Abstract
Oxidative stress is one of the earliest events in the pathogenesis of Alzheimer’s disease (AD) and can markedly exacerbate amyloid pathology. Modulation of antioxidant and anti-inflammatory pathways represents an important approach for AD therapy. Synthetic triterpenoids have been found to facilitate antioxidant response and reduce inflammation in several models. We investigated the effect of the triterpenoid, 2-Cyano-3,12-Dioxooleana-1,9-Dien-28-Oic acid-MethylAmide (CDDO-MA) in Tg19959 mice, which carry the human amyloid precursor protein with two mutations. These mice develop memory impairments and amyloid plaques as early as 2–3 months of age. CDDO-MA was provided with chow (800 mg/kg) from 1 to 4 months of age. CDDO-MA significantly improved spatial memory retention and reduced plaque burden, Aβ42 levels, microgliosis, and oxidative stress in Tg19959 mice.
Keywords: Alzheimer’s disease, amyloid, antioxidant, memory, oxidative stress, triterpenoid
Oxidative stress has a key role in the pathogenesis of neurodegenerative disorders such as Alzheimer’s disease (AD) (Lin and Beal 2006). Reactive oxygen species (ROS) can damage lipids, proteins, and nucleic acids, giving rise to pathological consequences (Butterfield and Lauderback 2002; Nunomura et al. 2007; Moreira et al. 2008). Oxidative stress is present at early stages in AD patients (Pratico et al. 2002; Keller et al. 2005) as well as in AD animal models (Pratico et al. 2001; Drake et al. 2003; Manczak et al. 2006). Oxidative stress can markedly affect amyloid deposition. In vitro lipid oxidation products promote the misfolding and fibrillogenesis of Aβ (Liu et al. 2008). In vivo deficiency of manganese superoxide dismutase in AD transgenic mice increases plaque deposition (Li et al. 2004) and phosphorylation of tau (Melov et al. 2007), and accelerates the onset of behavioral abnormalities (Esposito et al. 2006).
In the CNS, well-functioning antioxidant machinery is necessary to prevent ongoing oxidative stress. Therefore, induction of endogenous antioxidant pathways could represent a promising therapeutic approach for AD. Synthetic triterpenoids (TPs), derivatives of 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO), have been found to be potent inducers of nuclear factor-E2-related factor 2 (Nrf2)/antioxidant response element (ARE) signaling (Liby et al. 2005, 2007). In response to oxidative stress, the transcription factor, Nrf2, binds to promoters with AREs, inducing expression of a battery of genes that coordinate a protective response (Kang et al. 2005; Lee et al. 2005). TPs also suppress inflammatory stress (Dinkova-Kostova et al. 2005). Inflammation is an important factor as it may exacerbate oxidative stress; activated microglia may be a source of ROS in addition to neurons. All together, oxidative stress and inflammation may alter neuronal and glial function and accelerate the time course of disease.
In this study, we investigated the effect of the TP, CDDO-methylamide (MA), in Tg19959 mice, which carry the human amyloid precursor protein (APP) with two mutations (KM670/671NL and V717F). At approximately 3 months of age, these mice develop amyloid deposition starting in the neocortex, the amydgala, and the hippocampus (Chishti et al. 2001). At the same time, reactive microglia and then activated astrocytes surrounded the plaques (Dudal et al. 2004). The mice also exhibited learning and memory impairments in the Morris water maze (Chishti et al. 2001). Amyloid plaques, levels of Aβ40 and Aβ42, and behavioral deficits increased with age (Hyde et al. 2005). At 6–7 months of age, they showed working memory deficits in the six-arm radial water maze (Lovasic et al. 2005) and cholinergic dysfunction associated with neuronal damage and axonal loss (Bellucci et al. 2006). We now report that administration of the TP, CDDO-MA, from 1 to 4 months of age improves spatial memory retention and reduces plaque burden, levels of Aβ42, inflammation, and oxidative stress in Tg19959 mice.
Materials and methods
Animals and treatment
Tg19959 mice were obtained from Dr. George Carlson (McLaughlin Research Institute, Great Falls, MT, USA). They were originally produced by pronuclear injection of FVB × 129S6F1 embryos with a cosmid insert containing APP695 with two familial AD mutations (KM670/671NL and V717F), under the control of the hamster PrP promoter. Mice were backcrossed into and maintained on a hybrid B6/SJL background by crossing APP mutant males to B6/SJL females from Jackson Laboratory (Bar Harbor, ME, USA). Offsprings were genotyped by PCR of tail DNA. Mutant APP-positive mice were randomly assigned to receive either control chow (LabDiet 5002, Purina-Mills, Richmond, IN, USA) or chow containing TP (800 mg of CDDO-MA/kg of chow). The chow was pelleted by Purina-Mills. CDDO-MA was synthesized as previously described (Honda et al. 2002).
Tg19959 mice were fed with CDDO-MA or control chow beginning at 1 month of age for 3 months. Behavioral analyses were performed at 4 months of age, and brain histopathology and biochemistry were then assessed on the same animals. All experiments were approved by the Institutional Animal Care and Use Committee.
Behavioral studies
Exploration and locomotor activity were evaluated in an open-field made of opaque black plastic (45 cm × 45 cm; height: 20 cm). At the beginning of the test, each mouse was placed in one of the corners. The total distance traveled was recorded during 5 min per day and averaged over 3 days using a video tracking system (Ethovision 3.0; Noldus Technology, Attleborough, MA, USA).
Exploration and anxiety were measured in an elevated plus maze. The apparatus, made of opaque beige plastic, consisted of four arms (length: 30 cm; width: 5 cm; height: 39 cm) in a cross-shaped form and a central region (5 cm × 5 cm). Two of these arms were enclosed on three sides by walls (height: 15 cm) whereas the other arms were not. The two enclosed arms faced each other as did the two open arms. At the beginning of the test, mice were placed in the central region. Time spent in the open arms was measured during a single trial of 5 min.
Spatial learning and memory were assessed in the Morris water maze. An opaque basin (diameter: 84 cm; height of the wall: 51 cm) was filled with water (23°C). The water was opacified with non-toxic and odor-free white tempera paint.
In order to familiarize the animals with the task, each mouse was placed on the hidden platform (diameter: 10 cm) in the center of the basin for 20 s. Then, four consecutive practice trials were performed, where the animals were released 3–5 cm away from the platform and were allowed to find it within 20 s. For each trial, mice were forced to stay 20 s on the platform before being removed by the experimenter. This procedure was repeated the next day.
During the acquisition period, extra-maze visual cues such as light fixtures and wall posters were arranged in the room. The hidden platform was located in the middle of the northwest (NW) quadrant, 1 cm beneath water level. Each day, mice were placed next to and facing the wall of the basin in four different starting positions: north (N), east (E), south (S), and west (W), corresponding to four successive trials. Latencies and total distances before reaching the platform were recorded for 5 days with a video tracking system (Ethovision). After each trial, animals were placed in a plastic holding cage filled with paper towels to keep them dry and warm, with an inter-trial interval of 20 min. Whenever the mouse failed to reach the platform within the maximally allowed time of 60 s, it was placed on the platform by the experimenter for 5 s.
A probe trial was assessed 24 h after the acquisition period, removing the platform from the pool. The mice were released on the north side for a single trial of 60 s, during which the percentage of time spent in each quadrant was measured for the first 15 s. To ensure that any differences were not due to visual deficits, the visible platform version of the water maze was performed. In this cued version, a pole (13 cm) was added on the platform. Animals were tested during four trials where the platform was located in the southeast (SE) quadrant. The duration of a single trial was 60 s with an inter-trial interval of 20 min. Latencies and total distances before reaching the platform were recorded and averaged over the four trials.
Motor coordination was evaluated in an accelerated rotorod (Economex, Columbus Instruments, Columbus, OH, USA). The beam of the apparatus (diameter 4 cm, width 8 cm, height 38 cm) was made of ribbed plastic, and plates were flanked on either side to prevent any escape. Mice were placed on the top of the revolving beam for four successive trials per day, with 20 min inter-trial intervals. The rod accelerated gradually from 4 to 40 rpm over 2 min. Latencies before falling from the rod were recorded over 2 days and averaged.
Tissue harvest and preparation
After anesthesia by i.p. injection of sodium pentobarbital, mice were transcardially perfused with ice-cold 0.9% sodium chloride. Brains were removed and dissected on ice. The entire right hemibrain was snap-frozen in liquid nitrogen and used for measurement of CDDO-MA levels. The left hemibrain was sectioned coronally. Frontal sections were snap-frozen in liquid nitrogen and stored at −80°C for biochemical assays. Parietal sections were fixed in 4% paraformaldehyde in 1 mM phosphate buffer pH 7.4 for 24 h and stored in cryoprotectant solution (30% glycerol, 30% ethylene glycol in 20 mM phosphate buffer, pH 7.4) until further processing for histochemical studies.
Brain levels of CDDO-MA
Cyano-3,12-dioxooleana-1,9-dien-28-oic acid methylamide levels were measured by mass spectrometry. For extraction, 0.5 mL acetonitrile was added to 100–150 mg of tissue before homogenization on ice using a Tissue-Tearor (Fisher Scientific, Pittsburgh, PA, USA). Samples were then centrifuged at 20 000 g for 10 min. The acetonitrile extracts were diluted 1 : 1 with 20 mM ammonium acetate, pH 7.4, and centrifuged at 20 000 g for 5 min. The supernatants (100 μL injection volume) were loaded onto a Waters 2695 HPLC (Waters Corporation, Milford, MA, USA) and analyzed by reverse phase chromatography on a Waters XTerra MS C18 5 μm particle column using an 8 min gradient from 46% to 94% acetonitrile. CDDO-MA and its metabolites were detected using a single quadrupole mass spectrometer with electrospray ionization (Waters Micromass ZQ). Analysis was carried out using Waters MassLynx 4.1 software. Standard curves were generated by spiking control tissue extracts with at least six different concentrations of CDDO-MA. All calculated values were within the limits of the spiked standards.
Immunohistochemistry and quantitation
Regions analyzed included the retrosplenial/motor cortex and CA1/dentate region of the hippocampus. The retrosplenial/motor cortex was analyzed in five sections (350 μm apart) per mouse beginning at the level of bregma −1.06 to bregma −1.94. The CA1/dentate region was analyzed in five sections (350 μm apart) per mouse beginning at the level of bregma −1.34 to bregma −2.7. Sections were pre-treated with 50% formic acid for 5 min before labeling with anti-Aβ42 rabbit polyclonal antibody, AB5078P (1 : 1000, Chemicon, Temecula, CA, USA). Immunostaining with AB5078P was similar to that seen with the mouse monoclonal anti-Aβ antibody 6E10 (1 : 1000, Covance, Emerville, CA, USA) (Fig. S3). However, 6E10 also binds APP and β-C-terminal fragments in addition to Aβ, and not surprisingly produced more background staining. Because Aβ42 is considered to be the pathogenic species of interest, quantitation was performed with AB5078P. Sections were also labeled with anti-CD-40 rat monoclonal antibody against activated microglia (1 : 100, Serotec, Raleigh, NC, USA). Double staining for activated microglia and plaques was carried out using CD-40 and Congo Red. Double staining for microglial phagocytic activity and plaques was carried out using anti-CD-68 mouse monoclonal antibody ED-1 (1 : 500, Serotec, Oxford, UK) and Congo Red. Immunolabeling was detected by the avidin–biotin complex peroxidase method and visualized with diaminobenzidine incubation for 5 min (Vector Laboratories, Burlingame, CA, USA).
Sections were viewed with the 10× objective on a Nikon Eclipse E600 microscope (Nikon Instruments, Melville, NY, USA), and digital images were captured using Stereo Investigator 4.35 (Microbrightfield, Burlington, VT, USA). Quantitative analysis was performed using NIH Image 1.63 (National Institute of Health, Bethesda, MD, USA). For Aβ42 deposits, the percent area occupied by plaques and the plaque count (number per 0.75 mm2) were calculated. For CD40 immunoreactivity, the percent area occupied by microglial cells was measured.
ELISA for levels of Aβ42 and Aβ40
Aβ42 and Aβ40 levels were analyzed from brain tissues previously homogenized in 6% sodium dodecyl sulfate (SDS). Aβ42 and Aβ40 ELISAs were performed using commercial kits (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions.
Western blotting
Snap-frozen brain sections were homogenized by sonication in 6% SDS containing protease inhibitor cocktail (Complete Protease Inhibitor Cocktail tablet, Roche Diagnostics, Mannheim, Germany) added fresh. Protein concentration was measured (DC Protein Measurement Kit, Bio-Rad Laboratories, Hercules, CA, USA). Homogenates were electrophoresed through 4–12% Tri-Bis NuPage or 10–20% Tris–Tricine polyacrylamide gels (Invitrogen). After transfer to polyvinylidene difluoride, membranes were blocked in 5% non-fat dry milk/phosphate buffered saline and 0.05% Tween 20 (PBST) and exposed overnight to primary antibody at 4 °C. Horseradish peroxidase-conjugated secondary antibody binding was visualized with enhanced chemiluminescence. α-Tubulin was used as a loading control.
Primary antibodies and concentrations were rabbit polyclonal anti-heme oxygenase-1 (HO-1) SPA-895 (1 : 500, Stressgen, Ann Arbor, MI, USA); goat polyclonal anti-NAD(P)H:quinone oxidoreductase 1 (1 : 1000, Novus Biologicals, Littleton, CO, USA); sheep polyclonal anti-Cu/Zn superoxide dismutase (1 : 1000, Calbiochem, San Diego, CA, USA); rabbit polyclonal anti-catalase (1 : 1000, Abcam, Cambridge, MA, USA); rabbit polyclonal anti-glutathione reductase (1 : 500, Abcam); rabbit polyclonal anti-glutathione peroxidase 1 (1 : 500, Abcam); rabbit polyclonal anti-APP 369 (1 : 1000, gift from S. Gandy, Thomas Jefferson University, Philadelphia, PA, USA); mouse monoclonal anti-Aβ 6E10 (1 : 1000, Covance); rabbit polyclonal anti-insulin degrading enzyme (IDE) (1 : 500, Calbiochem); rabbit polyclonal anti-neprilysin CD10 (1 : 500, Santa Cruz Biotechnology, Santa Cruz, CA, USA); mouse monoclonal anti-α-tubulin (1 : 10000, Sigma, St. Louis, MO, USA).
Films were scanned at 600 dpi, and densitometry was quantified with Scion Image 4.0.2 (Scion Corp., Frederick, MD, USA). Ratios were calculated using densitometric values for the protein of interest divided by densitometric values for α-tubulin.
Measurement of oxidized proteins
Brain sections were lysed in 6% SDS containing protease inhibitor, and protein concentrations were determined (DC Protein Measurement Kit, Bio-Rad Laboratories). Protein carbonyl levels were measured using the Oxyblot Protein Oxidation Detection Kit (Chemicon) according to the manufacturer’s protocol, with the following modifications: 5% non-fat dry milk/PBST was used as blocking solution and antibody diluent; the membrane was blocked for 1 h; and the primary antibody incubation was overnight. A 20-μg aliquot of protein was analyzed in each lane. Bands were visualized by enhanced chemiluminescence. Films were scanned at 600 dpi, and Scion Image 4.0.2 (Scion Corp.) was used for densitometry. Quantification was expressed as the ratio of carbonyls to tubulin. Membranes were stained with 0.1% Ponceau S solution to verify equal amount of proteins.
To determine the direct effect of CDDO-MA on protein oxidation in vitro, bovine serum albumin (BSA, 200 μg) was oxidized by incubation for 2.5 h at 37°C in 50 μL of 10% dimethylsulfoxide, 100 lM FeCl3, 25 mM ascorbate, 25 mM HEPES (pH 7.2), with 0, 20, 40, or 100 nM CDDO-MA. Five μL of each of these reaction mixtures (20 μg BSA) was then subjected to protein carbonyl measurement as described above.
Analysis of Aβ oligomers
For immunohistochemistry, sections were treated with 90% formic acid for 5 min prior to labeling with anti-oligomer antibody A11 (1 : 500; Invitrogen) (Kayed et al. 2003). Immunolabeling was detected by the avidin–biotin complex peroxidase method and visualized with diaminobenzidine incubation for 5 min (Vector Laboratories). Quantitative analysis of the percent area occupied by A11-immunoreactivity and the A11-immunoreactive patch count (number per 0.75 mm2) was carried out as previously described for Aβ immunohistochemistry and quantitation.
For western blot, 30 μg of protein from 6% SDS brain extracts were loaded into 10–20% Tris–Tricine polyacrylamide gels (Invitrogen). Synthetic Aβ42 oligomers prepared as previously described (Barghorn et al. 2005) were used as positive control. After transfer to polyvinylidene difluoride, membranes were blocked in 5% non-fat dry milk/PBST and exposed overnight at 4°C to the primary antibody 6E10 (1 : 1000). Horseradish peroxidase-conjugated secondary antibody binding was visualized with enhanced chemiluminescence. a-Tubulin (1 : 10000, Sigma) was used as a loading control for the western blots. Films were scanned at 600 dpi and densitometry was performed with Scion Image 4.0.2 (Scion Corp.).
Statistical analysis
anova was used to compare three groups: Tg19959 fed with CDDO-MA, Tg19959 fed with control chow, and wild-type mice fed with control chow. Post hoc Student–Neuman–Keuls (SNK) tests were used for further analyses between groups. When only two groups were involved, two-tailed t-tests were used to compare CDDO-MA-fed and control-fed Tg19959 mice (Statview 5.0.1, SAS Institute Inc., Cary, NC, USA).
Results
Detection of CDDO-MA in mouse brains
Detection of CDDO-MA in mouse brains was performed by mass spectrometry. Figure S1a shows chromatograms of CDDO-MA spiked in mouse brain homogenates at different concentrations, used to form a standard curve.
In our experiment, CDDO-MA provided via chow was present in brains of treated Tg19959 mice at 39 ± 11 nmol/kg. Thus, CDDO-MA crossed the blood brain barrier.
Potencies of CDDO-MA in cultured cells were previously reported by Dinkova-Kostova et al. 2005. CDDO-MA (called TP224 in their paper) acted at nanomolar concentrations to double the activity of NQO1 and to inhibit inducible nitric oxide synthase (NOS) by 50% (Dinkova-Kostova et al. 2005).
To ensure that CDDO-MA diet did not change food intake, body weights were recorded. No significant differences were found between Tg19959 mice fed CDDO-MA chow and Tg19959 mice fed control chow (Fig. S1b and c).
CDDO-MA improved spatial memory retention in Tg19959 mice
Mice were given 5 days of training to locate the position of a hidden platform in the Morris water maze. Latency and total distance before reaching the platform were recorded each day (Fig. S2). During this acquisition period, Tg19959 mice fed control chow were impaired compared with wild-type littermates. Tg19959 mice fed with control chow had longer latencies and distances (SNK, p < 0.05) than wild-type mice fed with control chow. Three month administration of CDDO-MA did not affect performance of the transgenic mice during the acquisition phase.
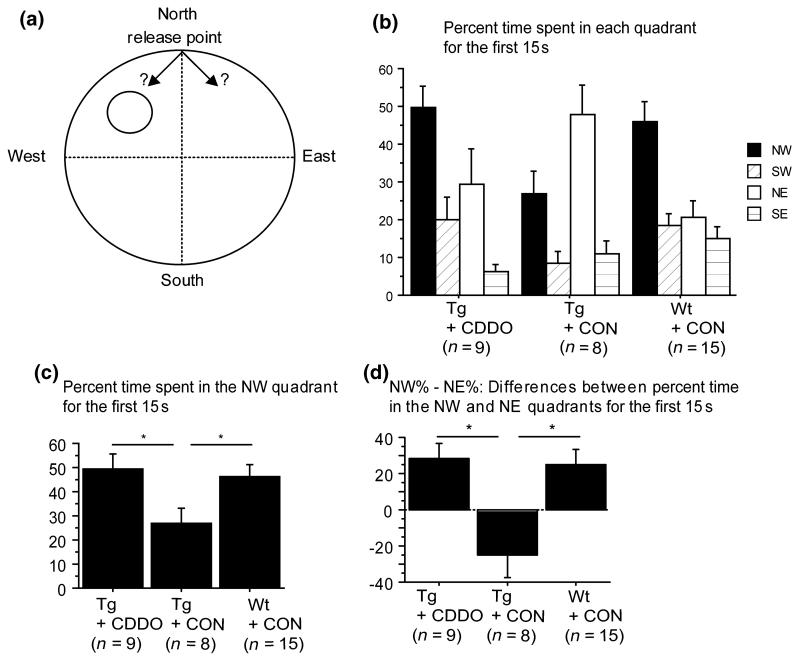
Spatial memory was assessed by a probe trial where the platform was removed from the maze and percent time spent in each quadrant was analyzed for the first 15 s of the trial (Fig. 1b). Because mice were released at the northern pole (N), the key distinction indicating memory of the platform was whether they turned left toward the NW (target) quadrant or right toward the NE quadrant (Fig. 1a). Preference for the target quadrant was therefore measured by examining the percent time spent in the NW quadrant (Fig. 1c) and the difference (d = NW% − NE%) in percent time spent in the NW versus the NE quadrants (Fig. 1d). Tg19959 mice fed control chow had memory impairment, compared with wild-type mice fed control chow, as shown by the percent time spent in the NW quadrant (Fig. 1c; SNK, p < 0.05) and the difference d (Fig. 1d; SNK, p < 0.05). In contrast, Tg19959 mice fed CDDO-MA significantly preferred the NW quadrant, compared with control fed Tg19959 mice, as shown by the percent time spent in the NW quadrant (Fig. 1c; SNK, p < 0.05) and the difference d (Fig. 1d; SNK, p < 0.05). Thus, CDDO-MA administration for 3 months improved spatial memory retention during the probe trial.
Fig. 1.
Cyano-3,12-dioxooleana-1,9-dien-2-8-oic acid methylamide (CDDO-MA) improved spatial memory retention in Tg19959 mice in the Morris water maze. (a) Scheme of strategies to search for the target quadrant during the probe trial. (b) Percent of the first 15 s spent in each quadrant. (c) Percent of the first 15 s spent in the NW (target) quadrant. (d) Differences between the percent time spent in the NW and NE quadrants during the first 15 s (means ± SE). Tg19959 mice fed CDDO-MA preferred the NW (target) quadrant, whereas Tg19959 mice fed with control chow did not (c and d; *p < 0.05). CON, control; NW, northwest; SW, southwest; NE, northeast; SE, southeast; Wt, wild type.
During the visible platform task (Table S1a), there were no significant differences in latency or distance traveled between groups. No impairments in swim speed were found during the probe trial (Table S1b).
Locomotor activity, exploration, and motor coordination were also analyzed (Table S2). Compared with wild-type, Tg19959 mice exhibited increased distance traveled in the open field (SNK, p < 0.05). CDDO-MA did not alter the motor behavior of Tg19959 mice.
CDDO-MA reduced plaque burden and microglia in the hippocampus of Tg19959 mice
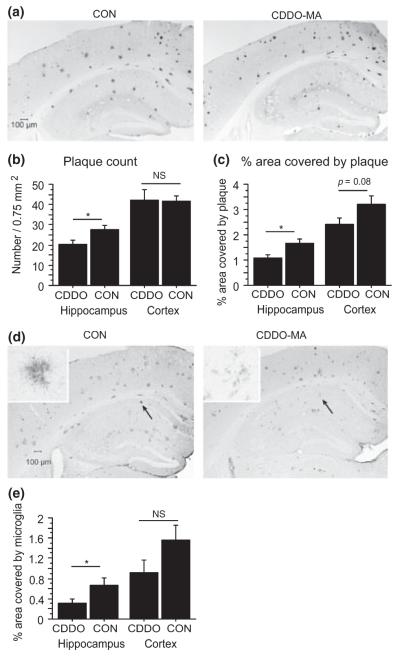
In Tg19959 brains, plaque burden was analyzed after staining with Aβ42 antibody AB5078P (Fig. S3). CDDO-MA reduced significantly hippocampal amyloid deposits (Fig. 2a) in Tg19959 mice, measured by plaque count (Fig. 2b; t-test p = 0.0211) and percent area occupied by plaque (Fig. 2c; t-test p = 0.0072). In cortex, CDDO-MA did not significantly reduce number of plaques (t-test p = 0.9352), though there was a trend for CDDO-MA to reduce percent area occupied by plaque (Fig. 2c; t-test, p = 0.08).
Fig. 2.
Cyano-3,12-dioxooleana-1,9-dien-28-oic acid methylamide (CDDO-MA) decreased plaque burden and microglia in the hippocampus of Tg19959 mice. (a) Representative photograph of plaques detected by Aβ42 antibody, AB5078P. (b) Plaque count and (c) percent area occupied by plaque in Tg19959 mice fed CDDO-MA or control (CON) chow (means ± SE). In hippocampus, CDDO-MA reduced significantly the plaque count (b; n = 6 per group; *p = 0.0211) and the percent area occupied by plaque (c; n = 6 per group; *p = 0.0072). (d) Representative photographs of microglial staining detected by CD40 antibody with high magnification views in the insets. (e) Area covered by microglia (means ± SE). In hippocampus, CDDO-MA reduced the area covered by microglia (n = 6 per group; *p = 0.0437). ns, not significant.
In addition, CDDO-MA significantly reduced inflammation in the hippocampus (t-test, p = 0.0437) but not in the cortex (t-test, p = 0.1236), measured by the percent area of microglial staining (Fig. 2d and e). To further analyze the relationship between microglia and plaques, we double-labeled sections of control fed and CDDO-MA fed mice with CD-40 antibody and Congo Red (Fig. S4a). With CDDO-MA, there was an overall reduction in staining for activated microglia but a local increase in staining in the immediate vicinity of plaques. We also assessed the phagocytic activity of microglia around plaques by double labeling with ED-1 antibody and Congo Red (Fig. S4b). With CDDO-MA, there was an increase in ED-1 staining in the immediate vicinity of plaques.
CDDO-MA decreased levels of SDS-soluble Aβ42 but did not affect APP processing
Levels of 6% SDS-soluble Aβ42 and Aβ40 were measured by ELISA (Fig. 3). Tg19959 mice fed CDDO-MA had decreased levels of Aβ42 compared with Tg19959 mice fed control chow (Fig. 3a; t-test, p = 0.0453). Aβ40 levels were not significantly changed by CDDO-MA treatment in Tg19959 mice (Fig. 3b; t-test, p = 0.1480).
Fig. 3.
Cyano-3,12-dioxooleana-1,9-dien-28-oic acid methylamide (CDDO-MA) decreased levels of sodium dodecyl sulfate-soluble Aβ42 but did not affect amyloid precursor protein (APP) processing. Levels of Aβ42 (a) and Aβ40 (b) by ELISA. Administration of CDDO-MA reduced significantly levels of Aβ42 in Tg19959 mice (a; *p = 0.0453). Levels of full length APP (c), α-CTFs (d), β-CTFs (e), neprilysin (f) and insulin degrading enzyme (g) in Tg19959 mice were measured by western blots. Data were expressed as ratios to tubulin (means ± SE). No differences were found between Tg19959 mice fed CDDO-MA and Tg19959 mice fed control (CON) chow. ns, not significant; CTF, carboxyterminal fragment of APP.
Because Aβ42 was reduced, we assessed whether CDDO-MA affected APP processing or Aβ degrading enzymes. Brain levels of full length APP (Fig. 3c), α-carboxyterminal fragments of APP (CTFs) (Fig. 3d), and β-CTFs (Fig. 3e) in Tg19959 mice were not changed with CDDO-MA. In addition, levels of neprilysin (Fig. 3f) and IDE (Fig. 3g) were not changed with CDDO-MA.
Effects of CDDO-MA on SDS-soluble Aβ oligomers in Tg19959 mice
Immunolabeling of Aβ oligomers was performed by using antibody A11 (Kayed et al. 2003). No significant differences were found between Tg19959 mice fed control versus CDDO-MA chow, as measured by density of A11 immunoreactive patches (Fig. 4b; t-test, p = 0.3372 for hippocampus, p = 0.6703 for cortex) and by percent area occupied by A11 immunoreactivity (Fig. 4c; t-test, p = 0.3265 for hippocampus, p = 0.5329 for cortex).
Fig. 4.
Effects of cyano-3,12-dioxooleana-1,9-dien-28-oic acid methylamide (CDDO-MA) on Aβ oligomers in Tg19959 mice. (a) Representative photograph of immunoreactivity detected by anti-oligomer antibody A11. (b,c) Count of A11-immunoreactive patches and area occupied by A11-immunoreactivity in Tg19959 mice fed CDDO-MA or control (CON) chow (means ± SE). No significant differences were found between Tg19959 mice fed control versus CDDO-MA chow (n = 5 per group). (d) Aβ oligomers by western blot with 6E10. Densitometric ratios of trimers (~12 kDa; the 14 kDa represents β-CTFs, as confirmed by 369 antibody) to tubulin (means ± SE). No differences in Aβ trimers were found after 3-month administration of CDDO-MA. CTF, carboxyterminal fragment of APP; ns, not significant.
Western blot with 6E10 antibody was also used to detect Aβ oligomers in Tg19959 mice. In 6% SDS extract, only trimers (~12 kDa) were identified and quantified. A 50-kD band consistent with dodecamers was also seen but was extremely faint even with prolonged exposure. No significant differences in trimers were found between Tg19959 mice fed CDDO-MA versus control chow (Fig. 4d; t-test, p = 0.6136).
CDDO-MA decreased oxidative stress in Tg19959 mice
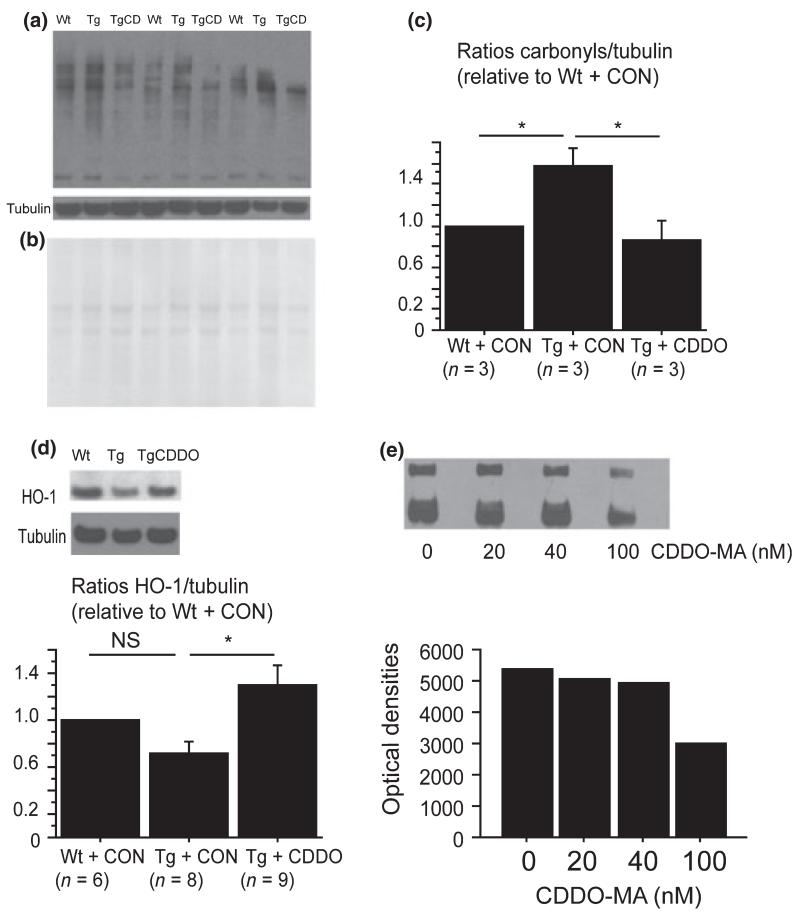
Oxidative stress is an important component in AD pathogenesis. To analyze the effect of CDDO-MA on oxidative stress, we examined levels of protein carbonyls, a marker of protein oxidation (Fig. 5a-c). Compared with wild-type mice, Tg19959 mice fed control chow had increased protein carbonyl levels measured by western blots (SNK, p < 0.05). After 3 month of CDDO-MA treatment, levels of protein carbonyls were significantly reduced in Tg19959 mice compared with those fed control chow (SNK, p < 0.05).
Fig. 5.
Cyano-3,12-dioxooleana-1,9-dien-2-8-oic acid methylamide (CDDO-MA) decreased oxidative stress in Tg19959 mice. (a) Protein carbonyls by western blot. (b) Ponceau S staining for protein levels. (c) Densitometric ratios of protein carbonyls to tubulin. Tg19959 mice had increased protein carbonyl levels compared with wild-type (Wt) mice (*p < 0.05). CDDO-MA administration for 3 months reduced protein carbonyl levels in Tg19959 mice (*p < 0.05). (d) Heme oxygenase-1 (HO-1) protein levels by western blot normalized to tubulin in Wt mice, Tg19959 mice fed control (CON) chow (Tg), and Tg19959 mice fed CDDO-MA (TgCDDO). Tg19959 mice fed CDDO-MA had increased HO-1 compared with Tg19959 fed CON chow (*p < 0.05). (e) Protein carbonyls by western blots of bovine serum albumin oxidized in the presence of 0, 20, 40, or 100 nM CDDO-MA. ns, not significant.
Because synthetic TPs have been shown to up-regulate endogenous antioxidant responses in vitro (Liby et al. 2005, 2007), we analyzed the effect of CDDO-MA on protein levels of several antioxidant enzymes – HO-1, NAD(P)H: quinone oxidoreductase 1, Cu/Zn superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase 1. Surprisingly, CDDO-MA administration did not affect levels of any of these enzymes (data not shown), except for HO-1 (Fig. 5d). CDDO-MA augmented the level of HO-1 in Tg19959 mice (Fig. 5d; SNK, p < 0.05).
Considering that CDDO-MA reduced brain protein carbonyl levels apparently without up-regulating a coordinated antioxidant program, we determined whether CDDO-MA could have direct antioxidant effects. BSA was oxidized in vitro by exposure to iron/ascorbate in the presence of 0, 20, 40, and 100 nM CDDO-MA. Levels of protein carbonyls were then measured. CDDO-MA decreased protein carbonyl levels at 40 nM and more markedly at 100 nM (Fig. 5e). This finding suggests that CDDO-MA could directly act as an antioxidant in Tg19959 mice.
Discussion
There is a large body of evidence demonstrating the involvement of oxidative stress in AD pathogenesis. Oxidative stress can exacerbate amyloid pathology (Li et al. 2004; Liu et al. 2008) and accelerate the onset of behavioral abnormalities (Esposito et al. 2006) in APP transgenic mice. Conversely, decreasing oxidative stress in APP transgenic mice through depletion of Nox2, the catalytic subunit of NAPDH oxidase, rescued behavioral and neurovascular function (Park et al. 2005, 2008).
Inflammation by glial activation is also an important factor involved in AD pathogenesis. Microglia can play a role in the clearance of amyloid plaques by phagocytosis (Jucker and Heppner 2008; Town et al. 2008), but can also be a source of ROS production through cytokine activation. Several therapeutic approaches have been taken to counteract cellular damage due to either oxidative stress or inflammation in AD models.
Recently, synthetic TPs, derivatives of CDDO, have been found in vitro and in vivo to be potent inducers of cytoprotective genes that increase resistance to oxidative stress (Kang et al. 2005; Lee et al. 2005; Liby et al. 2005, 2007). In addition, TPs markedly inhibited inflammatory stress induced by inducible NOS and cyclooxygenase 2 (Dinkova-Kostova et al. 2005). For these reasons, we examined whether the TP, CDDO-MA, could improve the AD-like phenotype of Tg19959 mice. We administered CDDO-MA to Tg19959 mice for 3 months and assessed its effects at 4 months of age. Compared with the decade-long course of human AD, this is a short-term study and focused early in disease pathogenesis.
In our study, CDDO-MA given for 3 months improved significantly, retention in the probe trial, even though it did not change learning during the acquisition phase. In Tg2576 mice, pomegranate juice improved learning but not retention (Hartman et al. 2006), thus also exhibiting differential effects but complementary to those of CDDO-MA. These and other studies (Daumas et al. 2008) indicate that learning and memory are related but distinct processes, both of which are impaired in AD.
Cyano-3,12-dioxooleana-1,9-dien-28-oic acid methylamide given for 3 months also reduced amyloid plaques and SDS-soluble Aβ42 levels in Tg19959 mice. These effects were not due to a decrease in APP production and processing, as shown by the lack of change in APP and CTF levels. The Aβ degrading enzymes neprilysin and IDE were also unchanged. CDDO-MA did not affect levels of oligomers detected by A11 or 6E10 antibodies. It should be noted that the detection of Aβ oligomers is complex and highly dependent on technical factors including the antibodies used. The A11 antibody is commonly used to assay Aβ oligomers, though it also recognizes oligomers of other proteins (Kayed et al. 2003; Yoshiike et al. 2008). We therefore complemented the A11 immunohistochemistry with western blotting using 6E10, which is specific for species containing the human Aβ sequence. Within the limitations of these techniques, the results were consistent, suggesting that 3-month administration of CDDO-MA did not affect oligomerization of Aβ in our model. Thus, the protective effects of CDDO-MA are likely to be exerted downstream of Aβ oligomers, perhaps reducing toxicity caused by inflammation or oxidative stress, or increasing phagocytosis of more aggregated species.
Importantly, CDDO-MA did reduce overall microgliosis and oxidative stress in Tg19959 mice. A recent study of CDDO-methyl ester reported the same effects in vitro (Tran et al. 2008). In cells treated with lipopolysaccharide and Aβ42, CDDO-methyl ester decreased microglial activation, cytokine levels, and reactive oxygen species, and enhanced phagocytic activity of microglia. In our CDDO-MA-treated mice, activation of microglia was reduced overall, but was increased in the immediate vicinity of plaques. A similar observation was reported in Tg2576 mice treated with curcumin, in which reactive microglia was decreased overall but increased in a ring around plaques (Lim et al. 2001). Thus, one possible mechanism for the protective effect of CDDO-MA could be decreased inflammation and increased microglial phagocytosis of Aβ and amyloid plaque, as supported by increased ED-1 staining around plaques.
A second possible mechanism to explain the beneficial effects of 3-month administration of CDDO-MA could involve its antioxidant properties. CDDO-MA directly inhibited protein oxidation in vitro and reduced protein carbonyl levels in Tg19959 mice. CDDO-MA has been shown to up-regulate Nrf2/ARE-regulated antioxidant enzymes in cultured cells in a time-dependent fashion. In our mice, we did not detect coordinated up-regulation of a battery of Nrf2/ARE genes, although we cannot completely rule out such up-regulation for technical reasons. For example, the mice were fed ad libitum and may not have been killed at an optimal time point after feeding. Moreover, there was up-regulation of HO-1. HO-1 is found in both neurons and glia and is induced by Aβ, oxidative stress, and pro-inflammatory stimuli. HO-1 catabolizes heme into biliverdin, Fe2+, and carbon monoxide (Schipper 2004). Biliverdin and bilirubin are free radical scavengers (Stocker 2004). Carbon monoxide is an activator of guanylate cyclase like nitric oxide (Verma et al. 1993), and recent data suggest that nitric oxide might be beneficial in AD – depletion of NOS exacerbated AD-like pathology and behavioral impairments in APP transgenic mice (Colton et al. 2008; Wilcock et al. 2008).
Three-month administration of CDDO-MA improved spatial memory retention and reduced Aβ levels and plaque deposition early in the disease course, probably by reducing inflammation, enhancing phagocytosis of Aβ and plaque, and decreasing oxidative stress. Thus, CDDO-MA and other more potent up-regulators of endogenous antioxidant and anti-inflammatory programs represent promising avenues of investigation for AD therapy.
Supplementary Material
Figure S1 Detection of Cyano-3,12-dioxooleana-1,9-dien-28-oic acid methylamide (CDDO-MA) in mouse brains and effect on body weight.
Figure S2 Cyano-3,12-dioxooleana-1,9-dien-28-oic acid methylamide (CDDO-MA) did not affect spatial learning in Tg19959 mice during the Morris water maze.
Figure S3 Staining of amyloid plaques in Tg19959 mice.
Figure S4 Relationship between amyloid plaques and activated microglia.
Table S1 Visible platform task and swim speed in the Morris water maze.
Table S2 Motor activity.
Acknowledgments
We thank Reata Pharmaceuticals (Dallas, TX, USA) for providing the TP, CDDO-MA, and Dr. Paul Zsabo for his help in the analysis of oligomers. This work was supported by National Institute of Health (NIH) grant AG20729.
Abbreviations used:
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- ARE
antioxidant response element
- BSA
bovine serum albumin
- CDDO
cyano-3,12-dioxooleana-1,9-dien-28-oic acid
- CDDO-MA
CDDO-methylamide
- CTF
carboxyterminal fragment of APP
- HO-1
heme oxygenase-1
- IDE
insulin degrading enzyme
- NOS
nitric oxide synthase
- Nrf2
nuclear factor-E2-related factor 2
- NW
northwest
- PBST
phosphate buffered saline and 0.05% Tween 20
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulfate
- SNK
Student–Neuman–Keuls
- TPs
triterpenoids
Footnotes
Disclosure statements
Michael Sporn receives grant support from Reata Pharmaceuticals. None of the other authors have actual or potential conflicts of interest for this study. All procedures concerning the use of animals were approved by the Weill Cornell Medical College Institutional Animal and Care Use Committee.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Barghorn S, Nimmrich V, Striebinger A, et al. Globular amyloid beta-peptide oligomer – a homogenous and stable neuropathological protein in Alzheimer’s disease. J. Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Bellucci A, Luccarini I, Scali C, Prosperi C, Giovannini MG, Pepeu G, Casamenti F. Cholinergic dysfunction, neuronal damage and axonal loss in TgCRND8 mice. Neurobiol. Dis. 2006;23:260–272. doi: 10.1016/j.nbd.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Biol. Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM, Wink DA, Davis J, Van Nostrand WE, Vitek MP. The effects of NOS2 gene deletion on mice expressing mutated human AbetaPP. J. Alzheimers Dis. 2008;15:571–587. doi: 10.3233/jad-2008-15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas S, Sandin J, Chen KS, Kobayashi D, Tulloch J, Martin SJ, Games D, Morris RG. Faster forgetting contributes to impaired spatial memory in the PDAPP mouse: deficit in memory retrieval associated with increased sensitivity to interference? Learn. Mem. 2008;15:625–632. doi: 10.1101/lm.990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Liby KT, Stephenson KK, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc. Natl Acad. Sci. USA. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging. 2003;24:415–420. doi: 10.1016/s0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- Dudal S, Krzywkowski P, Paquette J, Morissette C, Lacombe D, Tremblay P, Gervais F. Inflammation occurs early during the Abeta deposition process in TgCRND8 mice. Neurobiol. Aging. 2004;25:861–871. doi: 10.1016/j.neurobiolaging.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, Puolivali J, Scearce-Levie K, Masliah E, Mucke L. Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J. Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, Finn MB, Holtzman DM. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2006;24:506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Honda T, Honda Y, Favaloro FG, Jr, Gribble GW, Suh N, Place AE, Rendi MH, Sporn MB. A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg. Med. Chem. Lett. 2002;12:1027–1030. doi: 10.1016/s0960-894x(02)00105-1. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Kazdoba TM, Grilli M, et al. Age-progressing cognitive impairments and neuropathology in transgenic CRND8 mice. Behav. Brain Res. 2005;160:344–355. doi: 10.1016/j.bbr.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Jucker M, Heppner FL. Cerebral and peripheral amyloid phagocytes – an old liaison with a new twist. Neuron. 2008;59:8–10. doi: 10.1016/j.neuron.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid. Redox Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- Li F, Calingasan NY, Yu F, et al. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J. Neurochem. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Liby K, Hock T, Yore MM, et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu L, Komatsu H, Murray IV, Axelsen PH. Promotion of amyloid beta protein misfolding and fibrillogenesis by a lipid oxidation product. J. Mol. Biol. 2008;377:1236–1250. doi: 10.1016/j.jmb.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Lovasic L, Bauschke H, Janus C. Working memory impairment in a transgenic amyloid precursor protein TgCRND8 mouse model of Alzheimer’s disease. Genes Brain Behav. 2005;4:197–208. doi: 10.1111/j.1601-183X.2004.00104.x. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Melov S, Adlard PA, Morten K, et al. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G. Nucleic acid oxidation in Alzheimer disease. Free Radic. Biol. Med. 2008;44:1493–1505. doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Moreira PI, Takeda A, Smith MA, Perry G. Oxidative RNA damage and neurodegeneration. Curr. Med. Chem. 2007;14:2968–2975. doi: 10.2174/092986707782794078. [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J. Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice over-expressing the amyloid precursor protein. Proc. Natl Acad. Sci. USA. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch. Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Heme oxygenase expression in human central nervous system disorders. Free Radic. Biol. Med. 2004;37:1995–2011. doi: 10.1016/j.freeradbiomed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Stocker R. Antioxidant activities of bile pigments. Antioxid. Redox Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TA, McCoy MK, Sporn MB, Tansey MG. The synthetic triterpenoid CDDO-methyl ester modulates microglial activities, inhibits TNF production, and provides dopaminergic neuroprotection. J. Neuroinflammation. 2008;5:14. [Google Scholar]
- Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, Vitek MP, Colton CA. Progression of amyloid pathology to Alzheimer’s disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J. Neurosci. 2008;28:1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiike Y, Minai R, Matsuo Y, Chen YR, Kimura T, Takashima A. Amyloid oligomer conformation in a group of natively folded proteins. PLoS ONE. 2008;3:e3235. doi: 10.1371/journal.pone.0003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Detection of Cyano-3,12-dioxooleana-1,9-dien-28-oic acid methylamide (CDDO-MA) in mouse brains and effect on body weight.
Figure S2 Cyano-3,12-dioxooleana-1,9-dien-28-oic acid methylamide (CDDO-MA) did not affect spatial learning in Tg19959 mice during the Morris water maze.
Figure S3 Staining of amyloid plaques in Tg19959 mice.
Figure S4 Relationship between amyloid plaques and activated microglia.
Table S1 Visible platform task and swim speed in the Morris water maze.
Table S2 Motor activity.