SUMMARY
The cell-intrinsic mechanisms guiding naïve CD8+ T cells for clonal expansion and memory generation via Homeostatic Proliferation (HP) are unclear. Herein, we have shown that HP of naïve CD8+ T cells requires IL-7, but not IL-15 induced mTOR kinase activation. HP-induced mTOR enhances transcription factor T-bet for functional maturation and CD122 expression, which sensitizes for an IL-15 dependent memory transition by favoring transcription factor Eomesodermin over T-bet. Inhibition of mTOR blocks T-bet and CD122 expression but preserves memory in an IL-15-independent manner by promoting Eomesodermin expression. The ability of rapamycin to augment HP-induced memory was cell-intrinsic as silencing mTOR in CD8+ T cells generated identical outcomes. Strikingly, HP-induced CD8+ T cell memory generated by IL-15 dependent or independent mechanisms demonstrated identical tumor efficacy. These results indicate a central role for mTOR in HP-induced CD8+ T cell responses and demonstrate the importance for CD8+ memory in HP-induced tumor efficacy.
Key words/phrases: IL-7, mTOR, rapamycin, T-bet, Eomesodermin, CD8+ T cell, homeostatic proliferation, memory, IL-15
INTRODUCTION
The stimulation of naïve CD8+ T cells with antigen, co-stimulation and cytokines induce vigorous proliferation and associated differentiation that can give rise to various effector and/or memory phenotypes (Ahmed and Gray, 1996). Alternatively, naïve CD8+ T cells can undergo proliferation in the absence of antigen, in response to space (self-antigen-MHC, cytokines like interleukin-7 (IL-7) and/or IL-15) secondary to lymphopenia (genetic deficiency, infection, irradiation or unprimed neonatal animals) (Ernst et al., 1999; Goldrath and Bevan, 1999; Kieper and Jameson, 1999; Le Campion et al., 2002). The antigen independent proliferation exhibited by naive CD8+ T cells has been termed as homeostatic proliferation (HP) and it plays an important role in maintaining normal CD8+ T cell numbers (Cho et al., 2000; Ernst et al., 1999; Goldrath and Bevan, 1999; Kieper and Jameson, 1999; Murali-Krishna et al., 1999). Additionally, lymphopenia-induced proliferation of naïve CD8+ T cells induce functional maturation resulting in sub-optimal effector functions (IFN-γ and/or cytolytic T lymphocyte (CTL) activity) and transition to memory-like cells (Goldrath et al., 2000; Hamilton et al., 2006; Surh and Sprent, 2008).
The critical role of extrinsic cues like self-antigen-MHC Class I and IL-7 in HP of CD8+ T cells has been demonstrated (Ernst et al., 1999; Goldrath and Bevan, 1999; Tan et al., 2001). It is envisioned that weak but frequent T cell receptor (TCR) interactions with abundant self-peptide-MHC class I molecules and IL-7 under conditions of lymphopenia induces cell cycling and HP of naïve CD8+ T cells (Li et al., 2006; Schluns et al., 2000; Tan et al., 2001). Although, the other common γ-chain (γc) cytokine; IL-15, has also been implicated in HP of naive CD8+ T cells, it was unable to compensate for IL-7 deficiency in HP of naive CD8+ T cells (Tan et al., 2001). However, IL-15 is important for sustained CD8+ T cell proliferation and accumulation in a lymphopenic setting (Sandau et al., 2007) and the reduction in HP-induced CD8+ T cells expressing CD62L and CD44hi phenotype in IL-15Rα deficient mice indicates the need for IL-15 in promoting CD8+ T cell memory but not initiation of HP (Kennedy et al., 2000; Lodolce et al., 1998; Ramsey et al., 2008). Together, the γc cytokines; IL-7 and IL-15, act in concert to maintain CD8+ T cell homeostasis, but their precise mechanisms of action and collaboration are not completely understood.
The binding of IL-7 to IL-7Rα on naïve CD8+ T cells can initiate a cascade of signals that lead to phosphorylation of signal transducers and activators of transcription 5 (STAT5) and the kinase cascades involving phosphatidylinositol 3-kinase (PI3K)-protein kinase B (PKB, also called Akt) to prevent naïve T cell atrophy via mammalian target of rapamycin (mTOR) and suggest a role for IL-7 in CD8+ T cell survival and growth during lymphopenia induced HP (Plas et al., 2001; Rathmell et al., 2001; Wofford et al., 2008). The fact that activated CD8+ T cells up-regulate CD122 (IL-2 receptor β chain), the signaling component of the IL-15R complex provides an explanation for the reliance on IL-15 for memory CD8+ T cell generation (Intlekofer et al., 2005; Judge et al., 2002; Ku et al., 2000). However, CD122 is a direct gene target of the transcription factor T-bet, whose expression has been inversely co-related with CD8+ T cell memory generation (Intlekofer et al., 2005; Joshi et al., 2007; Matsuda et al., 2007). It is reasonable to suggest that IL-15 rescues effector cells for memory; yet this notion has not been formally tested, particularly in HP-induced generation of CD8+ T cell memory.
Although, the antigen stimulated and HP-induced CD8+ T cells seem to undergo proliferation, clonal expansion and functional maturation for memory (Haluszczak et al., 2009; Hamilton et al., 2006; Suzuki et al., 2008), several distinctions have been noted. The early activation markers typically associated with antigen stimulated CD8+ T cells are not observed with HP-induced stimulation, and the HP-induced functional maturation is weaker and transient. In addition, HP-induced CD8+ T cells display higher CD44 and CD122, and also retain expression of CD62L (Goldrath et al., 2000; Murali-Krishna et al., 1999). The HP-induced memory cells display weaker ability to mount antigen-recall responses and are therefore considered to be less potent than antigen induced memory cells (Cheung et al., 2009). Nevertheless, HP-induced CD8+ T cell responses have been shown to impart considerable efficacy against infectious and tumor challenge (Dummer et al., 2002; Hamilton et al., 2006).
Recent evidence demonstrates an essential role for mTOR in memory CD8+ T cell differentiation. Rapamycin treatment of lymphocytic choriomeningitis virus (LCMV) infected mice was shown to increase the quantity and quality of memory CD8+ T cells in a T cell-intrinsic manner (Araki et al., 2009). We have further demonstrated the ability of mTOR to instruct effector versus memory cell fate of antigen-specific CD8+ T cells by regulating the expression of transcription factors, T-bet and Eomesodermin (Eomes) (Rao et al., 2010). However, its role in integrating self-MHC and cytokine signals (IL-7 and/or IL-15) to regulate lymphopenia induced homeostatic proliferation, functional maturation, and memory function has not been reported.
The increasing use of bone marrow transplantation (BMT), radiation and chemotherapy behooves us to better understand the cell-intrinsic mechanisms that regulate HP-induced CD8+ T cell clonal expansion and memory. In this study, we present the first evidence for a central role of mTOR in bridging IL-7 and IL-15 mediated HP-induced CD8+ clonal expansion, memory functions, and tumor efficacy. This understanding will enable new approaches to harness homeostatic proliferation for immunity and restrict self-reactivity.
RESULTS
HP induced CD8+ T cell clonal expansion and functional maturation is IL-7 but not IL-15 dependent
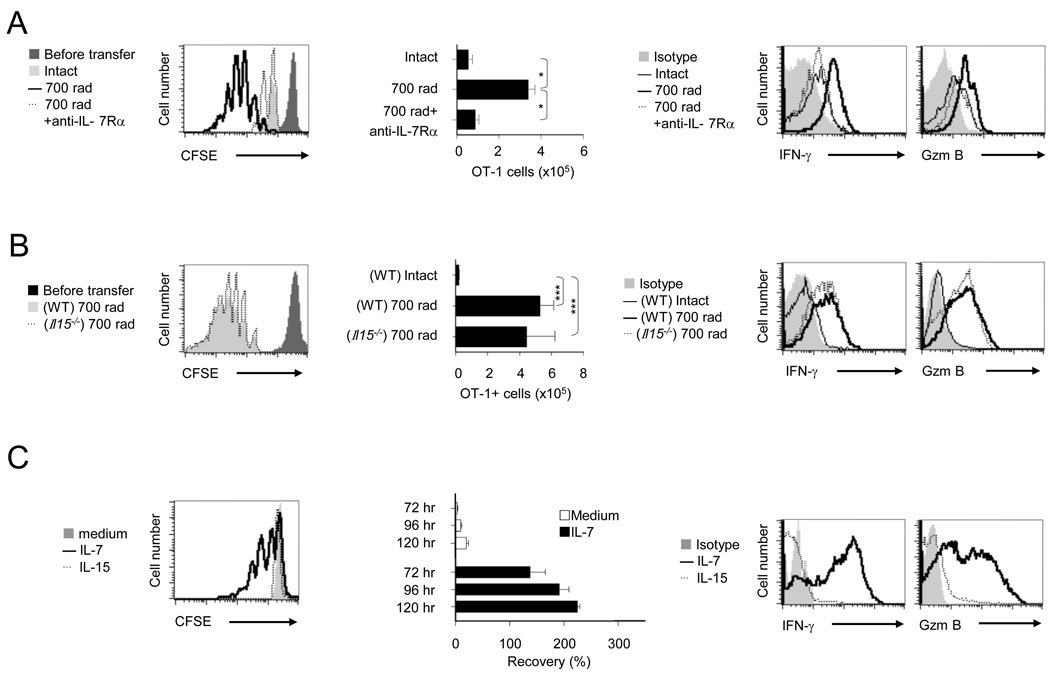
The homeostatic cytokines, IL-7 and IL-15 play a crucial role in lymphopenia-induced HP that restores CD8+ T cell numbers and promotes memory generation (Surh and Sprent, 2008; Takada and Jameson, 2009). To explore potential contributions of these cytokines in regulating HP-induced CD8+ T cell proliferation, expansion and functional maturation, we monitored carboxy fluorescein diacetate succinimidyl ester (CFSE) labeled naïve OT-1 cells after adoptive transfer into irradiated B6, B6 Il15−/− or B6 mice treated with anti-IL-7Rα. Consistent with previous findings, we demonstrated that IL-7 blockade considerably reduced lymphopenia induced OT-1 proliferation and clonal expansion on day 5 (Figure 1A). Interestingly, IL-15 deficient (B6 Il15−/− recipients showed no reduction in OT-1 proliferation or expansion (Figure 1B). Furthermore, the OT-1 cells transferred to recipients with IL-7 blockade failed to undergo functional maturation (IFN-γ and granzyme-B production) (Figure 1A), but no difference in OT-1 functional maturation was observed in Il15−/− recipients (Figure 1B). To confirm our in vivo observations and demonstrate the sufficiency of IL-7 to induce proliferation (CFSE), clonal expansion, and functional maturation of naïve CD8+ T cells, we tested the addition of IL-7 to OT-1 cells in an in vitro system. As shown in Figure 1C, IL-7 was sufficient to induce OT-1 proliferation and functional maturation by day 5 (Figure 1C and data not shown). In addition, IL-7 but not IL-15, at the concentration of 10 ng/ml induced cell proliferation and IFN-γ production, which was almost identical for TCR transgenic (Tg) CD8+ T cells (OT-1) or polyclonal CD8+ T cells from B6 mice (Figure S1A). These results establish an essential role for IL-7 in the initiation of lymphopenia-induced naïve CD8+ T cell proliferation for functional maturation. In addition, the extent of lymphopenia caused by radiation dosage, regulated CD8+ T cell clonal expansion and functional maturation as CFSE labeled naïve OT-1 Thy1.1+ cells that were transferred into mice irradiated with 0, 175 and 700 rad showed dose dependent increases in CFSE dilution, cell number and functional maturation (IFN-γ and in vivo CTL) (Figures S1B).
FIGURE 1. Lymphopenia induced CD8+ T cell HP and functional maturation is IL-7, but not IL-15 dependent.
(A) CFSE-labeled naïve OT-1 cells (1 × 106) were transferred into intact or irradiated (700 rads) syngeneic recipients on day 0. Some radiated recipients received either an isotype control (700 rad) or anti-IL-7Rα (100 µg per mouse, 700 rad+anti-IL-7Rα) on day -1 and day 2. The CD8α+Thy1.1+ cells were gated and evaluated by flow cytometric analysis of recipient spleens on day 5 for; proliferation-CFSE dilution (left), the clonal expansion-absolute number of OT-1 cells (middle) (as described in methods) and effector functions-IFN-γ and Granzyme B (Gzm B) expression detected by ICS (right).
(B) The day 5 response of CFSE-labeled naive OT-1 cells in intact or irradiated syngeneic B6 or Il15−/− recipients were evaluated by flow cytometry for; proliferation-CFSE dilution (left), absolute numbers of OT-1 cells (middle), and the expression of IFN-γ and Gzm B (right).
(C) In vitro CFSE-labeled naïve OT-1 cells cultured with IL-7 (10 ng/ml), IL-15 (10 ng/ml) or medium were analyzed for proliferation-CFSE dilution (left), cell recovery (middle), and the expression of IFN-γ and Gzm B (right) at 120 hr. The bars present SD of three individual samples and the results shown are representative of three independent experiments with similar outcomes (*, p<0.05; ***, p<0.001).
IL-7 induces mTOR to promote HP-induced clonal expansion and functional maturation of CD8+ T cells
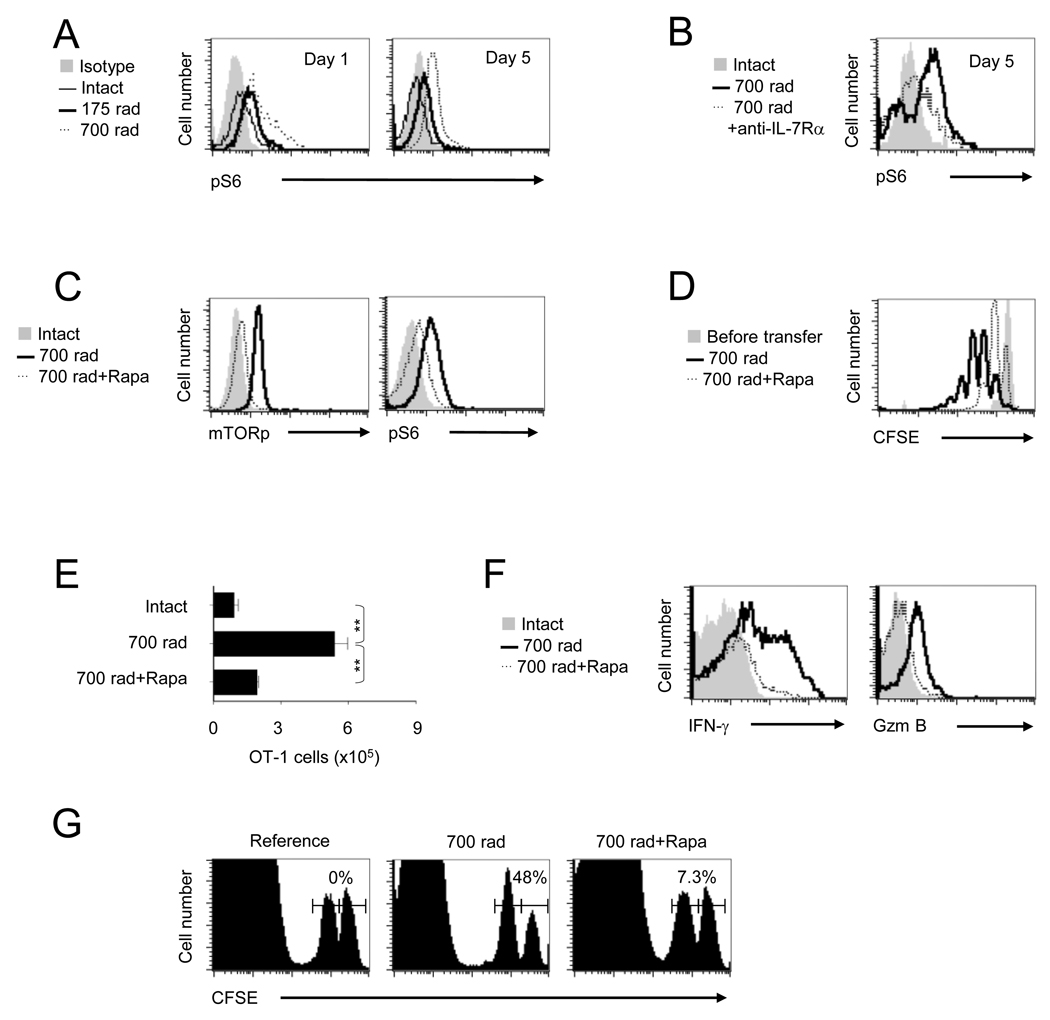
The energy sensitive kinase mTOR, has been shown to regulate memory generation in antigen stimulated CD8+ T cells (Araki et al., 2009; Rao et al., 2010). Since HP-induced functional maturation results in CD8+ T cell memory, we explored the potential role for mTOR kinase in lymphopenia associated CD8+ T cell response by testing the ability of HP to induce mTOR activity, by evaluating the phosphorylation of its downstream target, ribosomal protein S6 (pS6); as a functional read-out of mTOR activity (Burnett et al., 1998). As shown in Figure 2A, HP dramatically induced mTOR activity (pS6) in OT-1 cells which was blocked by anti-IL-7Rα treatment (Figure 2B). In agreement, the addition of IL-7, but not IL-15 to naive OT-1 cells in vitro, induced a dose dependent (1 or 10 ng/ml) increase of mTOR phosphorylation (mTORp) and pS6 (Figures S2A and S2B). These results demonstrate that HP induces an IL-7 dependent increase in mTOR activity, which may play a potential role in regulating CD8+ T cell homeostasis under conditions of lymphopenia.
FIGURE 2. IL-7 induced mTOR activity is essential for HP induced functional maturation of CD8+ T cells.
(A and B) OT-1 cells detected in the spleens of recipients receiving different doses of irradiation; 0, 175 or 700 rads (A), or 700 rads irradiation followed by anti-IL-7Rα (100 µg) on day -1 and day 2 (B) were evaluated for phosphorylation of S6 by ICS and flow cytomtery.
(C – F), OT-1 detected in recipients treated with 700 rad or 700 rad plus daily injection of rapamycin (0.75 mg/Kg/day) from day 0 – 4 were evaluated on day 5 for phosphorylation of mTOR and S6 (C), CFSE dilution (D), absolute numbers of OT-1 cells (E), and IFN-γ and Granzyme B expression by ICS and flow cytometry (F).
(G) In vivo CTL activity produced by normalized numbers of OT-1 cells, the percentage of specific lysis is indicated above histograms. The error bars are SD of three independently obtained values. A representative of three experiments with identical results is shown (**, p<0.01).
We next inquired whether rapamycin; a specific inhibitor of mTOR complex-1, could block HP-induced CD8+ T cell responses. Administration of rapamycin from day 0 – 7 (750 µg/Kg/day) blocked lymphopenia-induced mTORp and pS6 in OT-1 cells (Figure 2C), and concomitantly decreased proliferation and clonal expansion (Figures 2D and 2E). The relatively high dose of rapamycin (750 µg/Kg/day) has been previously used in vivo to block Graft-versus-host diseases (GVHD) (Blazar et al., 1998) and was found to be optimum in a titration study for blocking mTOR in OT-1 cells; in vivo (data not shown). Notably, the lymphopenia induced-IL-7 dependent increases in OT-1 functional maturation; IFN-γ, granzyme-B production and in vivo CTL activity, was reduced upon mTOR inhibition (Figures 2F and 2G). This was further corroborated by the loss of IL-7 induced mTOR activity, proliferation, clonal expansion and functional maturation of OT-1 cells upon rapamycin treatment in vitro (Figures S2C and S2D). Interestingly, the IL-7 induced mTOR activity (pS6) was PI3K dependent (Figure S2E), supporting the previously noted observation that IL-7 induced CD8+ T cell responses are PI3K dependent. These observations indicate an essential role for IL-7 induced mTOR for regulating HP-induced CD8+ T cell expansion and functional maturation.
HP promotes mTOR dependent T-bet expression for CD8+ T cell functional maturation
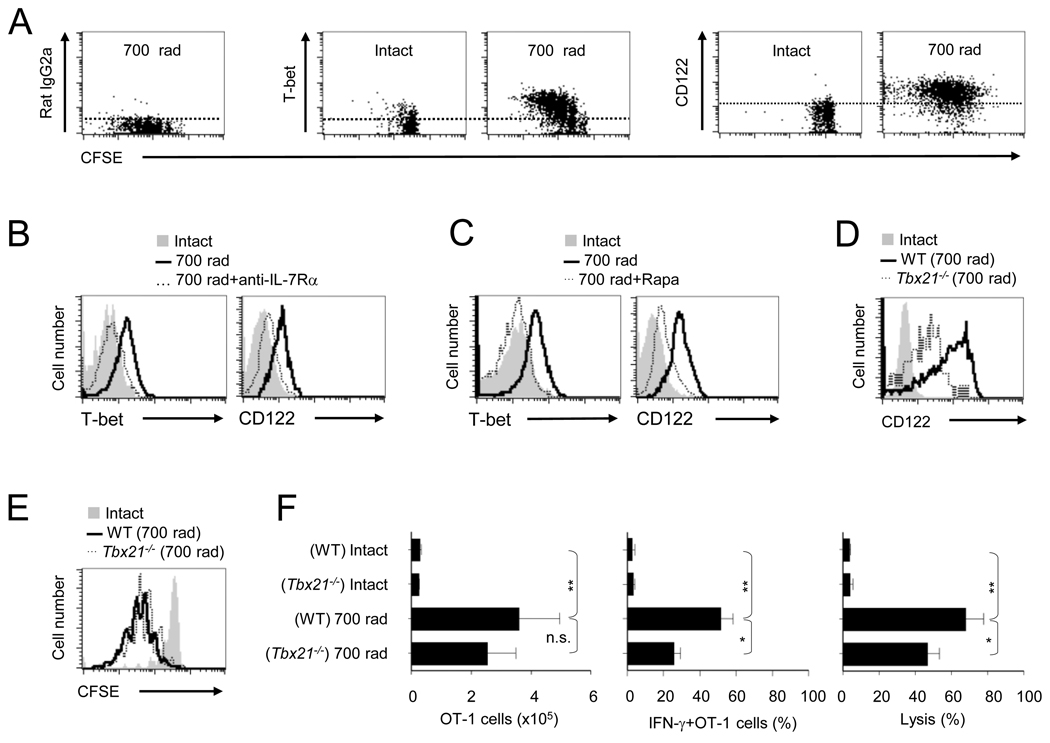
The acquisition of type I effector functions in antigen-experienced CD8+ T cells is governed by the master regulatory transcription factor, T-bet (Szabo et al., 2000). To test the notion that T-bet plays a role in IL-7 dependent HP-induced CD8+ T cell functional maturation, we transferred CFSE labeled naïve OT-1 cells into irradiated recipients and evaluated the ability of HP to induce T-bet expression in OT-1 cells on day 5 post-transfer. As predicted, HP induced T-bet expression; which corresponded with the extent of OT-1 cell proliferation (Figure 3A). Moreover, the increase in T-bet was accompanied by an increase in CD122 expression (Figure 3A), which required IL-7 in vivo, as anti-IL-7Rα abrogated the increase in T-bet and CD122 expression (Figure 3B). These results demonstrate an essential role for T-bet in HP-induced CD8+ T cell responses and CD122 expression.
FIGURE 3. HP-IL-7 induced mTOR activity is essential for T-bet mediated functional maturation.
(A – F) Naïve WT or Tbx21−/− OT-1 cells were detected by flow cytometry on day 5 after transfer into intact or irradiated (700 rads) recipients that were either untreated (A,D,E,F) or treated with anti-IL-7Rα (100 µg) on day -1 and day 2 (B), or with rapamycin (0.75 mg/Kg/day) (C) and evaluated for; (A) CFSE dilution, T-bet and CD122 expression. (B and C) T-bet and CD122 expression in recipients treated with anti-IL-7Rα (B) or in radiated recipients treated with rapamycin (C). (D) The expression of CD122 in WT or Tbx21−/− OT-1 cells. (E) CFSE dilution of WT or Tbx21−/− OT-1 cells. (F) The number of OT-1 (CD8α+Thy1.1+) cells (left), percentage of IFN-γ+ OT-1 cells (middle), and in vivo cytolysis (right). The error bars are SD of three independently obtained values. The data shown is representative of three experiments (* p<0.05; **p<0.01; n.s., not significant).
To characterize the role of mTOR-T-bet-CD122 pathway in HP-induced CD8+ T cell functional maturation, we evaluated T-bet and CD122 expression on OT-1 cells in rapamycin treated recipients. Clearly, rapamycin treatment blocked mTOR induced T-bet and subsequent CD122 expression (Figure 3C), which was confirmed by our in vitro observations (Figure S3A). By in vivo monitoring, we compared HP-induced CD8+ clonal expansion, functional maturation and CD122 expression by WT and Tbx21−/− (T-bet−/−) OT-1 Thy1.1+ cells on day 5. The T-bet deficiency reduced CD122 expression (Figure 3D), but not proliferation (Figure 3E) or OT-1 cell numbers (Figure 3F). Notably in the absence of T-bet, HP-induced functional maturation was diminished as reflected by IFN-γ production and in vivo CTL activity (Figure 3F). Surprisingly, the Tbx21−/− OT-1 cells showed similar cell surface phenotype (day 5; CD44, CD62L, IL-7Rα, IL-15Rα, and KLRG1) except for reduced CD122 expression (Figures S3B and 3D). These results identify a previously unappreciated role for T-bet in HP-induced CD8+ T cell functional maturation and CD122 expression, and suggest a potential role for mTOR kinase in bridging IL-7 and IL-15 mediated CD8+ T cell homeostasis.
T-bet programs IL-15 dependent HP-induced memory CD8+ T cells
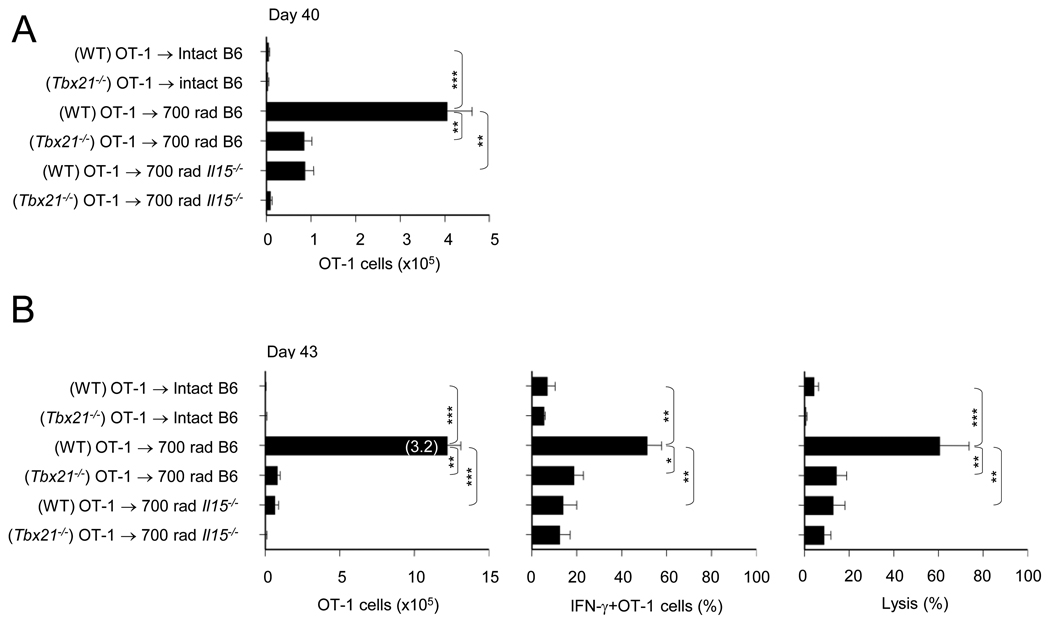
The fact that T-bet is essential for CD122 expression (Figure 3D) and IL-15 is important for CD8+ T cell accumulation during HP (Sandau et al., 2007), suggests a pivotal role for mTOR in integrating IL-7 and IL-15 signals to regulate HP-induced CD8+ T cell memory. To test this possibility, we adoptively transferred naïve wild-type (WT) or Tbx21−/− OT-1 Thy1.1+ cells into intact or irradiated B6 recipients and monitored their persistence (day 40), and the ability to mount antigen recall-responses (day 43), as a measure of memory function. In comparison to Tbx21−/− OT-1 cells, the WT OT-1 cells underwent significant clonal expansion, persisted up to day 40 (Figure 4A), and produced a considerable antigen-recall response; clonal expansion and effector functions, after rechallenge (Figure 4B). In contrast, the Tbx21−/− OT-1 cells failed to persist in irradiated B6 recipients (Figure 4A) and subsequently produced poor antigen recall responses (Figure 4B). These results demonstrate an essential requirement for T-bet expression in HP-induced memory CD8+ T cell responses and identify an important role for T-bet in CD8+ T cell persistence.
FIGURE 4. HP-induced T-bet augments IL-15 dependent CD8+ T cell memory.
(A) Naïve WT or Tbx21−/− OT-1 cells transferred to radiated B6 or Il15−/−B6 recipients were evaluated for the absolute numbers of OT-1 (CD8α+Thy1.1+) cells on day 40.
(B) OT-1 cell numbers (3 days post re-challenge, day 43) (left), the percentage of IFN-γ+ OT-1 cells (middle), and in vivo cytolysis (right). Numbers in parenthesis (left panel) is the OT-1 fold increase. The error bars represent SD of values from three mice/experimental group and a representative of two independent experiments is shown (**, p<0.01; ***, p<0.001).
To determine whether T-bet mediates HP-induced CD8+ T cell memory via IL-15, we transferred naïve WT or Tbx21−/− OT-1 cells into irradiated WT or Il15−/− hosts and assessed their persistence and antigen recall responses at day 40 and 43. Consistent with an earlier report demonstrating the belated requirement for IL-15 in HP-induced CD8+ T cell responses (Sandau et al., 2007), WT OT-1 cells transferred into Il15−/− hosts failed to persist up to day 40 and produce antigen recall-responses (Figures 4A and 4B). Moreover, the absence of T-bet in OT-1 cells and IL-15 in recipients further abrogated the HP-induced CD8+ T cell response (in comparison to WT recipients) (Figures 4A and 4B). These results demonstrate the requirement of HP-IL-7-mTOR induced T-bet expression for CD8+ T cell memory via the cell extrinsic factor; IL-15. These observations are further corroborated by our in vitro experiments, in which IL-7 conditioned OT-1 cells underwent a dose dependent proliferation in response to IL-15 and this response to IL-15 was T-bet dependent (Figure S4A). In addition, the cell recovery of WT OT-1 cells in Il15−/− hosts was equivalent to the cell recovery of Tbx21−/− OT-1 cells in WT hosts, reiterating the interdependency of cell intrinsic regulation of CD122 expression by T-bet and cell extrinsic requirement for IL-15 in programming HP-induced CD8+ T cell persistence for memory.
To confirm whether T-bet expression was involved with the previously noted role for STAT5 in IL-15 mediated CD8+ T cell homeostasis (Kelly et al., 2003), we evaluated STAT5 phosphorylation (pSTAT5) in OT-1 cells treated with varying doses of IL-15 after conditioning with IL-7. The WT OT-1 cells showed an IL-15 dose-dependent increase in pSTAT5 after IL-7 pretreatment, which was lost in Tbx21−/− OT-1 cells (Figure S4B). These results support the augument that HP-IL-7 induced mTOR activity, enhances T-bet mediated IL-15 sensitivity for CD8+ T cell memory.
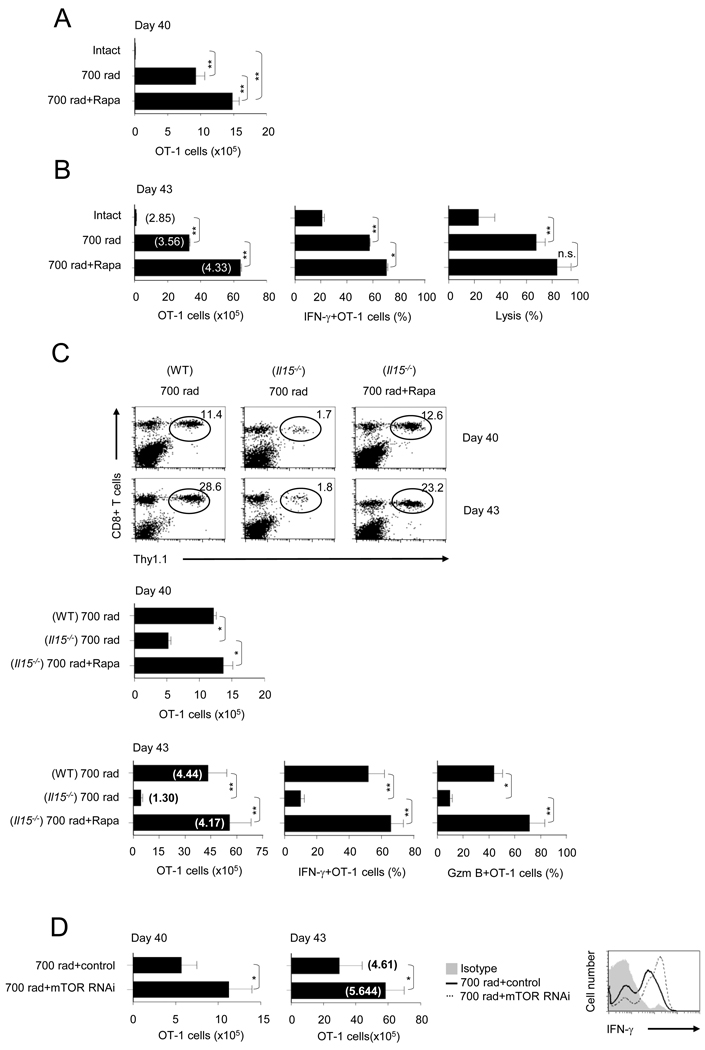
Inhibition of mTOR promotes IL-15 independent CD8+ T cell memory
The observations made thus far suggest that HP-induced mTOR activity promotes T-bet mediated CD8+ T cell functional maturation and CD122 expression for IL-15 dependent memory. Therefore, we predicted that along with the loss of T-bet expression and functional maturation, mTOR blockade would abolish HP-induced CD8+ T cell memory and provide a mechanistic basis for the efficacy of rapamycin in BMT induced GVHD and loss of tumor efficacy. Unexpectedly, rapamycin treatment (day 0 – 7) led to a considerable increase in OT-1 cell persistence until day 40 (Figure 5A). Unlike antigen-experienced OT-1 cells, OT-1 cells derived from either rapamycin treated or non-treated irradiated hosts expressed CD44int, CD62hi, IL-7Rαhi, and KLRG1lo on day 5 (Figure S5). Moreover, the OT-1 cells from untreated and treated recipients expressed comparable phenotypes reflective of their transition to memory precursor-like cells in a kinetic manner; except for their expression of CD122 and IL-7Rα (Figure S5). The early loss in CD122 expression (day 10) was reversed by day 20 and maintained till day 40, whereas the inhibition of mTOR enhanced the expression of IL-7Rα at day 40 (Figure S6). When re-challenged with antigen plus incomplete Freund’s adjuvant (IFA), rapamycin treated recipients did not show a difference in the fold expansion of the OT-1 cells, but due to greater persistence of OT-1 cell numbers, the rapamycin treated recipients showed enhanced memory functions (Figure 5B). The memory OT-1 cells at day 40 in irradiated recipients that were treated with or without rapamycin, produced type I effector response upon antigen re-challenge (IFN-γ and in vivo CTL) (Figure 5B). This result indicates that, transient blockade of HP-induced mTOR by short duration of rapamycin treatment, leads to decreased CD8+ T cell HP, clonal expansion and effector functions, but enhanced sustenance for memory.
FIGURE 5. Inhibition of mTOR promotes IL-15 independent HP-induced CD8+ T cell memory.
(A – C) WT or Il15−/− irradiated OT-1 recipients that were either untreated or treated with rapamycin (0.75 mg/Kg/day) from day 0 – 7 were evaluated on day 40 for the absolute numbers of OT-1 cells (A) and antigen-recall responses measured on day 43 (B). Number of OT-1 cells (left), percentage of IFN-γ+ OT-1 cells (middle), and in vivo cytolysis (right). (C) Top, the percentage of OT-1 cells (CD8+ Thy1.1+) in WT or Il15−/− recipients on day 40 or day 43. The circles represent the OT-1 population and the numbers indicate the percent frequency. Middle, number of OT-1 cells on day 40. Bottom, number of OT-1 cells on day 43, percentage of IFN-γ+ OT-1 cells and percentage of granzyme B+ OT-1 cells on day 43. The numbers in parenthesis indicate the fold-increase in OT-1 numbers post-immunization.
(D) RNAi mediated inhibition of mTOR promotes HP-induced CD8+ memory T cells. Naïve OT-1 cells activated in vivo in irradiated B6 hosts were transduced with retroviruses expressing RNAi for mTOR or empty control vector. OT-1 cells were then transferred into irradiated B6 hosts. The number of OT-1 cells was evaluated on day 40 post transduction and antigen-recall responses were measured on day 43. The numbers in parenthesis indicate the fold-increase in OT-1 numbers post-immunization (middle panel). The error bars are the SD of values obtained from three animals/experimental groups and one of two independent experiments is shown (*, p<0.05; **, p<0.01; ***, p<0.001; n.s., not significant).
Since, mTOR blockade results in reduced T-bet mediated CD122 expression but augments memory, we reasoned that rapamycin mediated CD8+ T cell memory is distinct in its requirement for IL-15. To test this idea, we monitored OT-1 cells transferred into irradiated B6 Il15−/− recipients for persistence and antigen-recall responses with or without rapamycin treatment. As shown in Figure 5C, the frequency and the absolute numbers of OT-1 cells were decreased on day 40 in Il15−/− compared to intact radiated recipients. However, rapamycin treatment restored persistence (day 40) and antigen-recall responses (day 43) of OT-1 cells in irradiated Il15−/− recipients (Figure 5C).
To directly confirm whether mTOR acts intrinsically in CD8+ T cells to promote HP induced memory responses, we silenced mTOR in OT-1 cells by using a retrovirus carrying mTOR RNAi and examined their persistence and recall responses after adoptive re-transfer into irradiated recipients. The OT-1 cells transduced with mTOR RNAi constructs showed remarkable increase in persistence (cell numbers on day 40) (Figure 5D, left panel), antigen-recall responses, fold increase in OT-1 cell numbers and production of IFN-γ (Figure 5D, middle and right panels). These data establish a CD8+ T cell-intrinsic role of mTOR in regulating HP induced memory differentiation.
Inhibition of mTOR regulates transcriptional factor expression for CD8+ T cell memory generation
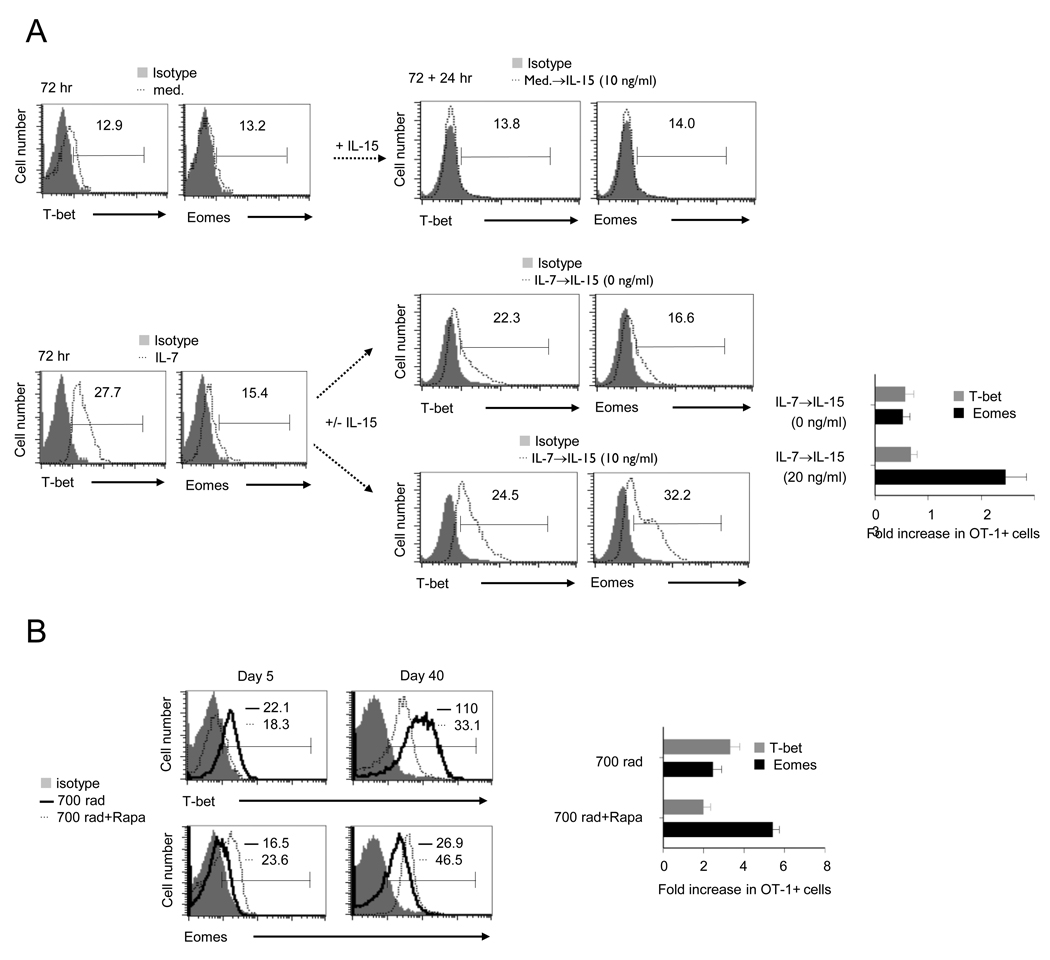
The balanced expression of the master transcription factors T-bet and Eomes determines effector versus memory functional fates in CD8+ T cells (Intlekofer et al., 2005). Moreover, we have shown that inhibition of mTOR favors Eomes over T-bet expression for increased CD8+ T cell memory generation in antigen stimulated CD8+ T cells (Rao et al.). Based on these findings, we postulated that mTOR mediated IL-15 dependent and IL-15 independent CD8+ T cell memory is due to differential expression patterns of T-bet and Eomes in OT- 1 cells. To verify this, we first examined the relative T-bet and Eomes protein expression in IL-7 conditioned OT-1 cells in vitro, and as expected IL-7 induced higher amount of T-bet than Eomes expression (Figure 6A). Remarkably, the IL-7 conditioned OT-1 cells when treated with IL-15 failed to change the expression of T-bet, but dramatically enhanced Eomes expression (Figure 6A). Moreover, rapamycin treatment altered HP-induced transcriptional profile of OT-1 cells by inhibiting T-bet for increased Eomes expression on day 5. The expression of T-bet was restored by day 20, however, higher amount of Eomes expression were noted in rapamycin-treated recipients (Figure 6B and Figure S6), which suggesting that HP-induced memory requires IL-7-mTOR for T-bet expression that sensitizes OT-1 cells for IL-15 mediated increases in Eomes expression. In contrast, rapamycin blockade of mTOR switches T-bet for Eomes expression without the need for extrinsic programming by IL-15. Notably, irrespective of the pathway employed, the relative patterns of T-bet and Eomes expression is associated with HP-induced CD8+ T cell memory.
FIGURE 6. IL-15 regulates the expression of T-bet and Eomes in IL-7 conditioned CD8+ T cells.
(A) Naive OT-1 cells stimulated with IL-7 (10 ng/ml) for 72 hr were re-cultured with IL-15 at various concentration for additional 24 hr. The expression of T-bet and Eomes in OT-1 cells at 72 hr (left) and 72+24 hr (middle). Numbers above the lines indicate mean fluorescence intensity (MFI). The fold-increase in OT-1 cells expressing Eomes or T-bet post-treatment of IL-15 (72+24 hr/72 hr, right). The data are representative of three independent experiments.
(B) Left, splenocytes from irradiated recipients or irradiated recipients followed the treatment of rapamycin (0.75 mg/Kg/day, day 0–day 7) were stained for T-bet and Eomes by ICS on day 5 or day 40. Numbers above the lines indicates mean fluorescence intensity (MFI). Right, the fold-increase in OT-1 cells expressing Eomes or T-bet (day 40/day 5). The error bars represent SD of values obtained from three animals/experimental groups. The data are representative of two independent experiments.
Collectively, the above data indicate that mTOR regulates CD8+ T cell memory differentiation in an IL-15 dependent and independent manner. The ability of rapamycin treatment to induce IL-15 independent CD8+ T cell memory was accompanied by a transient decrease in endogenous CD4+ and CD8+ T cell numbers which were rapidly resurrected (by day 20) in rapamycin-treated recipients (data not shown). Thus, the reduction in clonal expansion and functional maturation of OT-1 cells upon rapamycin treatment was not an indirect effect of enhanced reconstitution kinetics of endogenous T-cells, although the contributions of the myeloid compartment requires more testing.
HP induced CD8+ T cell memory has anti-tumor efficacy
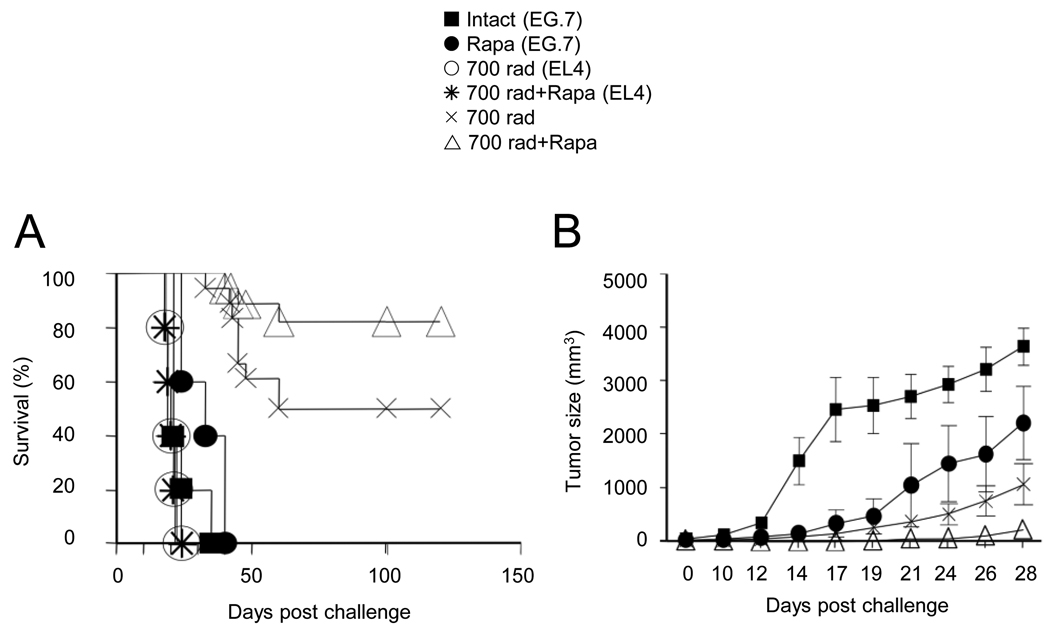
Previously it has been reported that lymphopenia can generate CD8+ T cell mediated tumor immunity (Dummer et al., 2002). To compare the tumor efficacy of HP-induced CD8+ T cell memory cells produced with or without rapamycin treatment, B6 mice received EL4 (parental) or EG.7 (EL-4 cells transfected with OVA) thymoma tumor cells on day -7 and naïve OT-1 cells on day 0 were monitored the subcutaneous tumor growth and survival. As noted, transfer of OT-1 cells into radiated recipient’s prolonged survival and reduced the size of the EG.7 tumor, but not the EL-4 tumor burden. Importantly, rapamycin administration in radiated recipients also enhanced survival rates (p=0.0816) and delayed EG.7 tumor growth (p=0.0167) (Figures 7A and 7B). It is noteworthy that rapamycin alone (in the absence of radiation) also provided some tumor protection in both s.c. and i.p. models, suggesting its potential ability to directly affect tumor cell growth, perhaps by regulating angiogenesis (Brown et al., 2003; Guba et al., 2002). Thus demonstrating that mTOR mediated CD8+ T cell memory induction; with or without clonal expansion and functional maturation, is a major determinant for HP-induced tumor efficacy.
FIGURE 7. mTOR inhibitor enhances HP-mediated tumor protection.
(A and B) B6 mice inoculated with 3 × 106 EL4 or EG.7 by i.p. or s.c. injection on day -7 were irradiated (700 rad) on day -2 and received 1×106 naïve OT-1 cells on day 0. Rapamycin was injected into indicated recipients from day 0 – 7 post-transfer. (A) Tumor-free survival curve. (B) Tumor size (mean ± s.e.m.) curve. Data in this figure were pooled from three independent experiments.
DISCUSSION
The generation of HP-induced CD8+ T cell memory has been shown to require self-peptide-MHC class I interactions as well as an abundance of the cytokines, IL-7 and IL-15. Although, HP-induced CD8+ T memory cells are relatively weaker in their protective recall responses (Cheung et al., 2009; Hamilton et al., 2006), they are capable of empowering host immunity to tumor and infectious challenge in vivo (Dummer et al., 2002; Ma et al., 2003; Suzuki et al., 2008). To exploit HP-induced CD8+ T cell memory responses for protection against infectious diseases and cancer, we need to better understand the cell intrinsic pathways that guide naïve CD8+ T cell differentiation for memory in response to lymphopenia. Herein, we demonstrate that mTOR plays an essential role in regulating self-peptide-MHC Class I, IL-7 and IL-15 mediated gene programs that promote HP-induced CD8+ T cell functional maturation and memory responses. In addition, we demonstrate that mTOR inhibition produces cell intrinsic changes in transcriptional programs to promote CD8+ T cell memory in an IL-15 independent manner. Notably, HP-induced CD8+ T cell memory generated by cell extrinsic (IL-15 dependent) or intrinsic (mTOR inhibition, IL-15 independent) pathways produce similar expression patterns of transcription factors and achieve equivalent tumor immunity at later time-points. This is quite surprising since mTOR inhibition by rapamycin augments CD8+ T cell memory functions but dampens early CD8+ T cell clonal expansion and transient effector maturation.
Our results demonstrate an essential role for IL-7 activated mTOR in HP-induced functional maturation; as reflected by IFN-γ secretion, Granzyme B and cytolytic activity. Indeed, several studies have previously reported that IL-7, but not IL-15 can promote survival and HP of naïve CD8+ T cells (Goldrath et al., 2002; Schluns et al., 2000; Tan et al., 2001; Tan et al., 2002; Webb et al., 1999). Although, Janus kinase 3 (Jak3) and STAT5 dependent pathways have been implicated in Akt and PI3K mediated survival and expansion of cytokine-induced CD8+ T cells (Cantley, 2002; Pallard et al., 1999; Venkitaraman and Cowling, 1994), the precise molecular mechanisms by which IL-7 regulates CD8+ T cell functional maturation and memory generation are not well understood. Our results indicate that lymphopenia associated IL-7 abundance induces mTOR in naïve CD8+ T cells for HP. This was confirmed by our in vitro studies in which IL-7 in a dose dependent manner induced mTOR activity and subsequent proliferation, clonal expansion and functional maturation. The fact that IL-15 was not required in vivo and was unable to induce mTOR dependent naïve CD8+ T cell proliferation was somewhat contradictory to recent data showing robust proliferation and functional responses of CD8+ T cell derived from B6 mice when treated with IL-15 (1 µg/ml), but not IL-7 (1 µg/ml) (Cho et al.). These discrepancies may be due in part to the dose of cytokines used and the source of naïve CD8+ T cells (non-Rag2−/− background). Indeed, using comparable experimental settings, we have confirmed that IL-7 at 10 ng/ml and IL-15 at 1 µg/ml can induce HP, clonal expansion and IFN-γ production by polyclonal B6 and TCR Tg OT-1 cells (data not shown). However, the relevance of this in vivo is somewhat questionable as no differences in early OT-1 HP were noted in IL-15 deficient radiated recipients. The fact that naive CD8+ T cells express low amount of CD122, further suggests that “high dose” IL-15 (1 µg/ml) would be required for their HP response, which could be available under certain conditions in tissue microenvironments, but was not observed under radiation induced lymphopenia we generated. Therefore, we conclude that radiation associated abundant IL-7, induces mTOR dependent HP leading to CD122 expression and IL-15 responsiveness in CD8+ T cells.
In contrast to antigen-dependent differentiation, the mechanisms by which HP induces CD8+ T cell memory differentiation have not been fully characterized. Our findings are the first to demonstrate that the IL-7 induced mTOR activity induces T-bet expression which is required for increased CD122 expression and integration of IL-15 mediated signals for memory transition. The role of IL-15 to mediate transition of HP-induced effector CD8+ T cells to memory is further corroborated by the noted increase in Eomes expression, demonstrating the ability of mTOR to bridge IL-7 and IL-15 mediated cell-extrinsic factors to program CD8+ T cell memory generation by regulating transcriptional programs.
Recently, a central role for mTOR in antigen induced CD8+ T cell memory generation has been demonstrated (Araki et al., 2009; Pearce et al., 2009; Rao et al., 2010). Therefore, it was logical to expect that cell-intrinsic programs were important in HP-induced CD8+ T cell memory generation, but it was not entirely clear whether they would be sufficient. Based on the in vivo use of rapamycin to regulate mature CD8+ T cell responses (750 µg/kg/day for 8 days) (Araki et al., 2009; Phung et al., 2007), we inhibited mTOR activity and validated the notion that HP-induced CD8+ T cell memory can be produced without the requirement for extrinsic factors like IL-15. The ability of rapamycin treatment to switch T-bet for Eomes expression at early time points (day 5), was associated with the imprinted memory fate, which could not be reversed by subsequent re-expression of T-bet along with persistent Eomes expression. In congruence with the skewed pattern of T-bet and Eomes expression in antigen induced CD8+ T cells (Intlekofer et al., 2005; Rao et al., 2010), our findings indicate that HP-induced mTOR activity regulates T-bet versus Eomes expression for regulating CD8+ T cell functional fate.
Interestingly, the re-expression of T-bet at later time-points leads to CD122 expression in OT-1 cells, which is not functionally required, as rapamycin promotes HP-induced CD8+ T cell memory in the absence of IL-15. This information has considerable value for understanding the impact of rapamycin treatment on BMT associated GVHD and graft-versus-tumor (GVT) effects, as it can assist in the development of new strategies to enhance the therapeutic index of BMT in the clinic. It was also quite intriguing that although rapamycin decreased OT-I clonal expansion at day 5, the numbers had recuperated by day 40 and their ability to produce antigen-recall responses was considerably better than IL-15 dependent HP-induced memory CD8+ T cells (absence of rapamycin treatment). It can be envisaged that despite their similarity in cell surface phenotype and functional outcomes, the CD8+ memory T cells generated by IL-15 dependent or independent pathways represent distinct sub-types of memory cells. Although, only two functional types have been described; effector-memory and central-memory, this may represent an over simplification which can be characterized further by detailed analysis directed at understanding the functional attributes of rapamycin versus non-rapamycin generated CD8+ memory T cells.
The inherent ability of HP-induced (IL-15 dependent-extrinsic) and rapamycin treated (IL-15 independent-intrinsic) memory CD8+ T cells to produce comparable anti-tumor effects indicates that HP-induced tumor immunity is dependent on CD8+ memory T cells, irrespective of their method of production. Additionally, these findings lend support to the notion that the tumor efficacy of CD8+ T cells is dependent more on memory than effector functions or vigorous proliferation at early time-points (Gattinoni et al., 2009; Rao et al., 2010). This understanding urges reconsideration of established paradigms guiding active immune based therapies for cancer. Finally, the better understanding of cell intrinsic and extrinsic pathways that regulate HP-induced CD8+ memory T cell generation provide new targets for exploring means to favor GVT over GVHD.
EXPERIMENTAL PROCEDURES
Mice and reagents
The C57BL/6 (B6) mice (National Cancer Institute-Fredrick), CD8+ TCR transgenic mice with Thy1.1congenic marker (OT-1), Tbx21−/− OT-1 (T-bet deficient) were bred and maintained according to IACUC approved protocol at RPCI. OT-1 mice and Tbx21−/− OT-1 mice were >20 generations backcrossed to recombination activating gene (Rag)-2-deficient mice (The Jackson Laboratory). The Il15−/−B6 mice were purchased from Taconic. RmIL-7 and rmIL-15 was purchased from Peprotech (Rocky Hill, NJ). Rapamycin was purchased from Sigma Aldrich (St. Louis, MO) and ChemieTek (Indianapolis, IN). The rapamycin was diluted with PBS and used at 0.75 mg/kg/day in vivo by intraperitoneal (i.p.) injection and at 20 ng/ml in vitro. The PI3K inhibitor (LY294002) was purchased from Calbiochem whereas phorbol ester PMA, ionomycin and brefeldin A were purchased from Sigma-Aldrich.
Flow cytometry
All mAbs used for flow cytometry were purchased from BD PharMingen except anti-IL-7Rα (A7R34), anti-Eomes (Dan11mag), anti-T-bet (eBio4B10) and anti-Granzyme B (16G6) from eBioscience, anti-CD8α (53-6.7) from Biolegend. The hybridoma secreting anti-IL-7Rα (clone SB199) was kindly provided by Dr. P. Kincade (University of Oklahoma). The following antibodies were purchased from Cell Signaling: anti-mTORp (Ser 2481), anti-pS6 (Ser 235/236) and phospho-STAT5 (pSTAT5). Intracellular staining (ICS) and flow cytometry for IFN-γ, T-bet, Eomes, Granzyme B (Gzm B), mTORp, and pS6K was performed as described (Rao et al.). Expression of IFN-γ was determined after a 5hr antigen re-stimulation. Using single-cell suspensions from spleens and/or lymph nodes were stained and analyzed by flow cytometry. Donor OT-1 cells were detected as CD8α and Thy1.1 double positive and gated for further analysis. Flow cytometry was performed on a FACS Calibur and data analyzed using CellQuest software (BD Biosciences).
Cell culture, stimulation and proliferation
The LN cells from OT-1 Rag−/− mice were negatively selected for CD8+ T cells (Cedarlane Lab Ltd, ON). The OT-1 cells after enrichment were >90% CD8+TCR Vα+ and >95% CD44loCD122lo by flow cytometric analysis. The OT-1 cells treated with or without rapamycin for 30 min before addition of IL-7 were harvested at indicated time for staining and evaluation by flow cytometry. In few experiments, IL-7 stimulated OT-1 cells were harvested (72 hr) and re-stimulated with various doses of IL-15 for an additional 24 hr and then evaluated by flow cytometry. Proliferation was measured by the addition of 1 μCi [3H] thymidine per well for the last 12 hr of culture.
Adoptive transfer and IL-7 blockade
Lymphopenia was induced in naïve age and sex matched B6 recipient mice by radiation (0, 175 or 700 rads) and the mice were maintained on antibiotics thereafter. Unless otherwise stated, purified naïve OT-1 cells (1 × 106) labeled with 5 µM CFSE (Invitrogen) were (i.v.) adoptively transferred into syngeneic recipients (2 days after radiation) as described (Kieper et al., 2001). Intact mice were also given immunization by s.c. injection of recombinant poxvirus expresses chicken ovalbumin on day 0 (immunization). In some experiments, the purified anti–IL-7Rα (100 µg per mouse twice a week) was injected to achieve IL-7 blockade in vivo.
Evaluation of in vivo OT-1 cells responses
The total number of adoptively transferred OT-1 cells (CD8α+Thy1.1+) in recipient mice was determined by multiplying the total cell counts into the percentage of CD8α+Thy1.1+ T cells detected by flow cytometry at the indicated time points. The in vivo CTL assays were performed after normalizing OT-1 cell numbers and re-transfer into intact target bearing mice as previously described (Nelson et al., 2000)
Plasmid and retrovirus based RNAi
The pMKO.1 GFP retroviral vector (plasmid 10676) was obtained from Addgene (Cambridge, MA). The pMKO.1 GFP retroviral vector encoding RNAi for mTOR was a kind gift of Dr. Rafi Ahmed (Emory University, Atlanta, GA) (Araki K, et al. Nature 2009). The pMKO.1 or its derivative was transfected into a viral packaging cell line along with the plasmid pCL-Eco. Naïve OT-1 (Thy1.1+) cells adoptively transferred into 700 rad irradiated mice were isolated by anti-CD90.1 MicroBeads, on a MiniMACS Separator (Miltenyi Biotec) on day 5, following by spin-transduction at 30°C for 2 hr with freshly collected retrovirus. The OT1 cells were sorted for GFP+ cells (1 × 106) and were adoptively transferred into 700 rad irradiated B6 recipients 3 days post-transduction (matched for radiation treatment). The persistence of OT-1 cells and antigen recall response was evaluated on days 40 and 43.
In vivo tumor challenge
B6 mice inoculated with EL4 thymoma or EG.7-OVA thymoma (3 × 106 cells per mouse) by s.c. or i.p. injection on day -7, were radiated (700 rad) on day -2 and naïve OT-1 cells (1 × 106 per mouse) adoptively transferred on day 0. Rapamycin (0.75 mg/Kg/day) was injected into indicated recipients (i.p.) every day between day 0 ~ 7 after OT-1 cell transfer. Tumor volume were calculated by measuring diameters of the tumor in the X and Y plane using digital calipers and entering measurements into the equation tumor volume tumor volume (mm3) = (major axis) × (minor axis)2 × 0.52.
Statistical analysis
The unpaired student’s t test was applied with GraphPad Prism software to all data points. Tumor survival between various groups was compared using Kaplan Meier survival curves and log-rank statistics. Significance is indicated as follows: * p< 0.05; ** p<0.01; and ***p<0.001.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Rafi Ahmed for providing us with mTOR RNAi retrovirus. Dr.’s T. Tomasi and N. Bangia for critical reading of the manuscript. Supported in part by NIH (CA104645), OCRF and Alliance Foundation of Roswell Park Cancer Institute (to P.A.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Vallera DA. Rapamycin inhibits the generation of graft-versus-host disease- and graft-versus-leukemia-causing T cells by interfering with the production of Th1 or Th1 cytotoxic cytokines. J Immunol. 1998;160:5355–5365. [PubMed] [Google Scholar]
- Brown VI, Fang J, Alcorn K, Barr R, Kim JM, Wasserman R, Grupp SA. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc Natl Acad Sci U S A. 2003;100:15113–15118. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Cheung KP, Yang E, Goldrath AW. Memory-like CD8+ T cells generated during homeostatic proliferation defer to antigen-experienced memory cells. J Immunol. 2009;183:3364–3372. doi: 10.4049/jimmunol.0900641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 32:214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Spolski R, Imada K, Bollenbacher J, Lee S, Leonard WJ. A role for Stat5 in CD8+ T cell homeostasis. J Immunol. 2003;170:210–217. doi: 10.4049/jimmunol.170.1.210. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc Natl Acad Sci U S A. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieper WC, Prlic M, Schmidt CS, Mescher MF, Jameson SC. Il-12 enhances CD8 T cell homeostatic expansion. J Immunol. 2001;166:5515–5521. doi: 10.4049/jimmunol.166.9.5515. [DOI] [PubMed] [Google Scholar]
- Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- Le Campion A, Bourgeois C, Lambolez F, Martin B, Leaument S, Dautigny N, Tanchot C, Penit C, Lucas B. Naive T cells proliferate strongly in neonatal mice in response to self-peptide/self-MHC complexes. Proc Natl Acad Sci U S A. 2002;99:4538–4543. doi: 10.1073/pnas.062621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WQ, Jiang Q, Aleem E, Kaldis P, Khaled AR, Durum SK. IL-7 promotes T cell proliferation through destabilization of p27Kip1. J Exp Med. 2006;203:573–582. doi: 10.1084/jem.20051520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–766. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Ma J, Urba WJ, Si L, Wang Y, Fox BA, Hu HM. Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur J Immunol. 2003;33:2123–2132. doi: 10.1002/eji.200324034. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, George TC, Hagman J, Gapin L. Temporal dissection of T-bet functions. J Immunol. 2007;178:3457–3465. doi: 10.4049/jimmunol.178.6.3457. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- Nelson D, Bundell C, Robinson B. In vivo cross-presentation of a soluble protein antigen: kinetics, distribution, and generation of effector CTL recognizing dominant and subdominant epitopes. J Immunol. 2000;165:6123–6132. doi: 10.4049/jimmunol.165.11.6123. [DOI] [PubMed] [Google Scholar]
- Pallard C, Stegmann AP, van Kleffens T, Smart F, Venkitaraman A, Spits H. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 1999;10:525–535. doi: 10.1016/s1074-7613(00)80052-7. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- Ramsey C, Rubinstein MP, Kim DM, Cho JH, Sprent J, Surh CD. The lymphopenic environment of CD132 (common gamma-chain)-deficient hosts elicits rapid homeostatic proliferation of naive T cells via IL-15. J Immunol. 2008;180:5320–5326. doi: 10.4049/jimmunol.180.8.5320. [DOI] [PubMed] [Google Scholar]
- Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- Sandau MM, Winstead CJ, Jameson SC. IL-15 is required for sustained lymphopenia-driven proliferation and accumulation of CD8 T cells. J Immunol. 2007;179:120–125. doi: 10.4049/jimmunol.179.1.120. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ogawa S, Tanabe K, Tahara H, Abe R, Kishimoto H. Induction of antitumor immune response by homeostatic proliferation and CD28 signaling. J Immunol. 2008;180:4596–4605. doi: 10.4049/jimmunol.180.7.4596. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaraman AR, Cowling RJ. Interleukin-7 induces the association of phosphatidylinositol 3-kinase with the alpha chain of the interleukin-7 receptor. Eur J Immunol. 1994;24:2168–2174. doi: 10.1002/eji.1830240935. [DOI] [PubMed] [Google Scholar]
- Webb LM, Foxwell BM, Feldmann M. Putative role for interleukin-7 in the maintenance of the recirculating naive CD4+ T-cell pool. Immunology. 1999;98:400–405. doi: 10.1046/j.1365-2567.1999.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.