using standard methodology.22,25,30 Recombinant MSP-119 (Wellcome genotype), AMA-1 (3D7 genotype), and a synthetic peptide CSP (NANP4) were coated onto ELISA plates overnight at 4°C at a concentration of 0.5 mg/mL, respectively. Plates were washed using PBS plus 0.05% Tween 20 (PBS/T) and blocked with 1% (w/v) skimmed milk powder (Marvel, UK) in PBS/T. Samples were added in duplicate to each plate at a serum dilution of 1:200 for CSP, 1:2000 for AMA-1, and 1:750 for MSP-119 in 1% bovine serum albumin (BSA) in PBS/T.22 A positive control of pooled hyperimmune serum collected from adults resident in a malaria-endemic area was included in duplicates on each plate to allow standardization of day-to-day and plate-to-plate variation; serum from malaria-naive Europeans was included in each assay as negative controls. After overnight incubation at 4°C the plates were washed and horseradish peroxidase-conjugated rabbit anti-human IgG (Dako Ltd., High Wycombe, United Kingdom) (1/5,000 in PBS/T) was added to all wells. All plates were developed using OPD/H2O2 substrate solution and reactions were stopped with 2 M H2SO4. Plates were read immediately at 492 nm and optical density (OD) values recorded.
Data analysis.
All data were double entered and validated in Microsoft Access (Redmond, WA); inconsistencies were verified against the original questionnaire. Data were imported into Stata 11.0 (Stata Statistical Software, StataCorp, College Station, TX) for statistical analysis. Fever was defined as a temperature ≥ 37.5°C; submicroscopic parasitemia was defined as parasitemia by PCR in the absence of microscopically confirmed parasite carriage. Parasite density was presented as geometric mean in microscopically positive parasite carriers only with the 25th and 75th percentile (interquartile range, IQR). Duplicate OD results in ELISA assays were averaged and normalized against the positive control sample on each plate. A cut-off above which samples were deemed antibody positive was defined using a mixture model as previously described.22,30 Briefly, the distribution of normalized OD values was fitted as the sum of two Gaussian distributions (a narrow distribution of seronegatives and a broader distribution of seropositives) using maximum likelihood methods. The mean OD of the Gaussian corresponding to the seronegative population plus three standard deviations was used as the cut-off for seropositivity.22 The seroconversion rate (SCR or λ) was estimated by fitting a simple reversible catalytic model to the measured seroprevalence, stratified into yearly age groups, using maximum likelihood methods. For these models only individuals' ≥ 1 year of age were included to avoid the effect of maternally derived antibodies in infants. Evidence for temporal changes in SCR was explored by fitting models in which the SCR is allowed to change at a single time point.23,24 The significance of the change was identified using likelihood ratio tests against models with no change, and profile likelihoods were plotted to determine confidence intervals (CIs) for the estimated time of the change.23 The titer of the antibody response was estimated by using the formula dilution/(maximum OD/[OD test serum-minimum OD] − 1), where the maximum OD was the maximum value of the standard curve and the minimum OD the lowest value of the negative control. The titer was used as an indicator of antibody density in the analyses. Categorical variables were analyzed using the χ2 test or χ2 test for trend. Student's t test, analysis of variance, or non-parametric equivalents were used when comparing continuous variables. Logistic and linear regression models were used to adjust binary and continuous variables for potential confounding. Titer was log10 transformed for analysis and the exponentiated regression coefficients with 95% CI were presented.
Results
Parasite carriage by microscopy and PCR.
A total of 883 individuals were enrolled from four parishes (202–251 individuals per parish) with overall parasite prevalence by microscopy of 37.5% (219/851). Only P. falciparum was detected by microscopy. Fever was significantly more common among participants recruited at health facilities or the hospital compared with those recruited at school (odds ratio [OR] = 1.54; 95% CI = 1.03–2.31, P = 0.04); parasite prevalence by microscopy was not significantly different (OR = 0.77; 95% CI = 0.55–1.06, P = 0.11). Parasite prevalence by microscopy (P < 0.001) and parasite density (P < 0.001) decreased with age (Table 1). There was no statistically significant difference in the prevalence or density of parasites between parishes after adjusting for age (P = 0.15). There was a strong positive association between parasite prevalence by RDT and microscopy (P < 0.0001). Nevertheless, 19.6% (65/331) of individuals that were parasite positive by RDT were negative by microscopy; 52.3% (34/65) of these reported antimalarial drug use in the previous 2 weeks, possibly indicating HRP-2 antigen persistence after a successfully treated infection.25 Samples that were positive by microscopy but negative by RDT (N = 50) were characterized by a low parasite density, geometric mean density 238 parasites/μL (IQR = 80–600), significantly lower than that in RDT-positive parasite carriers (P < 0.001).
Table 1.
Characteristics of enrolled individuals*
| N | Abedi | Akere | Apac town | Atopi | |
|---|---|---|---|---|---|
| 251 | 217 | 213 | 202 | ||
| Age, median (IQR) | 15 (5.3–25) | 13 (4.3–29) | 19 (5.5–32) | 13 (5.6–30) | |
| Fever, % (n/N) | < 5 years | 30.0 (18/60) | 27.1 (16/59) | 46.9 (23/49) | 14.3 (6/42) |
| 5–14 years | 12.9 (8/62) | 17.7 (9/51) | 36.6 (15/41) | 9.1 (6/66) | |
| ≥ 15 years | 6.5 (8/124) | 3.9 (4/102) | 9.5 (11/116) | 3.4 (3/88) | |
| Parasite prevalence by RDT, % (n/N) | < 5 years | 56.7 (34/60) | 73.3 (44/60) | 71.4 (35/49) | 81.0 (34/42) |
| 5–14 years | 59.4 (38/64) | 44.2 (23/52) | 51.2 (21/41) | 64.2 (43/67) | |
| > 15 years | 16.8 (21/125) | 17.3 (18/104) | 14.1 (17/121) | 15.4 (14/91) | |
| P. falciparum parasite prevalence by microscopy, % (n/N) | < 5 years | 54.4 (31/57) | 50.9 (29/57) | 59.6 (28/47) | 63.4 (26/41) |
| 5–14 years | 59.7 (37/62) | 42.0 (21/50) | 51.3 (20/39) | 65.7 (44/67) | |
| ≥ 15 years | 20.2 (25/124) | 22.0 (22/100) | 11.3 (12/106) | 23.3 (21/90) | |
| P. falciparum parasite density, GM (IQR) | < 5 years | 6,063 (480–77,880) | 2,844 (400–32,640) | 8,102 (1,380–43,720) | 2,874 (520–11,120) |
| 5–15 years | 753 (120–3,320) | 1,235 (440–2,120) | 1,404 (460–3,740) | 918 (310–2,740) | |
| ≥ 15 years | 215 (80–520) | 453 (80–1,520) | 232 (60–540) | 456 (160–800) | |
| P. falciparum parasite prevalence by PCR, % (n/N) | < 5 years | 66.7 (38/57) | ND | ND | ND |
| 5–14 years | 69.4 (43/62) | ND | ND | ND | |
| ≥ 15 years | 42.62 (52/122) | ND | ND | ND |
IQR = interquartile range; RDT = rapid diagnostic test; GM = geometric mean; ND = not done; PCR = polymerase chain reaction.
The prevalence of infections was also determined by PCR in the parish of Abedi, where 241 DNA samples were analyzed for the presence of different malaria species. Parasites were detected by PCR in 107 samples after Chelex extraction; the QIAamp DNA Mini Kit (QIAGEN) was used to extract DNA from 131 samples that were negative after Chelex extraction and yielded another 32 PCR-positive samples, giving parasite prevalence by PCR of 57.7% (139/241). The vast majority of infections detected by PCR were P. falciparum mono-infection (87.8%, 122/139), whereas 8.6% (12/139) of the infections were with P. ovale, either as mono-infection (N = 6) or as co-infection with P. falciparum (N = 5) or as co-infection with P. falciparum and P. malariae (N = 1). Five mixed infections with P. falciparum and P. malariae were detected (3.6% of all infections, 5/139). Plasmodium vivax was not detected in any of the 241 samples analyzed by PCR. Individuals with non-falciparum malaria species detected by PCR, either as mono- or mixed-infection with P. falciparum, were on average younger than those with P. falciparum mono-infection by PCR (P = 0.035). Overall, 35.1% (52/148) of the samples negative by microscopy were positive by PCR; four samples were positive by microscopy but negative by PCR. Plasmodium falciparum parasite prevalence by PCR was highest in children < 15 years of age and showed an overall negative association with age (Figure 1, P < 0.001). The proportion of infections that was below the microscopic threshold for detection increased from 16.4% to 19.3% in children < 15 years of age to 48.7–53.1% in older age groups (Figure 1, P < 0.001).
Figure 1.
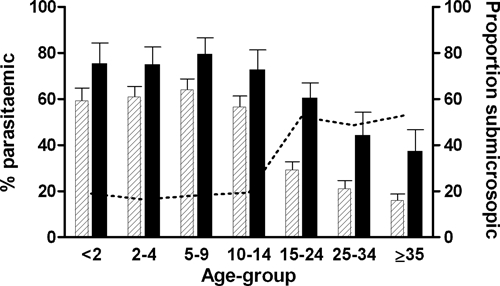
Plasmodium falciparum parasite carriage by microscopy and polymerase chain reaction (PCR). The prevalence of P. falciparum infection by microscopy (dashed bars) and PCR (black bars) is given for individuals < 2 years (microscopy, N = 87/PCR, N = 24), 2–4 years (N = 122/N = 34), 5–9 years (N = 111/N = 33), 10–14 years (N = 108/N = 28), 15–24 years (N = 155/N = 61), 25–34 years (N = 124/N = 29), and ≥ 35 years (N = 144/N = 32). Error bars indicate the upper limit of the 95% confidence interval around the proportion. The dotted line indicates the proportion of infections that is below the microscopic threshold for detection.
Fever and parasite carriage in children by microscopy and PCR.
Fever, defined as a temperature ≥ 37.5°C, was detected in 24.4% (42/172) of children < 10 years of age without microscopically detectable parasites, compared with 33.6% (85/253) of children of the same age group with parasites (P = 0.04). The prevalence of fever in submicroscopic parasite carriers was not different from that of non-infected individuals (P = 0.23). The proportion of febrile children increased with increasing parasite densities (Figure 2) but there was no statistically significant increase in fever prevalence until densities exceeded 10,000 parasites/μL. The 45.5% (13/33) of children with 10,000–49,999 parasites/μL had a current fever (temperature ≥ 37.5°C), which was significantly higher than that of children without parasites (OR = 2.58; 95% CI = 1.20–5.56, P = 0.016). For children with parasite densities ≥ 50.000 parasites/μL, the prevalence of fever was 65.9% (29/44), OR = 5.98 (95% CI = 2.93–12.21, P < 0.001). The association between parasite density and fever prevalence did not improve when fever was defined as a temperature ≥ 38.0°C and/or if children who reported using antipyretics were excluded.
Figure 2.
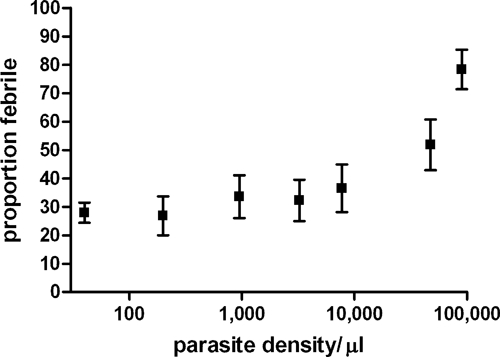
The occurrence of fever for different parasite densities in children < 10 years of age. The prevalence of fever (temperature ≥ 37.5°C) is given for children < 10 years of age with no parasites (N = 172), with < 400 parasites/μL (N = 50), 400–1,500 parasites/μL (N = 46), 1,500–5,000 parasites/μL, 5,000–15,000 parasites/μL (N = 39), 15,000–80,000 parasites/μL (N = 35), and ≥ 80,000 parasites/μL (N = 35). Error bars indicate the upper and lower limits of the 95% confidence interval around the proportion.
Malaria-specific antibody responses.
Antibodies against CSP, AMA 1, and MSP-119 were measured in 825 individuals. The overall seroprevalence was 25.2% (95% CI = 22.2–28.3) for CSP, 63.8% (95% CI = 60.4–67.0) for AMA-1, and 44.2% (95% CI = 40.8–47.7) for MSP-119. Seroprevalence generally increased with age (P < 0.001) for all the antigens (Figure 3) and this increase was most pronounced for AMA-1 where antibody prevalence rapidly rose to 74.3% (95% CI = 65.1–82.2) in children aged 5–10 years and 81.7% (95% CI = 72.9–88.6%) in children aged 10–15 years, and gradually decreased with age in older age groups (OR = 0.92; 95% CI = 0.96–0.99, P = 0.001). This decrease in older age groups may reflect a reduction in immune boosting by blood-stage infections in individuals with effective anti-parasite immunity. The age-seroprevalence curves did not indicate more than one force of infection over time; this was checked by allowing the SCR to differ at a single time point. For none of the antigens did multiple SCRs improve the fit of the age-seroprevalence curves. The overall SCR rate (λ) was estimated at 0.025 (95% CI = 0.019–0.033) for CSP, 0.260 (95% CI = 0.208–0.326) for AMA 1, and 0.056 (95% CI = 0.044–0.072) for MSP-119 (Figure 3). Microscopically confirmed parasitemia was associated with a higher odds of being AMA-1 seropositive for children 1–5 years of age (OR = 3.2, 95% CI = 1.54–6.61, P = 0.002) but not for older individuals (P = 0.46), after adjustment for age within the age strata (Table 2). A similar trend of a higher odds of being seropositive for parasitemic compared with non-parasitemic children < 5 years of age was seen for CSP (OR = 3.25; 95% CI = 0.67–15.8, P = 0.14) and MSP-119 (OR = 1.62; 95% CI = 0.79–3.32, P = 0.19), although not statistically significant. The titer of AMA-1 antibodies was also higher in the presence of microscopically confirmed infections (1.92-fold increase; 95% CI = 1.38–2.66, P < 0.001, after adjustment for age). A similar association was observed for CSP antibody titer (1.25-fold increase; 95% CI = 1.00–1.57, P = 0.05), whereas this trend was not significant for MSP-119 (1.26-fold increase; 95% CI = 0.88–1.83, P = 0.20). The presence of submicroscopic infections did not significantly influence the prevalence of AMA-1 (OR = 1.45; 95% CI = 0.32–6.60, P = 0.63), MSP-119 (OR = 0.37; 95% CI = 0.04–3.17, P = 0.37), or CSP (OR = 2.47; 95% CI = 0.23–26.25, P = 0.45) antibody responses in children 1–5 years of age compared with uninfected children of the same age. Similarly, antibody titer was not significantly elevated for AMA-1 (1.08-fold increase; 95% CI = 0.54–2.15, P = 0.83), MSP-119 (0.47-fold decrease; 95% CI = −0.17–0.76, P = 0.12), or CSP (0.11-fold decrease; 95% CI = −0.44–0.45, P = 0.64) in the presence of a submicroscopic infection in children < 5 years of age. These estimates will have been affected by small numbers, only 11 children < 5 years of age carried parasites at submicroscopic densities.
Figure 3.
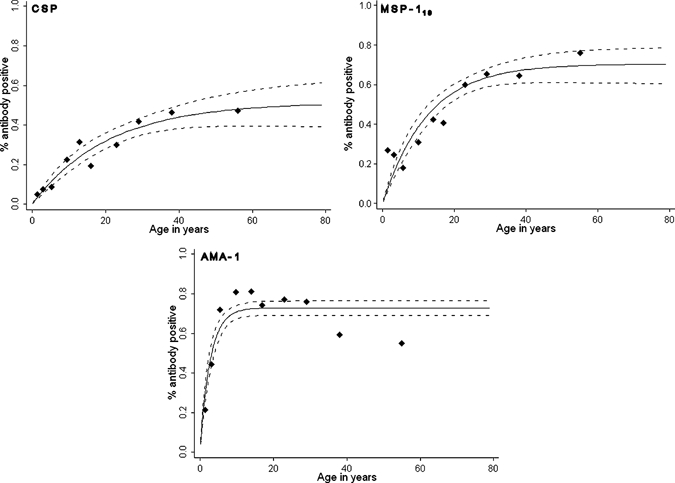
Age-seroprevalence plots for circumsporozoite protein (CSP), merozoite surface protein-119, and apical membrane antigen-1. Dots indicate the observed antibody prevalence for different age groups, the solid line the best fit based on age as a continuous variable, the dotted line the upper and lower limit of the 95% confidence interval (CI). The serocoversion rate λ was estimated at 0.025 (95% CI = 0.019–0.033) for CSP, 0.056 (95% CI = 0.044–0.072) for MSP-119, and 0.260 (95% CI = 0.208–0.326) for AMA 1.
Table 2.
Antibody prevalence and density in relation to parasite carriage by microscopy*
| CSP | Titer, median (IQR) | AMA-1 | Titer, median (IQR) | MSP-119 | Titer, median (IQR) | ||
|---|---|---|---|---|---|---|---|
| Prevalence, % (n/N) | Prevalence, % (n/N) | Prevalence, % (n/N) | |||||
| 1–5 years | Parasite-negative | 2.5 (2/79) | 31.1 (13.2–52.4)† | 25.0 (20/80)‡ | 194.5 (66.2–766.9)‡ | 19.2 (15/78) | 42.6 (11.4–119.5) |
| Parasite carrier | 7.8 (8/103) | 37.9 (21.4–61.5)† | 44.7 (46/103)‡ | 552.7 (167.9–1,429.3)‡ | 29.0 (29/100) | 63.9 (16.8–195.2) | |
| 5–14 years | Parasite-negative | 22.7 (20/88) | 71.2 (45.4–111.8) | 78.3 (72/92) | 1,215.9 (800.0–1,719.8) | 31.5 (29/92) | 55.3 (23.4–202.7) |
| Parasite carrier | 21.4 (25/117) | 60.5 (28.9–97.4) | 78.2 (93/119) | 1,391.3 (880.7–1,804.1) | 27.1 (32/118) | 64.0 (25.5–167.1) | |
| ≥ 15 years | Parasite-negative | 36.4 (112/308) | 93.0 (51.3–147.2) | 67.7 (216/319) | 1,063.6 (608.1–1561.2) | 62.3 (198/318) | 248.2 (94.1–478.8) |
| Parasite carrier | 34.7 (25/72) | 79.6 (49.3–152.9) | 76.3 (58/76) | 1,297.7 (769.6–1686.6) | 56.2 (41/73) | 190.5 (70.3–427.9) |
Median titer includes all individuals, also seronegatives. The prevalence or (log10-adjusted) titer were compared between parasite-positive and parasite-negative individuals by microscopy; these analyses were done for different age categories and adjusting for age within that category.
P = 0.05.
P < 0.01.
Discussion
In this work, we describe the current malaria situation in Apac, northern Uganda. Plasmodium falciparum parasite prevalence was high, ≥ 50% by microscopy in children < 5 years of age, and infections with P. malariae and especially P. ovale were common. Parasite carriage often occurred asymptomatically, at densities ranging from submicroscopic concentrations to densities > 5,000 parasites/μL. These parameters are consistent with continuing holoendemic malaria transmission and, unsurprisingly, the age-dependent prevalence of malaria-specific antibodies did not indicate recent changes in transmission intensity.
In our surveys, P. falciparum parasite prevalence in children 2–9 years of age was 58%. This was slightly lower than the previously reported parasite prevalence of 70–90% in the same age group living in neighboring parishes in Apac District in 1995–199917–19,31 but within the 50–100% range reported in world malaria maps.32 When PCR was used for parasite detection, overall P. falciparum parasite prevalence was 55.2% in all age groups (37.5% by microscopy) and 69.3% in children 2–9 years of age (57.3% by microscopy). The prevalence of submicroscopic parasite carriage is in perfect agreement with the recent meta-analysis by Okell and colleagues,33 who reported a median PCR parasite prevalence of 58.6 (IQR = 51.4–74.0%) for areas where the microscopical parasite prevalence is 25–50%. The relative proportion of parasite carriers that harbored parasites at submicroscopic densities increased with age34; this is likely to be a reflection of acquired immunity that allows adults to control infections more effectively. However, even in the youngest age group a substantial proportion of infections were not detected by microscopy, as was previously shown in areas of intense35 and low endemicity.36 Microscopy will be sufficiently sensitive to detect clinically relevant parasite densities, although our data clearly indicate that not every episode of parasitemia with fever equals clinical malaria.37,38 One-quarter of children < 10 years of age presented with fever in the absence of parasites and the proportion of febrile children did not change considerably until malaria parasite densities exceeded 10,000 parasites/μL. Our data were insufficient to define a pyrogenic threshold parasite density or malaria-attributable fraction of fever episodes; only 77 children had a parasite density ≥ 10,000 parasites/μL. One could argue that a single blood film per individual is insufficient for calculating the pyrogenic threshold density or malaria-attributable fraction, because the density of peripheral parasitemia within a single individual can fluctuate widely between times of the day and between days.39
The prevalence and density of antibody responses was influenced by microscopically detectable parasite densities.40,41 This was only apparent in children < 5 years of age and suggests that immune responses are less stable in this age group, fluctuating with concurrent infections.42 In older age groups immune responses were not influenced by concurrent parasitemia. Contrary to previous studies, we did not find evidence for a boosting of immune responses by submicroscopic parasite carriage.43,44 We observed few submicroscopic infections in children 1–5 years of age (N = 11), which will have affected our power to detect such an immune-boosting effect. These findings confirm previous indications that parasitization status can be an important consideration in longitudinal assessments of the protective role of immune responses.41,45
Age-seroprevalence plots can reveal recent reductions in transmission intensity when the age-seroprevalence curve shows an improved fit to the data when more than one SCR rate is assumed for specific time periods.23,24 We did not find any evidence for more than one force of infection and therefore have no reason to conclude a reduction in transmission intensity since the last surveys in the area.17 The used methods may not have picked up a steady, gradual decline in transmission intensity,25 but this was not suggested by our findings in relation to previous surveys. Our approach of convenient sampling at health facilities and a school where surrounding villagers were mobilized for screening has some drawbacks. Although it was previously shown that this approach is valid in obtaining an estimate of antimalarial antibody prevalence,23 it may have resulted in an overestimation of parasite carriage because of a selection of symptomatic or overly exposed individuals. Although this implies that some caution is required in extrapolating the results to the general population, we feel that the asymptomatic nature of the vast majority of infections makes it likely that our estimates are informative for the general population. The observation of persisting intense transmission are in agreement with a recent review that concluded that reductions in transmission intensity are not evident in all African settings8 and that transmission intensity may have remained unchanged or even increased in northwestern Uganda46 and neighboring countries in East and Central Africa.47–49 The failure to reduce the burden of malaria could reflect sub-optimal implementation of malaria control measures. Even in areas of intense malaria transmission intensity, considerable gains can be achieved by vector control and effective antimalarial treatment, as was illustrated by successful malaria control on Bioko island.50 Malaria control efforts in Apac were not reliably monitored in the last decade and affected by political unrest in preceding years. The ACTs were officially available in Apac from the year 2006, but especially the smaller health facilities were affected by supply shortages that affected their implementation51; recently, malaria initiatives in Apac have been intensified. Indoor residual spraying (IRS) with DDT was banned in Apac in 2008 by a court injunction launched by organic famers,52 but IRS with pyrethroids (Fendona, BASF, Midrand, South Africa) started again in May 2010 (i.e., after the current survey was completed). Community-wide distribution of long lasting ITNs took place in early 2009, reaching the majority of households. Our study illustrates that these control efforts are still profoundly needed in areas in Africa where transmission remains intense and malaria control continues to be a tremendous challenge.
ACKNOWLEDGMENTS
We are grateful to the Apac district's inhabitants for their participation to the study; we also thank Martin Ogwal of the Apac District Health Office for sharing details on malaria control programs in the study area.
Footnotes
Financial support: This study was supported by the FIGHTMAL project, receiving funding from the European Community's Seventh Framework Programme [FP7/2007-2013] under grant agreement PIAP-GA-2008-218164.
Authors' addresses: Carla Proietti, Eleanor M. Riley, Chris Drakeley, and Teun Bousema, London School of Hygiene and Tropical Medicine, Department of Immunology and Infection, Faculty of Infectious and Tropical Diseases, London, UK, E-mails: carla.proietti@lshtm.ac.uk, eleanor.riley@lshtm.ac.uk, chris.drakeley@lshtm.ac.uk, and teun.bousema@lshtm.ac.uk. Davide D. Pettinato and Andrea Crisanti, Imperial College London, Division of Cell and Molecular Biology, London, UK, E-mails: davidepettinato@googlemail.com and crisanti@unipg.it. Bernard N. Kanoi, Edward Ntege, and Thomas G. Egwang, Medical Biotech Laboratories, Kampala, Uganda, E-mails: bnkanoi@gmail.com, edwardsdoc@yahoo.com, and director.mbl@gmail.com.
References
- 1.Barnes KI, Durrheim DN, Little F, Jackson A, Mehta U, Allen E, Dlamini SS, Tsoka J, Bredenkamp B, Mthembu DJ, White NJ, Sharp BL. Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med. 2005;2:e330. doi: 10.1371/journal.pmed.0020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattarai A, Ali AS, Kachur SP, Martensson A, Abbas AK, Khatib R, Al Mafazy AW, Ramsan M, Rotllant G, Gerstenmaier JF, Molteni F, Abdulla S, Montgomery SM, Kaneko A, Bjorkman A. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinschmidt I, Schwabe C, Benavente L, Torrez M, Ridl FC, Segura JL, Ehmer P, Nchama GN. Marked increase in child survival after four years of intensive malaria control. Am J Trop Med Hyg. 2009;80:882–888. [PMC free article] [PubMed] [Google Scholar]
- 4.O'Meara WP, Bejon P, Mwangi TW, Okiro EA, Peshu N, Snow RW, Newton CR, Marsh K. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–1562. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceesay SJ, Casals-Pascual C, Erskine J, Anya SE, Duah NO, Fulford AJ, Sesay SS, Abubakar I, Dunyo S, Sey O, Palmer A, Fofana M, Corrah T, Bojang KA, Whittle HC, Greenwood BM, Conway DJ. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet. 2008;372:1545–1554. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graves PM, Osgood DE, Thomson MC, Sereke K, Araia A, Zerom M, Ceccato P, Bell M, Del Corral J, Ghebreselassie S, Brantly EP, Ghebremeskel T. Effectiveness of malaria control during changing climate conditions in Eritrea, 1998–2003. Trop Med Int Health. 2008;13:218–228. doi: 10.1111/j.1365-3156.2007.01993.x. [DOI] [PubMed] [Google Scholar]
- 7.Barnes KI, Chanda P, Ab Barnabas G. Impact of the large-scale deployment of artemether/lumefantrine on the malaria disease burden in Africa: case studies of South Africa, Zambia and Ethiopia. Malar J. 2009;8((Suppl 1)):S8. doi: 10.1186/1475-2875-8-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . World Malaria Report 2009. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- 10.Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts L. Shrinking the malaria map from the outside in. Science. 2010;328:849–851. doi: 10.1126/science.328.5980.849. [DOI] [PubMed] [Google Scholar]
- 12.Coghlan B, Brennan RJ, Ngoy P, Dofara D, Otto B, Clements M, Stewart T. Mortality in the Democratic Republic of Congo: a nationwide survey. Lancet. 2006;367:44–51. doi: 10.1016/S0140-6736(06)67923-3. [DOI] [PubMed] [Google Scholar]
- 13.Salama P, Spiegel P, Talley L, Waldman R. Lessons learned from complex emergencies over past decade. Lancet. 2004;364:1801–1813. doi: 10.1016/S0140-6736(04)17405-9. [DOI] [PubMed] [Google Scholar]
- 14.Hawkes M, Katsuva JP, Masumbuko CK. Use and limitations of malaria rapid diagnostic testing by community health workers in war-torn Democratic Republic of Congo. Malar J. 2009;8:308. doi: 10.1186/1475-2875-8-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gayer M, Legros D, Formenty P, Connolly MA. Conflict and emerging infectious diseases. Emerg Infect Dis. 2007;13:1625–1631. doi: 10.3201/eid1311.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . An Inter-Agency Field Handbook. Geneva, Switzerland: WHO; 2005. (Malaria control in complex emergencies). [Google Scholar]
- 17.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D'Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 18.Apio B, Nalunkuma A, Okello D, Riley E, Egwang TG. Human IgG subclass antibodies to the 19 kilodalton carboxy terminal fragment of Plasmodium falciparum merozoite surface protein 1 (MSP1(19)) and predominance of the MAD20 allelic type of MSP1 in Uganda. East Afr Med J. 2000;77:189–193. doi: 10.4314/eamj.v77i4.46620. [DOI] [PubMed] [Google Scholar]
- 19.Egwang TG, Apio B, Riley E, Okello D. Plasmodium falciparum malariometric indices in Apac district, northern Uganda. East Afr Med J. 2000;77:413–416. [PubMed] [Google Scholar]
- 20.Accorsi S, Fabiani M, Lukwiya M, Ravera M, Costanzi A, Ojom L, Paze E, Manenti F, Anguzu P, Dente MG, Declich S. Impact of insecurity, the AIDS epidemic, and poverty on population health: disease patterns and trends in northern Uganda. Am J Trop Med Hyg. 2001;64:214–221. doi: 10.4269/ajtmh.2001.64.214. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Disaster update: report 15110. 2004. http://www.who.int/disasters/repo/15110.pdf Available at. Accessed July 15, 2009.
- 22.Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SL, Carneiro I, Malima R, Lusingu J, Manjurano A, Nkya WM, Lemnge MM, Cox J, Reyburn H, Riley EM. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart L, Gosling R, Griffin J, Gesase S, Campo J, Hashim R, Masika P, Mosha J, Bousema T, Shekalaghe S, Cook J, Corran P, Ghani A, Riley EM, Drakeley C. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS ONE. 2009;4:e6083. doi: 10.1371/journal.pone.0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook J, Reid H, Iavro J, Kuwahata M, Taleo G, Clements A, McCarthy J, Vallely A, Drakeley C. Using serological measures to monitor changes in malaria transmission in Vanuatu. Malar J. 2010;9:169. doi: 10.1186/1475-2875-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corran P, Coleman P, Riley E, Drakeley C. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol. 2007;23:575–582. doi: 10.1016/j.pt.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization . Malaria Rapid Diagnostic Test Performance. Results of WHO product testing of malaria RDTs. 2008. Round 1. doi:10.2471/TDR.09.978-924-1598071. [Google Scholar]
- 27.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 28.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 29.Padley D, Moody AH, Chiodini PL, Saldanha J. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann Trop Med Parasitol. 2003;97:131–137. doi: 10.1179/000349803125002977. [DOI] [PubMed] [Google Scholar]
- 30.Corran PH, Cook J, Lynch C, Leendertse H, Manjurano A, Griffin J, Cox J, Abeku T, Bousema T, Ghani AC, Drakeley C, Riley E. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J. 2008;7:195. doi: 10.1186/1475-2875-7-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talisuna AO, Langi P, Mutabingwa TK, Van Marck E, Speybroeck N, Egwang TG, Watkins WW, Hastings IM, D'Alessandro U. Intensity of transmission and spread of gene mutations linked to chloroquine and sulphadoxine-pyrimethamine resistance in falciparum malaria. Int J Parasitol. 2003;33:1051–1058. doi: 10.1016/s0020-7519(03)00156-5. [DOI] [PubMed] [Google Scholar]
- 32.Malaria Atlas Project. 2009. http://www.map.ox.ac.uk/ Available at. Accessed July 13.
- 33.Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis. 2009;200:1509–1517. doi: 10.1086/644781. [DOI] [PubMed] [Google Scholar]
- 34.Steenkeste N, Rogers WO, Okell L, Jeanne I, Incardona S, Duval L, Chy S, Hewitt S, Chou M, Socheat D, Babin FX, Ariey F, Rogier C. Sub-microscopic malaria cases and mixed malaria infection in a remote area of high malaria endemicity in Rattanakiri province, Cambodia: implication for malaria elimination. Malar J. 2010;9:108. doi: 10.1186/1475-2875-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouedraogo AL, Schneider P, de Kruijf M, Nebie I, Verhave JP, Cuzin-Ouattara N, Sauerwein RW. Age-dependent distribution of Plasmodium falciparum gametocytes quantified by Pfs25 real-time QT-NASBA in a cross-sectional study in Burkina Faso. Am J Trop Med Hyg. 2007;76:626–630. [PubMed] [Google Scholar]
- 36.Shekalaghe SA, Bousema JT, Kunei KK, Lushino P, Masokoto A, Wolters LR, Mwakalinga S, Mosha FW, Sauerwein RW, Drakeley CJ. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop Med Int Health. 2007;12:547–553. doi: 10.1111/j.1365-3156.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 37.Schellenberg JR, Smith T, Alonso PL, Hayes RJ. What is clinical malaria? Finding case definitions for field research in highly endemic areas. Parasitol Today. 1994;10:439–442. doi: 10.1016/0169-4758(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 38.Koram KA, Molyneux ME. When is “malaria” malaria? The different burdens of malaria infection, malaria disease, and malaria-like illnesses. Am J Trop Med Hyg. 2007;77:1–5. [PubMed] [Google Scholar]
- 39.Delley V, Bouvier P, Breslow N, Doumbo O, Sagara I, Diakite M, Mauris A, Dolo A, Rougemont A. What does a single determination of malaria parasite density mean? A longitudinal survey in Mali. Trop Med Int Health. 2000;5:404–412. doi: 10.1046/j.1365-3156.2000.00566.x. [DOI] [PubMed] [Google Scholar]
- 40.Kinyanjui SM, Mwangi T, Bull PC, Newbold CI, Marsh K. Protection against clinical malaria by heterologous immunoglobulin G antibodies against malaria-infected erythrocyte variant surface antigens requires interaction with asymptomatic infections. J Infect Dis. 2004;190:1527–1533. doi: 10.1086/424675. [DOI] [PubMed] [Google Scholar]
- 41.Bull PC, Lowe BS, Kaleli N, Njuga F, Kortok M, Ross A, Ndungu F, Snow RW, Marsh K. Plasmodium falciparum infections are associated with agglutinating antibodies to parasite-infected erythrocyte surface antigens among healthy Kenyan children. J Infect Dis. 2002;185:1688–1691. doi: 10.1086/340420. [DOI] [PubMed] [Google Scholar]
- 42.Akpogheneta OJ, Duah NO, Tetteh KK, Dunyo S, Lanar DE, Pinder M, Conway DJ. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun. 2008;76:1748–1755. doi: 10.1128/IAI.01333-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giha HA, Nasr A, Iriemenam NC, Balogun HA, Arnot D, Theander TG, Troye-Blomberg M, Berzins K, ElGhazali G. Age-dependent association between IgG2 and IgG3 subclasses to Pf332-C231 antigen and protection from malaria, and induction of protective antibodies by sub-patent malaria infections, in Daraweesh. Vaccine. 2010;28:1732–1739. doi: 10.1016/j.vaccine.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Shekalaghe S, Alifrangis M, Mwanziva C, Enevold A, Mwakalinga S, Mkali H, Kavishe R, Manjurano A, Sauerwein R, Drakeley C, Bousema T. Low density parasitaemia, red blood cell polymorphisms and Plasmodium falciparum specific immune responses in a low endemic area in northern Tanzania. BMC Infect Dis. 2009;9:69. doi: 10.1186/1471-2334-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinyanjui SM, Bejon P, Osier FH, Bull PC, Marsh K. What you see is not what you get: implications of the brevity of antibody responses to malaria antigens and transmission heterogeneity in longitudinal studies of malaria immunity. Malar J. 2009;8:242. doi: 10.1186/1475-2875-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ndyomugyenyi R, Magnussen P. Trends in malaria-attributable morbidity and mortality among young children admitted to Ugandan hospitals, for the period 1990–2001. Ann Trop Med Parasitol. 2004;98:315–327. doi: 10.1179/000349804225003433. [DOI] [PubMed] [Google Scholar]
- 47.Mabiala-Babela JR, Samba-Louaka C, Mouko A, Senga P. Morbidity in a pediatric department (University Hospital of Brazzaville): 12 years later (1989–2001) Arch Pediatr. 2003;10:650–652. doi: 10.1016/s0929-693x(03)00290-2. [DOI] [PubMed] [Google Scholar]
- 48.Himeidan YE, Hamid EE, Thalib L, Elbashir MI, Adam I. Climatic variables and transmission of falciparum malaria in New Halfa, eastern Sudan. East Mediterr Health J. 2007;13:17–24. [PubMed] [Google Scholar]
- 49.Okiro EA, Alegana VA, Noor AM, Mutheu JJ, Juma E, Snow RW. Malaria pediatric hospitalization between 1999 and 2008 across Kenya. BMC Med. 2009;7:75. doi: 10.1186/1741-7015-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleinschmidt I, Torrez M, Schwabe C, Benavente L, Seocharan I, Jituboh D, Nseng G, Sharp B. Factors influencing the effectiveness of malaria control in Bioko Island, equatorial Guinea. Am J Trop Med Hyg. 2007;76:1027–1032. [PMC free article] [PubMed] [Google Scholar]
- 51.Zurovac D, Tibenderana JK, Nankabirwa J, Ssekitooleko J, Njogu JN, Rwakimari JB, Meek S, Talisuna A, Snow RW. Malaria case-management under artemether-lumefantrine treatment policy in Uganda. Malar J. 2008;7:181. doi: 10.1186/1475-2875-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis K. DDT stalemate stymies malaria control initiative. CMAJ. 2008;179:999–1000. doi: 10.1503/cmaj.081585. [DOI] [PMC free article] [PubMed] [Google Scholar]


