Abstract
α1-Antitrypsin deficiency is an inherited condition that causes liver disease and emphysema. The normal function of this protein, which is synthesized by the liver, is to inhibit neutrophil elastase, a protease that degrades connective tissue of the lung. In the classical form of the disease, inefficient secretion of a mutant α1-antitrypsin protein (AAT-Z) results in its accumulation within hepatocytes and reduced protease inhibitor activity, resulting in liver injury and pulmonary emphysema. Because mutant protein accumulation increases hepatocyte cell stress, we investigated whether transplanted hepatocytes expressing wild-type AAT might have a competitive advantage relative to AAT-Z–expressing hepatocytes, using transgenic mice expressing human AAT-Z. Wild-type donor hepatocytes replaced 20%–98% of mutant host hepatocytes, and repopulation was accelerated by injection of an adenovector expressing hepatocyte growth factor. Spontaneous hepatic repopulation with engrafted hepatocytes occurred in the AAT-Z–expressing mice even in the absence of severe liver injury. Donor cells replaced both globule-containing and globule-devoid cells, indicating that both types of host hepatocytes display impaired proliferation relative to wild-type hepatocytes. These results suggest that wild-type hepatocyte transplantation may be therapeutic for AAT-Z liver disease and may provide an alternative to protein replacement for treating emphysema in AAT-ZZ individuals.
Introduction
α1-Antitrypsin (AAT), an abundant plasma glycoprotein secreted primarily by hepatocytes, inhibits neutrophil elastase, a protease that can degrade constituents of the lung connective tissue (1). AAT deficiency affects 3.4 million individuals worldwide, causing both liver and lung disease (2). The classic form of AAT deficiency arises from the homozygous inheritance of the AAT-Z allele (AAT-ZZ), a variant of SERPINA1/AAT resulting from substitution of lysine for glutamate 342 (3). This mutation alters the folding of the protein, rendering it prone to polymerization and aggregation within the hepatocyte ER (3, 4). Inefficient secretion of AAT-Z reduces its plasma levels to approximately 15% of normal, allowing “uninhibited” leukocyte proteases to damage the pulmonary connective tissue matrix, causing emphysema (3). Liver disease is caused by a gain-of-function mechanism associated with the expression and accumulation of polymerized AAT-Z in hepatocytes. Genetic and/or environmental modifiers protect 80%–90% of the deficient population from severe liver disease, while in the remaining 10%–20%, the liver phenotype ranges from subclinical liver disease to cirrhosis and hepatoma (5). Although liver transplantation could, potentially, prevent the development of lung disease, because of the considerable morbidity of this procedure, it is not used for prophylaxis or treatment of the pulmonary component of the disease in the absence of cirrhosis and hepatic failure. Intravenous augmentation therapy with AAT purified from pooled human plasma or recombinant human AAT slows the disease progression in a subset of patients with moderate emphysema (6). Unfortunately, this expensive life-long therapy is not affordable by many patients. Furthermore, AAT administration does not affect the liver component of the disease.
Transplanting isolated hepatocytes offers several potential advantages over whole liver transplantation in patients with AAT-ZZ disease. Hepatocyte transplantation partially reconstitutes hepatic enzyme deficiencies in animal models of inherited liver-based metabolic disorders and has been used clinically to ameliorate the metabolic deficiency in a variety of liver-based metabolic diseases (7). Engraftment and function of the transplanted cells has been shown. Hepatocyte transplantation, being a minimally invasive procedure with little associated morbidity, should be much less expensive than AAT replacement therapy or whole liver transplantation. However, both animal experiments and clinical experience indicate that the number of hepatocytes that could be transplanted safely in one session is inadequate for fully curing most metabolic abnormalities. In mouse models of very rare metabolic disorders, such as fumaryl acetoacetate hydrolase (FAH) deficiency (model of human tyrosinemia-1), the rapid loss of host hepatocyte mass induces compensatory hyperplasia of the engrafted donor cells, which eventually replaces virtually all host hepatocytes (8). In the ATP7B-deficient LEC rat model of Wilson disease, in which hepatic copper accumulation causes severe liver injury, a modest level of proliferation of engrafted wild-type hepatocytes has been observed (9), while additional liver injury was required for extensive repopulation. In contrast, in most liver-based metabolic disorders, including those affecting the majority of patients with AAT-Z disease, liver injury is minimal. In laboratory animals, liver repopulation has been achieved by reducing the proliferative capacity of host hepatocytes by administering plant alkaloids (10) or controlled preparative hepatic irradiation (11) to provide a competitive advantage to engrafted hepatocytes in response to mitotic stimulation. In these circumstances, death of host hepatocytes is not required as a primary event, and a greater mitotic efficiency of the engrafted cells is sufficient for competitive replacement of the host hepatocytes.
We hypothesized that the stress of expression and accumulation of the misfolded AAT-Z protein in host hepatocytes would provide a competitive advantage to transplanted wild-type hepatocytes, leading to their proliferation and progressive replacement of the host cells. Here, we tested this hypothesis by transplanting hepatocytes from LacZ-transgenic ROSA26 (C57BL/6) mice into the liver of transgenic mice (PiZ) expressing human AAT-Z. The results provide evidence for competitive repopulation of the liver by wild-type donor hepatocytes and, therein, provide a basis for further studies of hepatocyte transplantation as a therapeutic option for AAT-ZZ individuals with either liver disease or emphysema.
Results and Discussion
PiZ mice have normal serum alanine aminotransferase (ALT), bilirubin, and albumin levels, but exhibit mild hepatic fibrosis.
Transgenic PiZ mice express human AAT-Z from its native promoter elements (12). Tissue distribution of transgene expression recapitulates that in humans (13). However, because the endogenous mouse AAT is expressed normally, these mice do not have the loss-of-function phenotype of the human deficiency state. Serum ALT (25 ± 10 IU/l), bilirubin (0.25 ± 0.20 mg/dl), and albumin (3.8 ± 0.25 g/dl) levels in the PiZ mice were not significantly different from corresponding values in wild-type C57BL/6 mice (27 ± 11 IU/l, 0.21 ± 0.18 mg/dl, and 3.7 ± 0.28 g/dl, respectively). In this respect, these mice resembled the majority of human patients homozygous for the AAT-Z mutation, in whom results of conventional serological liver function tests are within normal limits (1).
H&E staining of liver sections showed normal liver architecture and occasional intralobular lymphocytic infiltration. However, Sirius red and Masson’s trichrome staining showed mild hepatic fibrosis, with fine strands extending into the lobules from perivascular regions, confirming previously published results (14). As in human AAT-Z disease, diastase-resistant PAS-positive polymerized AAT-Z globules accumulate in the ER in clusters of hepatocytes, separated by globule-devoid cells. Consistent with previous reports (14), male mice exhibited a larger number of globule-containing hepatocytes (data not shown).
AAT-Z globule–containing hepatocytes have AAT-Z transgene and mRNA content similar to that of globule-devoid cells, but AAT-Z protein load is greater.
To determine the transgene content of globule-containing and the globule-devoid cells, we performed LASER capture microscopy on diastase/PAS-stained liver sections. DNA PCR showed that the AAT-Z transgene was present in both groups of hepatocytes. Quantitative RT-PCR revealed that both groups of hepatocytes expressed similar AAT-Z mRNA levels (data not shown). In contrast, Western blot analysis showed a much higher AAT-Z content in the globule-containing hepatocytes, which was consistent with the results of Lindblad et al., who also showed that the globule-containing cells had a higher rate of apoptosis than globule-devoid hepatocytes (15).
Together the results indicate that both globule-containing and globule-devoid cells express AAT-Z, but the globule-containing cells accumulate more polymerized AAT-Z, probably through posttranscriptional mechanisms. Despite the lesser accumulation of AAT-Z in globule-devoid hepatocytes, these cells may still be under sufficient intracellular stress, causing a proliferative disadvantage when in competition with transplanted wild-type hepatocytes (as shown below).
In vivo bioluminescence imaging showed spontaneous proliferation of transplanted wild-type donor hepatocytes in PiZ mouse livers.
To determine noninvasively whether wild-type donor hepatocytes proliferated in PiZ mouse livers, hepatocytes from C57BL/6 mice were transduced ex vivo using a recombinant lentivirus expressing firefly luciferase before transplantation. The transduction efficiency was approximately 30%. Fourteen days after transplantation, in vivo bioluminescence imaging showed that the transplanted cells were concentrated in the spleen. At later time points, marked increase in the signal over the liver indicated intrahepatic proliferation of engrafted hepatocytes (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI45260DS1).
Wild-type hepatocytes engraft in PiZ mouse livers as single cells and proliferate spontaneously over time.
Three days after transplantation of β-gal–expressing ROSA26 mouse hepatocytes, the donor cells were engrafted as single hepatocytes, without site preference in relation to the AAT-Z globule–containing hepatocytes. After 30 days, proliferating clusters of the engrafted hepatocytes were found (Figure 1A). With progression of repopulation (Figure 1A), AAT-Z globule–containing hepatocytes declined, so that after 90 days, very few of these cells remained. In contrast, after transplantation of ROSA26-derived hepatocytes into congeneic C57BL/6 recipients, with or without the injection of an adenoviral vector expressing human hepatocyte growth factor (Ad-HGF), the engrafted cells remained as single cells or microclusters of 2–3 hepatocytes (data not shown).
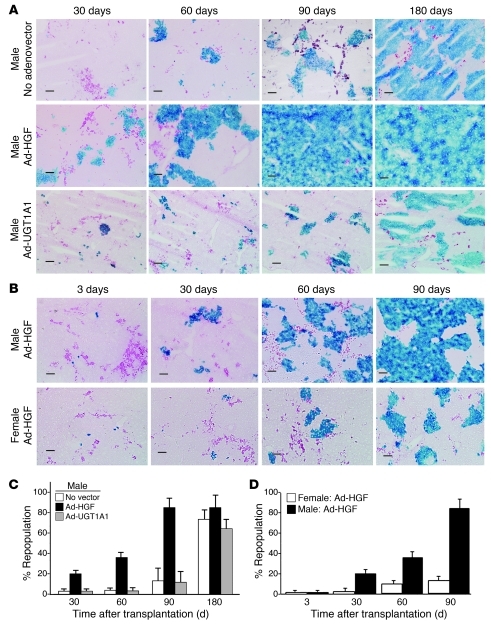
Figure 1. Kinetics of hepatic repopulation.
(A) Ad-HGF administration accelerated repopulation. ROSA26 mouse hepatocytes (1 × 106) were transplanted into male PiZ mice without (upper row) or with (lower row) Ad-HGF (1 × 1011 particles, i.v.). Liver sections were stained for E. coli β-gal (blue), and diastase plus PAS (magenta) to visualize AAT-Z globules. Scale bars: 100 μm. Data are from representative mice from each group (n = 6). (B) Repopulation was greater in male recipients. Male and female PiZ mice received Ad-HGF (n = 6). Hepatocyte transplantation and staining of liver sections were as in A. (C and D) Quantitative DNA PCR. Quantitative PCR for the E. coli lacZ gene was performed on DNA extracted from livers of recipient mice. Percentage of repopulation was calculated as described in the text. (C) Graphic presentation of data from experimental groups shown in A (mean ± SEM; n = 6 in each group), showing significantly higher repopulation in the Ad-HGF group at all time points (P < 0.05). (D) Data are from the experimental groups shown in B (mean ± SEM; n = 6 in each group), showing significantly higher repopulation in males 30, 60, and 90 days after transplantation (P < 0.05).
Ad-HGF accelerated liver repopulation.
To test the hypothesis that mitotic stimulation should accelerate liver repopulation by enhancing the difference between the proliferative rates of AAT-Z–expressing host hepatocytes and the wild-type donor cells, we determined the time course of repopulation with or without Ad-HGF (1011 i.v.) administration in male PiZ mice. In one control group, Ad-HGF was replaced with an adenovector expressing an unrelated gene, UGT1A1 (Ad-UGT1A1). Morphometric analysis showed that hepatic repopulation by donor hepatocytes was significantly greater at all time points in the Ad-HGF group, but not in recipients receiving Ad-UGT1A1 (Figure 1A). Ninety days after transplantation, in Ad-HGF–treated recipients, 70%–98% of the hepatocytes were replaced by the donor cells, but the host bile duct epithelial cells and the nonparenchymal cells persisted (Figure 1A). The effects of Ad-HGF administration (Figure 1C) on repopulation were corroborated by quantitative DNA PCR of the LacZ gene in hepatocytes isolated from the donor and recipient livers. Repopulation was estimated assuming 2 × 105 cells being present per mg liver weight and 60% of these cells being hepatocytes.
Interestingly, even in groups receiving no adenovector or Ad-UGT1A1, 180 days after transplantation, liver repopulation was 70% ± 9.2% (mean ± SEM) and 59% ± 10%, respectively (Figure 1C).
Hepatic repopulation was more extensive in male recipient mice than in females.
Initial engraftment of hepatocytes was equally efficient in male and female recipients (3 days; Figure 1B). However, 30, 60, and 90 days after transplantation, hepatic repopulation in males was more extensive than in females (Figure 1B). The morphometric analysis was corroborated by quantitative DNA PCR of the LacZ gene in hepatocytes isolated from the donors and in the recipient liver.
Rudnick et al. (14) reported higher levels of AAT-Z expression and a greater number of AAT-Z globule–containing hepatocytes in male PiZ mice than in females. Testosterone administration to female PiZ mice increased AAT-Z expression and the number of globule-containing hepatocytes (14). Therefore, the accelerated repopulation in male PiZ mice may be related to the effect of endogenous androgens on AAT-Z expression. However, within each sex, we did not find any correlation between the percentage of globule-containing cells in the initial biopsy and the rate of liver repopulation.
Hepatic AAT-Z content was progressively depleted after liver repopulation.
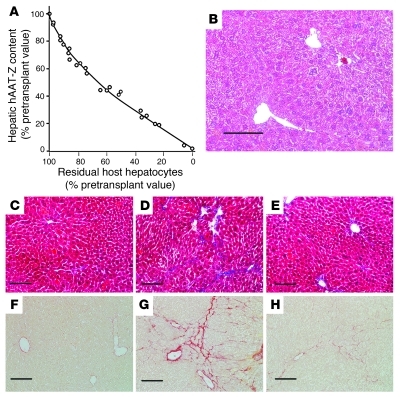
We analyzed liver tissue samples from the male and female PiZ mice at various time points after transplantation of 1 × 106 hepatocytes from ROSA26 donors, with or without Ad-HGF (1 × 1011 particles) administration. Western blot with densitometric analysis of band intensities showed that PiZ content of the liver declined progressively following hepatocyte transplantation (Figure 2A).
Figure 2. Depletion of hepatic human AAT-Z content and restoration of normal liver histology after repopulation.
(A) Male and female PiZ mice were transplanted with ROSA26 hepatocytes, with or without Ad-HGF administration (n = 6 in each group). Liver tissues were obtained by biopsy before and at various time points after transplantation and at the termination of the experiment. Hepatic human AAT-Z content was determined by Western blot/densitometry. Data (percentage of pretransplant values) are plotted against percentage of residual β-gal–negative host hepatocytes. The line of best fit is shown. (B) H&E staining of liver paraffin section after extensive (80%) hepatic repopulation by donor hepatocytes. (C–E) Masson’s trichrome–stained liver sections. (F–H) Sirius red–stained liver sections. (C and F) C57BL/6 mice; (D and G) untreated PiZ mice. (E and F) PiZ mice after 80% hepatic repopulation with wild-type hepatocytes. In Masson’s trichrome–stained sections, the area occupied by blue-stained collagen was quantified using the ImageJ program in conjunction with the Threshold Color plug-in. Scale bars: 100 μm.
Liver histology and function after repopulation.
Following extensive repopulation, H&E-stained liver sections showed normal architecture and hepatocyte morphology (Figure 2B). In untreated PiZ mice, Masson’s trichrome (Figure 2, C–E) and Sirius red (Figure 2, F–H) staining showed mild hepatic fibrosis, which resolved after extensive liver repopulation. Hepatic collagen content was estimated by image analysis of the blue-stained collagen in liver sections. In wild-type C57BL/6 and PiZ mice. hepatic collagen content was 0.45% ± 0.28% and 3.23% ± 1.25%, respectively (mean ± SD; n = 6, P < 0.01). After 40%–60% and 70%–90% repopulation, the collagen content decreased to 0.94% ± 0.45% and 0.55% ± 0.35%, respectively (n = 6). In PiZ mice with extensive liver repopulation (>70%), hepatic collagen content was not significantly different from that of C57BL/6 controls (P > 0.2). Serum bilirubin, ALT, and albumin levels remained normal (data not shown).
After transplantation, the donor hepatocytes divided more frequently, whereas the host hepatocytes exhibited increased apoptosis.
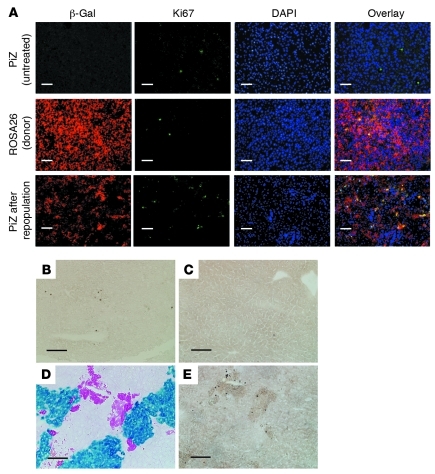
Progressive repopulation of the recipient mouse livers, without a change in the total liver size, suggested that the donor cell proliferation was counterbalanced by attrition of host hepatocytes, probably by apoptosis. To verify this, we quantified the number of dividing donor and host cells by immunofluorescent staining of the proliferation marker Ki67 and the number of host and donor cells undergoing apoptosis by TUNEL staining. For each section, 6 nonoverlapping fields, containing approximately 6000 hepatocytes, were counted. Dual immunofluorescent staining showed that resting PiZ mouse livers and livers from ROSA26 donor mice contained 3.8 ± 0.7 and 3.0 ± 0.8 Ki67-positive cells per 1000 (Figure 3A; n = 6, mean ± SD). After 40%–60% repopulation of the liver, the number of Ki67-positive cells increased (10 ± 2.2 per 1000) in the β-gal–positive donor cell clusters (Figure 3A), but not in the host hepatocytes (2.5 ± 0.8 per 1000) (n = 6, P < 0.02). TUNEL staining showed a higher level of apoptotic cells (4 ± 1.8 per 1000) in untreated PiZ mouse livers (Figure 3B) than in wild-type C57BL/6 mice (1.8 ± 0.5 per 1000) (Figure 3C). There was no increase in the number of TUNEL-positive cells in nontransplanted male and female PiZ mice receiving tacrolimus (1 mg/kg daily). After partial liver repopulation, the frequency of TUNEL-positive host hepatocytes increased 3.5-fold (14 ± 3.2 per 1000, n = 6, P < 0.01) over that in the untreated PiZ mice. No increase in TUNEL staining was found in the β-gal–positive donor cell clusters (1.6 ± 0.8 per 1000; Figure 3, D and E). These results suggest that the engrafted cells proliferated at a greater rate in the host PiZ mouse livers than in livers of wild-type mice, probably via growth signals received from the host. The increased apoptosis of host hepatocytes combined with greater proliferation of donor cells resulted in progressive repopulation of the host liver, while the liver size remained unaltered, consistent with tight physiological control of the liver to body size ratio. This phenomenon is reminiscent of the cell-cell competition observed during Drosophila wing development, where the proximity of more mitotically active cells causes death of less mitotically competent, but otherwise viable cells (16). This process is fundamentally different from liver repopulation in urinary plasminogen activator (uPA) transgenic mice and FAH-deficient mice, where the death of host hepatocytes results from intrinsic abnormalities and does not require the presence of wild-type donor cells (8). Notably, after extensive repopulation, serological tests for hepatocyte function and liver histology remained normal.
Figure 3. Proliferation and apoptosis of hepatocytes after partial repopulation.
(A) Ki67 and β-gal immunofluorescent staining. Paraffin sections of formalin-fixed liver samples at various time points after transplantation (as in Figure 1) were subjected to immunofluorescent staining for Ki67 and E. coli β-gal. To visualize the nuclei, sections were mounted with Vectashield containing DAPI (Vector Laboratories). Representative liver sections from recipient PiZ mice, donor ROSA26 mice, and PiZ mouse livers partly repopulated with the ROSA26 hepatocytes are shown. (B–E) TUNEL staining. TUNEL staining was performed on cryosections of formalin-fixed livers from livers of PiZ mice (B), ROSA26 donor mice (C), and PiZ mouse livers after partial repopulation with the donor ROSA26 hepatocytes (E). D shows a section consecutive to that shown in E, stained for β-gal activity (blue) and AAT-Z globules (magenta). Scale bars: 100 μm.
In summary, we show that engrafted wild-type hepatocytes proliferate preferentially over the recipient PiZ mouse hepatocytes. This is associated with enhanced apoptosis of the host hepatocytes, hepatic repopulation with donor hepatocytes, and resolution of the liver fibrosis that occurs in untreated PiZ mice. The repopulation was more extensive in male recipients and was accelerated by mitotic stimulation of hepatocytes. Our findings suggest that liver repopulation with transplanted normal allogeneic hepatocytes could be effective therapy for a subgroup of AAT-ZZ patients with liver disease and as an alternative to protein replacement for emphysema, even in the absence of severe liver disease.
Methods
Further information can be found in Supplemental Methods.
Hepatocyte transplantation.
Hepatocytes isolated by in situ collagenase perfusion of the livers of ROSA26 (C57BL/6 strain) mice expressing E. coli β-gal were engrafted into the livers of PiZ mice (transgenic for human AAT-Z) or C57BL/6 mice by intrasplenic injection. As PiZ mice are not fully congeneic to ROSA26 mice, the recipients received tacrolimus (1 mg/kg s.c. daily) to prevent allograft rejection. All animal experiments were performed with approval of the Animal Care and Use Committees of Albert Einstein College of Medicine and the University of Nebraska College of Medicine
Identification of donor cells.
The donor cells were visualized in the recipient livers by histochemical or immunofluorescence staining for E. coli β-gal.
Cell division and apoptosis were detected by immunofluorescence staining for Ki67 and TUNEL staining, respectively.
Statistics.
Sets of data in the various groups were compared using paired 2-tailed Student’s t test. P < 0.01 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by grants NYSTEM CO 24346 (to J. Roy-Chowdhury); NIH RO1 DK48794, and AI49472 and US Department of Defense W81XWH-09-1-0658 (to I.J. Fox); NIH RO1 DK079017-01 and a research grant from Oxalosis and Hyperoxaluria Foundation (to N. Roy-Chowdhury); and NIH R01 DK076918 (to D.H. Perlmutter). Authors thank John Murray, Albert Einstein College of Medicine, New York, New York, USA, for assistance with image analysis.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(5):1930–1934. doi:10.1172/JCI45260.
References
- 1.Crystal RG. α1-Antitrypsin deficiency, emphysema and liver disease: Genetic basis and strategies for therapy. J Clin Invest. 1990;85(5):1343–1352. doi: 10.1172/JCI114578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Serres FJ. Worldwide racial and ethnic distribution of alpha1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest. 2002;122(5):1818–1829. doi: 10.1378/chest.122.5.1818. [DOI] [PubMed] [Google Scholar]
- 3.Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z α1-antitrypsin accumulation in the liver. Nature. 1992;357(6379):605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 4.Perlmutter DH. The role of autophagy in alpha-1-antitrypsin deficiency. A specific cellular response in genetic diseases associated with aggregation-prone proteins. Autophagy. 2006;2(4):258–263. doi: 10.4161/auto.2882. [DOI] [PubMed] [Google Scholar]
- 5.The Alpha-1-Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of α1-antitrypsin. Am J Respir Crit Care Med. 1998;158(1):49–59. doi: 10.1164/ajrccm.158.1.9712017. [DOI] [PubMed] [Google Scholar]
- 6.Dirksen A, et al. A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999;160(5 pt 1):1468–1472. doi: 10.1164/ajrccm.160.5.9901055. [DOI] [PubMed] [Google Scholar]
- 7.Fox IJ, Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol. 2004;40(6):878–886. doi: 10.1016/S0168-8278(04)00155-2. [DOI] [PubMed] [Google Scholar]
- 8.Overturf K, Al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151(5):1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph B, Kapoor S, Schilsky ML, Gupta S. Bile salt–induced pro-oxidant liver damage promotes transplanted cell proliferation for correcting Wilson disease in the Long-Evans Cinnamon rat model. Hepatology. 2009;49(5):1616–1624. doi: 10.1002/hep.22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grompe M, Laconi E, Shafritz DA. Principles of therapeutic liver repopulation. Semin Liver Dis. 1999;19(1):7–14. doi: 10.1055/s-2007-1007093. [DOI] [PubMed] [Google Scholar]
- 11.Jiang J, et al. Correction of hyperoxaluria by liver repopulation with hepatocytes in a mouse model of primary hyperoxaluria type-1. Transplantation. 2008;85(9):1253–1260. doi: 10.1097/TP.0b013e31816de49e. [DOI] [PubMed] [Google Scholar]
- 12.Sifers RN, Carlson JA, Clift SM, DeMayo FJ, Bullock DW, Woo SLC. Tissue specific expression of the human alpha-1-antitrypsin gene in transgenic mice. Nucl Acids Res. 1987;15(4):1459–1475. doi: 10.1093/nar/15.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidvegi T, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329(5988):229–232. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- 14.Rudnick DA, et al. Analyses of hepatocellular proliferation in a mouse model of alpha-1-antitrypsin deficiency. Hepatology. 2004;39(4):1048–1055. doi: 10.1002/hep.20118. [DOI] [PubMed] [Google Scholar]
- 15.Lindblad D, Blomenkamp K, Teckman J. Alpha-1-antitrypsin mutant Z protein content in individual hepatocytes correlates with cell death in a mouse model. Hepatology. 2007;46(4):1228–1235. doi: 10.1002/hep.21822. [DOI] [PubMed] [Google Scholar]
- 16.de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117(1):107–116. doi: 10.1016/S0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.