Abstract
Targeted T cell immunotherapies using engineered T lymphocytes expressing tumor-directed chimeric antigen receptors (CARs) are designed to benefit patients with cancer. Although incorporation of costimulatory endodomains within these CARs increases the proliferation of CAR-redirected T lymphocytes, it has proven difficult to draw definitive conclusions about the specific effects of costimulatory endodomains on the expansion, persistence, and antitumor effectiveness of CAR-redirected T cells in human subjects, owing to the lack of side-by-side comparisons with T cells bearing only a single signaling domain. We therefore designed a study that allowed us to directly measure the consequences of adding a costimulatory endodomain to CAR-redirected T cells. Patients with B cell lymphomas were simultaneously infused with 2 autologous T cell products expressing CARs with the same specificity for the CD19 antigen, present on most B cell malignancies. One CAR encoded both the costimulatory CD28 and the ζ-endodomains, while the other encoded only the ζ-endodomain. CAR+ T cells containing the CD28 endodomain showed strikingly enhanced expansion and persistence compared with CAR+ T cells lacking this endodomain. These results demonstrate the superiority of CARs with dual signal domains and confirm a method of comparing CAR-modified T cells within individual patients, thereby avoiding patient-to-patient variability and accelerating the development of optimal T cell immunotherapies.
Introduction
As T cell immunotherapy extends into clinical application (1, 2), its benefits are being expanded by engineering T lymphocytes to express chimeric antigen receptors (CARs) that recognize specific antigens expressed on the cell surface of different types of tumor cells (3–8). CAR molecules usually combine the antigen-binding domain of the variable regions of a specific monoclonal antibody (scFv) with the CD3ζ endodomain of the TCR/CD3 complex (so-called first-generation CARs) (4). When expressed by T lymphocytes, CARs provide potent antigen-specific, non-MHC-restricted effector function against tumor cells in preclinical models (5). However, in the initial human trials, T lymphocytes expressing first-generation CARs showed limited expansion and relatively short persistence (3, 9, 10). This result likely reflects the failure of artificial CAR molecules to fully activate T cells after antigen engagement on tumor cells, especially when the tumor cells lack expression of costimulatory molecules (such as CD80 and CD86) that are required for sustained T cell activation, growth, and survival (11).
To provide the costimulation lacking in tumor cell targets and thereby overcome the above limitations, several groups have incorporated costimulatory endodomains, including CD28 (12), 4-1BB (13, 14), or OX40 (15), into CAR molecules (so-called second-generation CARs). Although preclinical studies suggest that this strategy can indeed augment the activation of CAR-modified T lymphocytes (5, 7, 12), there has been no direct demonstration of this effect in human subjects. To meet this challenge, we designed a clinical study in which patients with non-Hodgkin lymphomas (NHLs) were infused simultaneously with 2 autologous T cell products, each containing cells that expressed an identical CAR exodomain specific for the CD19 antigen (CD19-specific scFv) (16–18). In one product the CAR was coupled to the ζ-endodomain alone (CAR.CD19ζ), while in the second product the CAR was coupled to both the CD28 and ζ-endodomains (CAR.CD19-28ζ). With this study design, each patient acted as a “self-control,” allowing us to directly determine in vivo the effects of incorporating a costimulatory endodomain on the fate of the CAR-engineered T cells.
Results and Discussion
We enrolled 6 patients, aged 46 to 59 years, with relapsed or refractory NHL (Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI46110DS1). Each patient had active disease, measurable by physical examination or CT or PET imaging at the time of the T cell infusions. We generated 2 CAR-transduced T cell products for each patient, always from the same blood collection. Polyclonal T cell lines were generated after a period of culture (mean, 13 days; range, 6–18 days). Products that expressed either CAR.CD19ζ or CAR.CD19-28ζ transgenes were similar by functional and phenotypic analyses (Figure 1). Two patients were then treated with both preparations at each level of dose escalation (2 × 107/m2, 1 × 108/m2, or 2 × 108/m2 cells per dose). The infusions were well tolerated without any immediate adverse side effects.
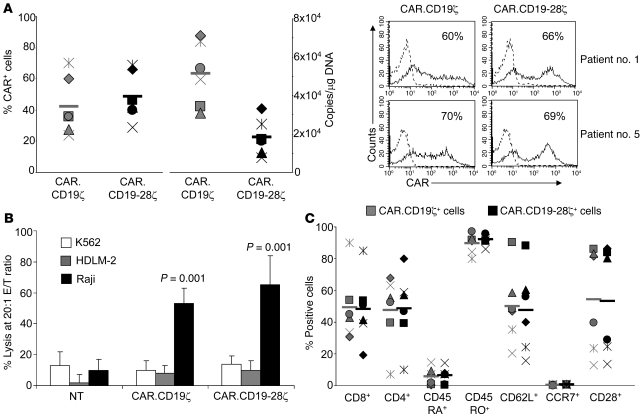
Figure 1. Transduction efficiency and phenotypic/function profile of T cell lines.
(A) FACS and Q-PCR analyses showing transduction efficiency with CAR.CD19ζ and CAR.CD19-28ζ vectors (left). Bars indicate mean values for peripheral blood samples from 6 patients (Supplemental Table 1). Each symbol represents an individual cell line. Representative histograms of T cells transduced with CAR.CD19ζ and CAR.CD19-28ζ vectors from patients number 1 and number 5 are also presented (right). Numbers represent the percentage of CAR+ cells. (B) Results of a 4-hour 51Cr-release assay at an effector/tumor cell (E/T) ratio of 20:1. Target cells were Raji (CD19+, CD80+, CD86–), a Burkitt lymphoma cell line; HLDM-2 (CD19–, CD80+, CD86+), a Hodgkin lymphoma cell line; and K562 (CD19–, CD80–, CD86–), an erythroid leukemia cell line that is susceptible to natural killer cell activity. Both CAR.CD19ζ+ and CAR.CD19-28ζ+ T cells specifically targeted CD19+ tumors. Data are mean ± SD for the 6 T cell lines. (C) Phenotypic composition of CAR.CD19ζ+ or CAR.CD19-28ζ+ T cells. These products contained both CD8+ and CD4+ CAR-expressing T cells that are predominantly CD45RO+CD62L+, with a fraction of them expressing CD28. Each symbol represents an individual cell line, and horizontal bars denote mean group values.
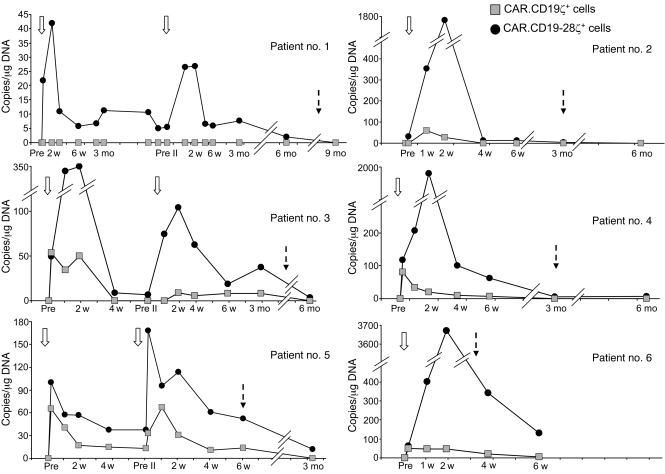
A pivotal question in CAR-mediated cancer immunotherapy is whether the introduction of dual signaling domains will enhance the expansion and persistence of genetically modified T cells in human subjects, as observed in response to tumor cells in vitro when these cells lack expression of costimulatory molecules (Supplemental Figure 1). We therefore assessed these end points in the peripheral blood of our patients by quantitative PCR (Q-PCR) assays specific for CAR.CD19ζ and CAR.CD19-28ζ transgenes. As illustrated in Figure 2, the molecular signals identifying CAR.CD19-28ζ+ T cells were detected at a low level as early as 3 hours after the first infusion (63.5 ± 15.5 copies/μg of DNA); this level increased to 218.2 ± 60.6 copies/μg of DNA and 1,285.8 ± 585.4 copies/μg of DNA at 1 and 2 weeks after infusion, respectively, representing a mean 6.82-fold change over the 3-hour value. The signals identifying CAR.CD19-28ζ+ T cells reached a nadir by 4 to 6 weeks after infusion (42.6 ± 19.5 copies/μg of DNA). Despite this decline, the remaining peripheral blood CAR.CD19-28ζ+ T cells retained the capacity to expand when restimulated ex vivo by engagement of their native TCRs (Supplemental Figure 2). Molecular signals derived from the CAR.CD19ζ+ T cells were also detected at 3 hours after infusion (41.3 ± 13.8 copies/μg of DNA), but they failed to expand thereafter (35.9 ± 8.2 copies/μg of DNA and 26.6 ± 7.7 copies/μg of DNA at 1 and 2 weeks after infusion, respectively), becoming virtually undetectable by 6 weeks after infusion (4.3 ± 2.2 copies/μg of DNA) (Figure 2), with only marginal reexpansion upon TCR stimulation in vitro (Supplemental Figure 2). By repeated measure ANOVA, CAR.CD19-28ζ signals were significantly higher than CAR.CD19ζ signals at every time point tested over the first 4 weeks after infusion (P < 0.0001). There was no evidence of a T cell–dose response based on the Q-PCR assay. However, the study is not powered enough to detect this difference, and no definite conclusion can be drawn on this matter. Since the T cell lines we infused were a mixture of CAR+ CD4+ and CD8+ cells, we asked whether both subsets had contributed to T cell expansion in vivo. Q-PCR analysis of DNA extracted from FACS-sorted CD4+ and CD8+ T cells from patients number 3 and number 5 indicated that both CD4+ and CD8+ T cells can contribute to the in vivo expansion of CAR.CD19-28ζ+ T cells (Supplemental Figure 3).
Figure 2. In vivo expansion and persistence of infused CAR.CD19ζ+ versus CAR.CD19-28ζ+ T cell lines as assessed by Q-PCR in peripheral blood.
Data points represent critical postinfusion intervals after the first or second infusion of modified T cells. Patients number 1, number 3, and number 5, who had stable disease or clinical benefit at 6 weeks after the first T cell infusion, received a second infusion of CAR-modified T lymphocytes. Patient number 1 received only CAR.CD19-28ζ+ T cells (2 × 107 cells/m2, the same as for the first infusion), because this was the only product available. Patient number 3 received both CAR.CD19-28ζ+ and CAR.CD19ζ+ T cells, but the cell dose was 60% of their first dose (1 × 108 cells/m2). Patient number 5 received both CAR.CD19-28ζ+ and CAR.CD19ζ+ T cells at the same dose administered during their first infusion (2 × 108 cells/m2). Open arrows indicate the time of T cell infusion, and dashed arrows indicate the time when chemotherapy was initiated for disease progression. Pre, before the first infusion; Pre II, before the second infusion.
Two patients (number 1 and number 3) with stable disease and one patient (number 5) with minimal progression at 1 site of disease after the first infusion were treated with the same T cell products 6 or more weeks after the first infusion to increase potential clinical benefits (Supplemental Table 1). The differential pattern of molecular signals over time, after the second infusion, was similar to that observed after the first infusion (Figure 2), such that only CAR.CD19-28ζ+ T cells showed any discernible expansion. This suggests that the decline of T cell persistence after the first infusion was unlikely to have been determined by rapid immune elimination of the gene-modified T cells (19).
Conceivably, the differences we observed in the expansion and survival of these CAR-modified T cells could be explained by the preferential accumulation of CAR.CD19ζ+ T cells in malignant tissues, as compared with that of CAR.CD19-28ζ+ T cells. We therefore analyzed tissue sections taken from a cutaneous lesion of patient number 5 at 2 weeks after the second T cell infusion. Phenotypic analysis of lymphocytes from this tissue revealed the presence of CAR+ T cells (Figure 3), which, by Q-PCR analysis, proved to be exclusively CAR.CD19-28ζ+ T cells, corresponding to the preponderance of these cells in peripheral blood.
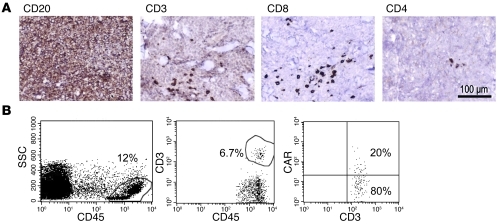
Figure 3. Detection of CAR+ T cells in a skin tumor biopsy.
(A) Immunohistochemical examination (diaminobenzidine with hematoxylin counterstaining) of a punch biopsy of a lymphoma skin lesion from patient number 5 at 2 weeks after T cell infusion showed that tumor cells were CD20+ (shown), CD10+, BCL2+, and BCL6+, consistent with involvement by follicular lymphoma with large cell transformation. Scattered CD3+ CD8+ cells infiltrated the tumor. Of note, the infused CAR.CD19-28ζ+ product consisted of 85% CD8+ cells. Scale bar: 100 μm. (B) FACS analysis of a cell suspension obtained from a fragment of the tumor biopsy. Viable cells represented approximately 45% of the preparation. The far left panel shows the gate on CD45+ cells, which represented 12% of the viable cells. The middle panel shows the CD3+ lymphocytes infiltrating the tumor, which accounted for 6.7% of CD45+ cells (0.8% of all viable cells). The far right panel illustrates that 20% of the gated CD3+ lymphocytes cells coexpressed the CAR, as assessed by the Fc-Cy5 monoclonal antibody, which binds to the IgG1-CH2CH3 spacer region of the CD19-specific CARs (~0.16% of all viable cells). SSC, side scatter.
Our results clearly demonstrate that CD28 costimulation improves the in vivo expansion and persistence of CAR-modified T cells in the peripheral blood as compared with that of T cells with a single signaling domain. Moreover, the presence of CAR.CD19-28ζ+ T cells in a cutaneous tumor biopsy suggests that the superior expansion and persistence conferred by the second-generation CAR in peripheral blood may extend to cells infiltrating tumor sites. Although the gains in T cell responses achieved with CD28 costimulation are encouraging, they still may be too short lived to produce meaningful clinical benefits. Indeed, despite transient stabilization of lymphoma in 2 patients, none showed evidence of sustained tumor regression at the cell doses used (Supplemental Table 1).
Infusion of CAR-modified T cells immediately after lymphodepletion or lymphoablative chemoradiotherapy, together with concomitant administration of cytokines such as IL-2, may further increase the persistence of the infused CAR.CD19-28ζ+ T cells and thus their clinical benefits (8, 20, 21). Nonetheless, it is clear that other signals besides CD28 costimulation will be required by T cells to allow them to pass through the physiological sequence of activation, proliferation, and survival check points to which T cells are subjected (22). One strategy to achieve this goal takes advantage of members of the tumor necrosis family (TNF) to recruit specific TNF-receptor-associated factor (TRAF) adapter proteins, which represents a fundamental departure from CD28 costimulation (23). OX40 and 4-1BB belong to the TNF receptor family, and their endodomains have been coexpressed with that of CD28 in CAR molecules to produce third-generation CARs, in an effort to recapitulate more complete costimulation of CAR-modified T cells upon binding to a specific antigen (14, 15).
Our method of comparing the behavior of CAR-modified T cells, with or without the CD28 costimulatory endodomain, in individual patients avoids many of the variables (such as disease status and prior treatment) that confound efforts to assess the responses to genetically altered T cell populations in consecutive or “matched” patients. Hence, this approach would allow direct analysis of the incremental benefits of incorporating critical costimulatory components into CARs even in small numbers of patients, thereby accelerating the development of optimal T cell products for cancer immunotherapy.
Methods
Patients.
This study was open to patients with recurrent or refractory NHL or those unable to receive or complete standard therapy. We obtained 30–60 ml peripheral blood for the production of gene-modified T cells, under current “good-tissue practice” conditions. The investigation was approved by the US Food and Drug Administration, the Recombinant DNA Advisory Committee, and the Institutional Review Board of Baylor College of Medicine. All participants gave informed consent upon enrollment.
T cell infusions.
All individuals received the T cell products at least 6 weeks after the last chemotherapy treatment. At the time of infusion, patients had measurable disease by imaging studies (CT or PET scan) or by physical examination (skin lesion in 1 patient). T cell products expressing either the CAR.CD19ζ or CAR.CD19-28ζ transgenes were administered simultaneously to each patient (Supplemental Table 1). Second infusions were allowed if there was evidence of clinical benefit (including stable disease or partial response) at 6 weeks after the first infusion as assessed by Response Evaluation Criteria in Solid Tumors (24). We assessed toxicity on the basis of patient interviews, physical examinations, and laboratory tests of organ function at 1, 2, 4, and 6 weeks and 3, 6, 9, and 12 months after infusion.
Generation of retroviral constructs.
The scFv domain targeting the CD19 antigen was provided by Heddy Zola (Child Health Research Institute, Women’s and Children’s Hospital, Adelaide, South Australia, Australia) (16). CAR.CD19ζ and CAR.CD19-28ζ vectors were generated as previously described (25). A spacer region derived from the human IgG1-CH2CH3 domain was cloned in-frame between the scFv and the signaling domains (25). The cassettes were cloned into the SFG retroviral backbone (3). Clinical grade packaging cell lines were generated with the use of PG13 cells (gibbon ape leukemia virus pseudotyping packaging cell line; CRL-10686, ATCC) as previously described (3). We used the highest-titer clone for each vector to establish a master cell bank, releasing the clones for clinical use only when safety testing for replication-competent retrovirus had been performed.
Generation and transduction of activated T cells.
To generate CAR-modified T lymphocytes, PBMCs were activated with immobilized OKT3 antibody (Ortho Biotech) and recombinant human IL-2 (rhIL-2) (100 U/ml; Proleukin Chiron) and then transduced by day 3 in 24-well plates precoated with a recombinant fibronectin fragment (FN CH-296, Retronectin Takara). After transduction, the T cell lines were expanded ex vivo in the presence of rhIL-2 (50–100 U/ml) added twice weekly, without any additional stimulation with OKT3 antibody or single cell cloning (3).
Immunophenotyping.
We stained T cell lines with monoclonal antibodies to CD3, CD4, CD8, CD62L, CD45RA, CD45RO, CCR7, and CD28 (Becton Dickinson). We detected the CD19-specific CAR with an Fc-specific cyanine-Cy5–conjugated (Fc-Cy5) monoclonal antibody, provided by Jackson ImmunoResearch Laboratories Inc., that recognizes the IgG1-CH2CH3 component of the CAR. We analyzed the cells using a FACScan Flow Cytometer (Becton Dickinson) equipped with a filter set for 4 fluorescence signals.
Chromium release assay.
We evaluated the cytotoxic specificity of T cell lines using a standard 4-hour 51Cr-release assay, as previously described (3).
Real-time Q-PCR.
We used Q-PCR to quantify the retrovirus integrants for both CAR.CD19ζ and CAR.CD19-28ζ transgenes in PBMCs collected before and at different time points after T cell infusions. After DNA extraction with the QIAamp DNA Blood Mini Kit (Qiagen), we amplified the DNA in triplicate with primers and TaqMan probes (Applied Biosystems) specific for each of the CAR.CD19ζ and CAR.CD19-28ζ transgenes, using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). The baseline range was set at cycles 6–15, with the threshold at 10 SDs above the baseline fluorescence. To generate DNA standards, we established serial dilution of DNA plasmids encoding each specific cassette.
Statistics.
Unless otherwise noted, data were summarized by mean and standard error of the mean. DNA measurements were logarithmically transformed to satisfy the normality assumption. The significance of differences between groups was determined by paired t test. A random effects model for repeated measures was also fitted to analyze the difference between CAR.CD19ζ and CAR.CD19-28ζ signals at each time point tested. A P value of less than 0.05 was considered statistically significant for all analyses.
Supplementary Material
Acknowledgments
This work was supported in part by Leukemia and Lymphoma Society Specialized Center of Research (SCOR; grant no. 7018) and by Department of Health and Human Services (grant 3P50CA126752).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(5):1822–1826. doi:10.1172/JCI46110.
References
- 1.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollard CM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110(8):2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pule MA, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21(2):215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9(10):704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116(7):1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Till BG, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kershaw MH, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 12.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 13.Imai C, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 14.Carpenito C, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12(5):933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson IC, et al. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol Immunol. 1997;34(16–17):1157–1165. doi: 10.1016/S0161-5890(97)00144-2. [DOI] [PubMed] [Google Scholar]
- 17.Cooper LJ, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101(4):1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 18.Brentjens RJ, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9(3):279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 19.Jensen MC, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16(9):1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudley ME, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 23.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3(3):221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 24.Cheson BD, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 25.Vera J, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108(12):3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.