Abstract
Recognizing the similarities between Huntington’s disease pathophysiology and the neurotoxicology of various metals, we hypothesized that they may exhibit disease-toxicant interactions revealing cellular pathways underlying neurodegeneration. Here we utilize metals and the STHdh mouse striatal cell line model of Huntington’s disease to perform a gene-environment interaction screen. We report that striatal cells expressing mutant Huntingtin exhibit elevated sensitivity to cadmium toxicity and resistance to manganese toxicity. This neuroprotective gene-environment interaction with manganese is highly specific, as it does not occur with iron, copper, zinc, cobalt, cadmium, lead, or nickel ions. Analysis of the Akt cell-stress signaling pathway showed diminished activation with manganese exposure and elevated activation after cadmium exposure in the mutant cells. Direct examination of intracellular manganese levels found that mutant cells have a significant impairment in manganese accumulation. Furthermore, YAC128Q mice, a Huntington’s disease model, showed decreased total striatal manganese levels following manganese exposure relative to wild-type mice. Thus, this disease-toxicant interaction screen has revealed that expression of mutant Huntingtin results in heightened sensitivity to cadmium neurotoxicity and a selective impairment of manganese accumulation.
Keywords: Huntington’s Disease, Manganese, Neuroprotection, Neurodegeneration, Neurotoxicity, Gene-Environment Interactions
Introduction
In Huntington’s disease (HD), degeneration of the medium spiny neurons within the corpus striatum occurs well prior to other affected brain regions such as the cortex (Imarisio et al. 2008). This selective degeneration occurs despite widespread expression of the disease-causing, polyglutamine-expanded protein, Huntingtin (HTT). Thus, other factors within the striatum may uniquely increase the vulnerability of this area to the pathophysiological mechanisms of HD. Interestingly, the toxicant 3-nitroproprionic acid (3NPA), a mitochondrial complex II inhibitor, exhibits an HD-like striatal specific neurodegeneration (Beal et al. 1993). One explanation of this common pathology is that both mutant HTT and 3NPA might impinge upon a shared pathophysiological vulnerability inherent to the striatum. Indeed, both HD and 3NPA toxicity cause mitochondrial dysfunction, oxidative stress, excitotoxicity, and altered iron and copper homeostasis (Dexter et al. 1991, Fox et al. 2007, Simmons et al. 2007).
We postulate that toxicants acting upon pathophysiological targets modulated in HD will exhibit disease-toxicant interactions, even if patients are not normally exposed to these toxicants. Furthermore, the identification of these interactions may uncover mechanisms of selective neurodegeneration due to environmental or genetic factors. Therefore, a disease-toxicant interaction screen may facilitate the identification of toxicants with common pathophysiological targets or mechanisms. The toxicological properties of metals are diverse, and include oxidative stress, deranged calcium signaling, activation of cell stress pathways, protein aggregation and altered energy metabolism (Zecca et al. 2004, Bush 2000). Here we exploit this broad toxicology to screen for gene-environment interactions between neurotoxic metals and the glutamine-expanded disease-causing HTT protein. We report the surprising discovery that expression of mutant HTT protects against manganese toxicity in part by substantially decreasing manganese accumulation during exposure.
Materials and Methods
Chemicals, reagents, and cell culture supplies
Cell culture media and supplements were obtained from Mediatech (Manassas, VA) unless indicated. Cell lines were grown in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), L-glutamine, 400µg/ml G418 and Penicillin-Streptomycin. Metals and toxicants used in survival assays were from Alfa Aesar (Ward Hill, MA) unless indicated: 3-nitroproprionic Acid (Sigma, St. Louis, MO), Fe(III) chloride (VWR, West Chester, PA), Mn(II) chloride, Cd(II) chloride, Co(II) chloride, Cu(II) chloride, Pb(II) chloride, Ni(II) sulfate, and Zn(II) chloride. Buffers and solutions for assays: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) salt (VWR, West Chester, PA), Sorenson’s Buffer (0.1 M glycine, 0.1 M NaCl2, pH 10.5), dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO). PBT (PBS with 0.1% Triton X-100 (Sigma, St. Louis, MO ). 4% paraformaldehyde in PBS (diluted from 16% solution, Electron Microscopy Sciences, Hatfield, PA) were used for cell imaging studies.
Antibodies
Antisera for western blotting include HTT (2166) 1:20000 (Millipore, Billerica, MA), Pan Akt 1:1000 (Cell Signaling, Beverly, MA), phospho-Akt 1:1000 (Cell Signaling, Beverly, MA), and actin 1:2000 (Developmental Studies Hybridoma Bank, Iowa City, IA). Appropriate secondary antibodies from Jackson Immunoresearch Laboratories (West Grove, PA) were used at 1:15000 accordingly.
Cell survival Assays
The clonal striatal cell lines – both mutant STHdhQ111/Q111 and wild-type STHdhQ7/Q7 – were a generous gift from Marcy Macdonald, PhD (Massachusetts General Hospital, MA), and grown at 33°C (Cattaneo & Conti 1998, Trettel et al. 2000). STHdhQ111/Q111 and wild-type STHdhQ7/Q7 cells were plated at equal density the evening before treatment. Toxins or metals were added to the culture media the next morning, and cells exposed for 26 to 30 hours. Cell viability was assessed by either trypan blue exclusion or MTT assay. Briefly, Trypan blue exclusion: Cells were harvested after exposure to various metals by trypsinization and exposed to trypan blue following published protocol (Ying et al. 2000). MTT assays were performed according to established protocols (Ehrich & Sharova 2000). Briefly, culture media was removed, and 500µL of 0.5% MTT salt in Minimal Essential Medium (MEM) (Invitrogen, Carlsbad, CA) containing FBS and Penicillin-Streptomycin was added to each well for 4 hours. Next, the MEM was removed and Sorenson’s Buffer was diluted 2.5ml into 20ml of DMSO. 200µl of Sorenson’s Buffer in DMSO was added to the empty wells to dissolve the precipitate. The plates were returned to the incubator until all the MTT salt precipitate could no longer be visualized under a microscope. 60µL from each well was removed and placed in a 96-well plate, and absorbance read at 570–590nm. Cell survival data was normalized by genotype to the vehicle-only exposed control included in each independent sample set.
Mutant HTT expression construct
The polyglutamine expansion of 128 repeats was created by PCR of the CAG repeat from the YAC128Q mouse model and subcloned into a full-length HTT cDNA construct (gift from Juan Botas, Baylor College of Medicine, TX). The new full-length mutant HTT cDNA was then subcloned into pCDNA3.1 (Invitrogen, Carlsbad, CA), a mammalian expression vector to make pHTT[128Q]. A control construct containing the full-length HTT cDNA in the reverse orientation, was used as a control vector in transfection experiments to control for the large size of pHTT[128Q] vector. The inverted control does not express the Htt protein, confirmed by western blot analysis (data not shown).
Cell viability analysis in transfected cells
Equal numbers of wild-type STHdhQ7/Q7 cells were plated onto glass coverslips approximately 10 hours prior to transfection using lipofectAMINE™ 2000 (Invitrogen, Carlsbad, CA). The pEGFP-N1 expression vector (Invitrogen, Carlsbad, CA) was co-transfected with pHTT[128Q] or the control vector at a 1:3 ratio to allow estimates of transfection efficiencies. Approximately 70% of cells were GFP positive. Cells were treated with MnCl2 24 hours post-transfection and analyzed 30 hours after exposure. Coverslips were harvested, washed in PBT, fixed with 4% paraformaldehyde, washed again, and mounted onto slides with ProLong Gold antifade reagent with DAPI nuclear stain (Invitrogen, Carlsbad, CA). DAPI positive cells were imaged by fluorescence microscopy using a Zeiss Axioplan microscope (Thornwood, NY) with a 10x objective by systematic unbiased sampling of non-overlapping fields. Number of surviving cells per field was quantified using NIH ImageJ by the threshold and analyze particles commands as previously described (Bowman et al. 2007).
Westerns blot
Equal numbers of wild-type and mutant STHdh cells were plated and treated with Mn(II) or Cd(II) for 3 or 30 hours. The cell pellets were lysed in RIPA buffer (50mM Tris, 150mM NaCl2, 0.1% SDS, 1.0% Nonidet 40, 12mM deoxcholic Acid, 1x protease inhibitor cocktail (Sigma), 1x phosphatase inhibitor cocktails I and II (Sigma) and loaded by equal cell number or protein for SDS-PAGE. Western blots were visualized with Thermo Scientific Pierce Supersignal West Dura Extended Duration Chemiluminescent Substrate (Waltham, MA) on an Ultralum Omega 12iC (Claremont, CA). Measurements of integrated density of protein bands was performed using ImageJ (NIH), with background correction calculated using a signal ratio error model, as described (Kreutz et al. 2007). Calculations of relative signal were normalized to untreated wild-type or mutant sample for each set, as indicated.
Animal manganese-exposure
All animal studies strictly followed protocols approved by the Vanderbilt University Institutional Animal Care and Use Committee and were adopted to minimize pain and distress of the animals. The FVB-Tg(YAC128)53Hay/J mouse line (YAC128Q) was obtained from JAX (#004938, Bar Harbor, ME) (Slow et al. 2003). The manganese exposure protocol followed a previously published paradigm (Dodd et al. 2005). In brief, 3-month old YAC128Q HD mutant mice and their wild-type littermates were injected subcutaneously with 50mg/Kg manganese chloride tetrahydrate. All injections were done blind to genotype. Injections were carried out on days 0, 3, and 7. On day 8, the animals were sacrificed by cervical dislocation, the brain regions were dissected, tails clipped for genotyping, and trunk blood collected into heparin coated tubes. Tissue collection and GFAAS were performed blind to both genotype and exposure. The tissues were then flash-frozen for analysis of manganese content. Analysis of manganese content by GFAAS was performed blind to genotype and exposure. Genotyping was done according to a previously published method (from JAX, 004938, Bar Harbor, ME) (Slow et al. 2003).
Graphite Furnace Atomic Absorption Spectroscopy (GFAAS)
Manganese and iron concentrations were measured with GFAAS (Varian Inc., AA240, Palo Alto, CA). STHdh cells were cultured and treated as described above for cell viability assays, harvested by trypsinization, washed multiple times in PBS and then flash-frozen until analysis. For analysis, cell pellets were thawed and digested in 200 µl ultrapure nitric acid for 24 hours in a sandbath (60°C). Brain tissue samples were diluted 1:5 (weight to volume) in 1X PBS and sonicated for 30 seconds. For protein analysis of the tissue samples, a 10 µl aliquot of the tissue homogenate was combined with 10 µl of RIPA lysis buffer. Homogenates were incubated on ice for 20 minutes before being centrifuged at 10,000 × g for 20 minutes at 4˚C. The supernatant was then transferred to new tubes, and the total protein concentration was determined by BCA assay (Pierce, Rockford, IL). An equal volume of ultrapure nitric acid was then added to the remaining homogenate and the sample was digested for 48 hours in a sandbath (60°C). Manganese content was determined by the following protocol: A 20 µl aliquot of the digested sample was brought to 1 ml total volume with 2% nitric acid for analysis. Bovine liver (NBS Standard Reference Material, USDC, Washington, DC) (10 µg Mn/g) was digested in ultrapure nitric acid and used as an internal standard for analysis (final concentration 10 µg Mn/L) as published previously (Anderson et al. 2009).
Statistical Analysis
Univariate, multivariate, and repeated measures ANOVA were performed using SPSS software (SPSS, Inc., Chicago, IL). Post-hoc analysis and pair-wise comparisons were done using Microsoft Excel (Redmond, WA) by Student’s t-tests (two-tailed), except for comparison of normalized data versus control which were performed by testing for non-overlap of the 95% confidence interval, error bars are expressed as standard error of the mean (SEM). Animal manganese-exposure data was analyzed by multivariate ANOVA using blood manganese levels as a covariate. The alpha level for all of the analyses was set at p≤0.05.
Results
A HD-toxicant interaction screen
To establish a gene-environment interaction model between metals and mutant htt, we utilized the HD mouse striatal cell line model developed by Marcy Macdonald. The model shares many phenotypic similarities with HD mouse models and human patients (Trettel et al. 2000, Imarisio et al. 2008). The striatal cell lines express full-length (wild-type or glutamine expanded) htt from the endogenous locus allowing comparisons of phenotypes between wild-type (STHdhQ7/Q7) and mutant (STHdhQ111/Q111) cells. The initial screen focused on cell survival as a basic toxicological phenotype. Previous research by Macdonald and colleagues has shown that the mutant cell line (STHdhQ111/Q111) line has increased sensitivity to the toxicant 3NPA (Ruan et al. 2004). Therefore, as a positive control, we examined cell survival in the striatal cell model following 30 hours exposure to 3NPA. We confirmed that the mutant STHdhQ111/Q111 line has a significant (p<0.05) decrease in cell survival relative to wild-type cells after 3NPA exposure (Fig. S1).
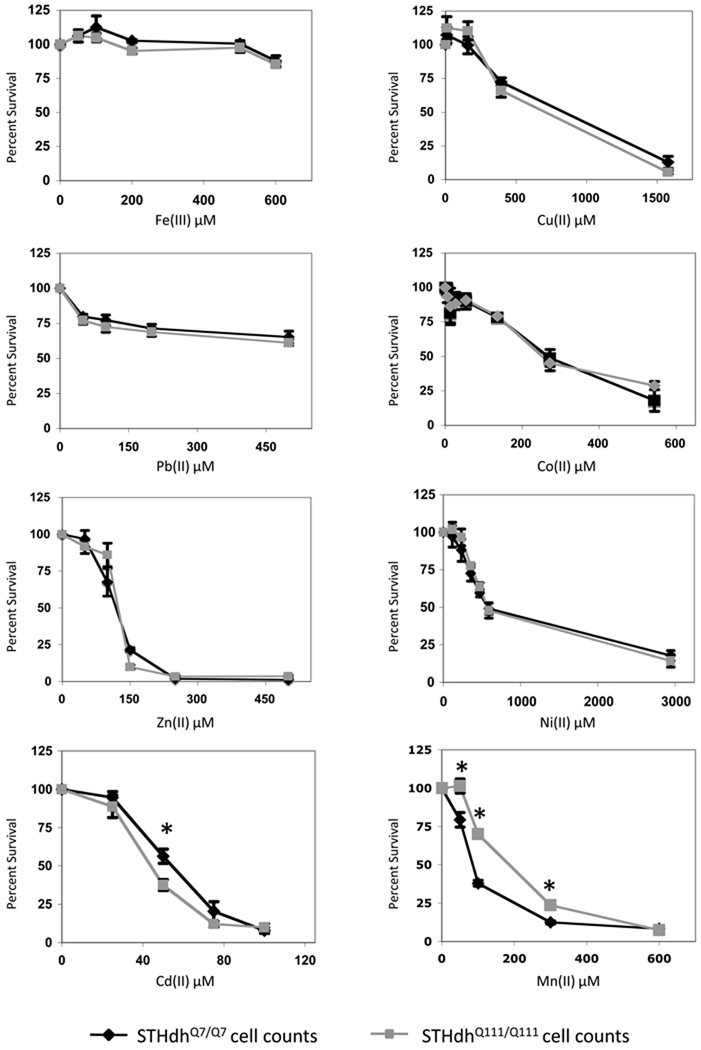
After validating the HD striatal model for detection of gene-environment interactions relevant to HD, we initiated a cell survival screen to determine whether expression of mutant htt modulates sensitivity to a diverse set of metal toxicants. We screened eight neurotoxic metal ions, Fe(III), Cu(II), Pb(II), Co(II), Zn(II), Ni(II), Cd(II) and Mn(II), by generating concentration response curves (Fig. 1). Metal concentrations were chosen to generate a survival curve spanning non-toxic to highly toxic exposures. We were unable to induce a high degree of cell death (>40%) for Fe(III) and Pb(II) to due to the insufficient solubility of these metals at higher concentrations. Nevertheless, statistical analysis of cell survival by two-way ANOVA revealed a significant effect of exposure on cell survival for each of the eight metals (p<0.001). Wild-type and mutant STHdh cell line survival curves were indistinguishable for Fe(III), Cu(II), Pb(II), Co(II), Zn(II) and Ni(II) (Fig. 1). In contrast, Cd(II) and Mn(II) both showed differential effects on wild-type and mutant cell survival (Fig. 1). The mutant STHdhQ111/Q111 cells displayed an increased sensitivity to Cd cytotoxicity. Statistical analysis by two-way univariate ANOVA found a significant effect of genotype (F(1,29)=5.31, p=0.029) on cell survival. Post-hoc analysis indicated that the wild-type line had significantly (p<0.05) higher survival at 50µM Cd(II) relative to mutant cells (Fig. 1). Analyses of Mn(II) toxicity in the STHdh cells revealed an unexpected gene-environment interaction, in which cells expressing mutant htt were resistant to Mn(II) toxicity. Statistical analysis by two-way repeated measures ANOVA found a significant effect of genotype (F(1,8)=323.2, p<0.001) on cell survival. Post-hoc analysis indicated that the mutant line had significantly (p<0.01) higher survival at 50µM, 100µM and 300µM Mn(II) relative to wild-type cells (Fig. 1).
Fig. 1.
Huntington’s disease - metal toxicity cell survival screen. Equal numbers of wild-type STHdhQ7/Q7 (black) or mutant STHdhQ111/Q111(grey) cells were exposed to the indicated metal ions. Cell survival was assessed 26–30 hours after exposure by MTT assay for all metals except Cu, which was assessed by trypan blue exclusion. The average absorbance (or mean cell counts for Cu) relative to the untreated control for each genotype is plotted as percent cell survival (± SEM), with Cu(II) chloride (4 experiments), Fe(III) chloride (3 experiments), Cd(II) chloride (4 experiments), Zn(II) chloride (3 experiments), Pb(II) chloride (3 experiments), Co(II) chloride (4 experiments), Ni(II) chloride (3 experiments) and Mn(II) chloride (2 experiments). Each experiment had between 3 and 6 independent samples at each genotype/metal concentration point. ANOVA showed a significant effect of exposure on cell survival for all metals (p<0.001), and finds a significant difference in cell survival between genotypes only for Cd(II) (p=0.029) and Mn(II) (p=0.001). Significant differences in survival (* p<0.05 post-hoc t-test) between wild-type and mutant cells at specific exposure levels are shown.
Mn(II) survival curve
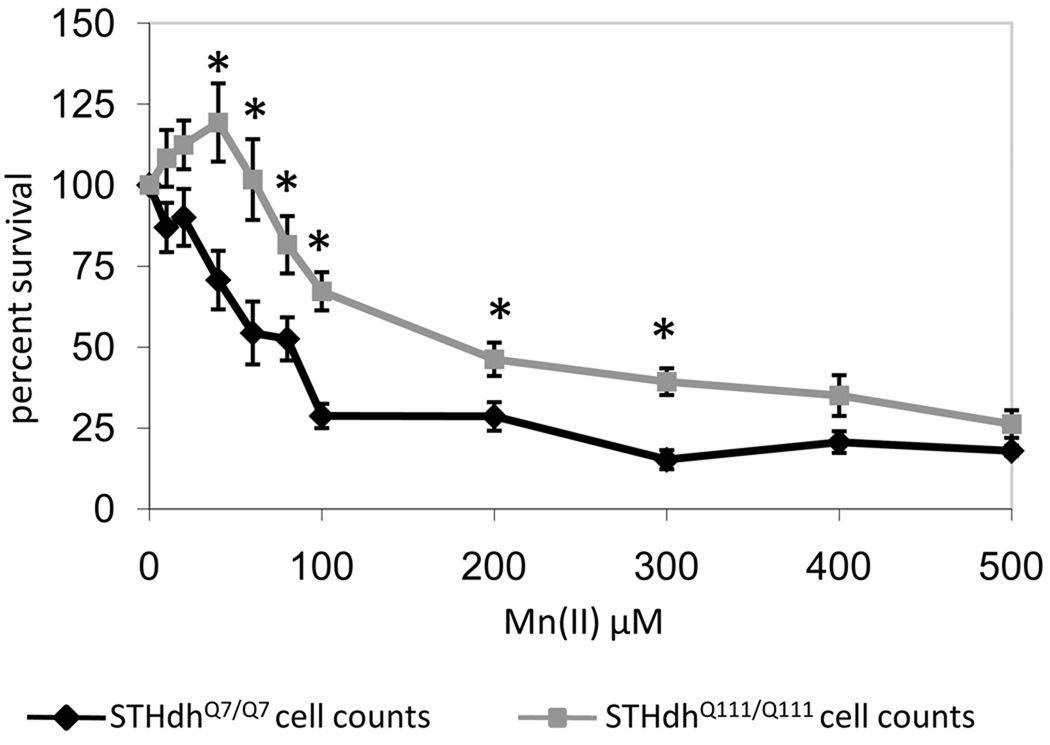
The MTT assay used in the initial screen is an indirect measure of cell survival. To determine if the HD-manganese interaction observed by MTT assay was directly due to changes in cell survival we used the trypan blue exclusion assay (Fig. 2). This independent and expanded survival curve for Mn(II) confirmed that the gene-environment interaction seen by MTT assay was due to changes in cell survival rather than just differences in mitochondrial reductase activity (Fig. 1 and Fig. 2). Statistical analysis by two-way univariate ANOVA found a significant effect of genotype (F(1,88)=59.3, p<0.001) on cell survival with Mn(II) exposure, in addition a two-way interaction between genotype and Mn(II) exposure was detected (F(10,88)=2.1, p=0.039) indicating that each genotype had a unique Mn-response curve. Over a broad range of Mn(II) exposures (40–300µM) the HD mutation limited Mn(II) cytotoxicity of the metal when compared to the wild-type cell line (p<0.05). At the highest concentrations of Mn(II) tested (400–500µM), the toxicity is not significantly different between wild-type and mutant cell lines. Therefore, the survival curve for Mn(II) shows a gene-environment interaction wherein mutant htt counters the toxic effects of Mn(II) exposure.
Fig. 2.
Mutant HD striatal cells are resistant to Mn(II) cytotoxicity. Equal numbers of wild-type STHdhQ7/Q7 (black) or mutant STHdhQ111/Q111 (grey) cells were exposed to increasing concentrations of manganese chloride. Cell survival was assessed 30 hours after exposure by trypan blue exclusion assay. The percent of viable cells relative to the untreated control is shown (± SEM. n=3 independent experiments). Significant differences in survival (* p<0.05 post-hoc t-test) between wild-type and mutant cells are shown.
40µM Mn(II) exposure does not significantly alter htt protein levels
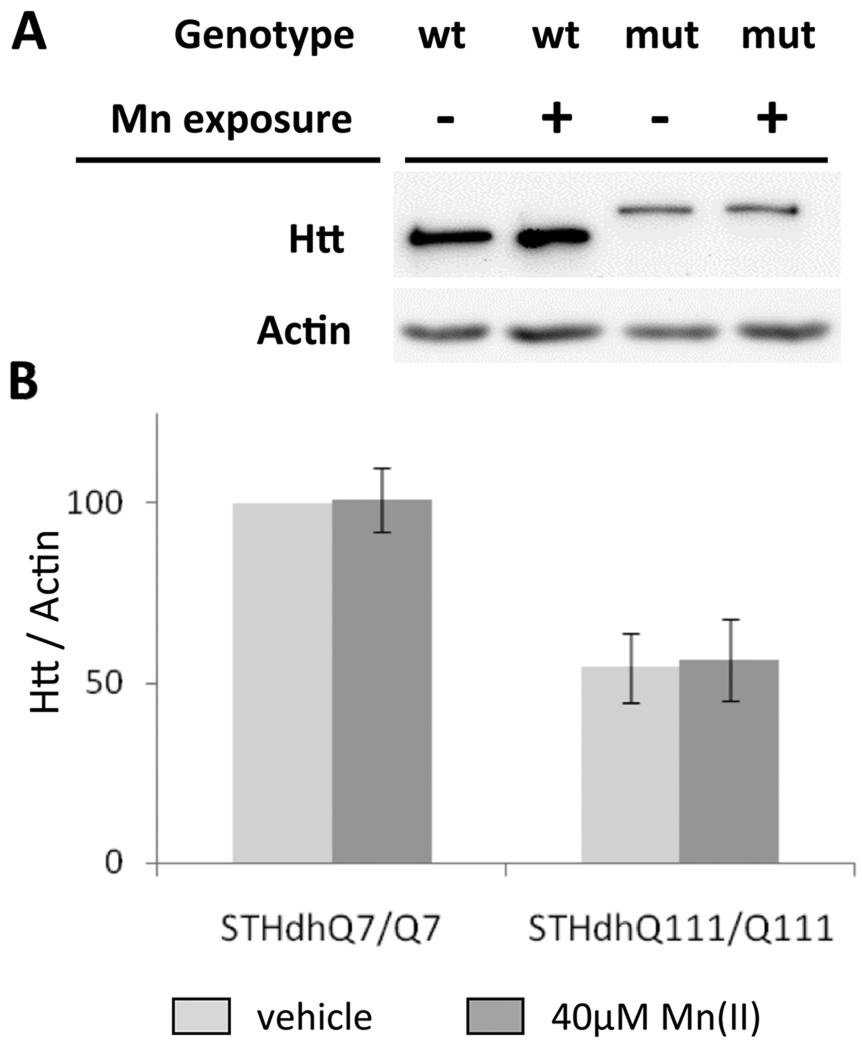
The htt gene has been shown to be Fe-responsive (Hilditch-Maguire et al. 2000) and Mn(II) exposure is known to alter cellular Fe levels (Zheng et al. 1999). To test the hypothesis that manganese exposure might influence cell viability by altering htt protein levels, we used western blot analysis to measure mutant and wild-type htt levels in the HD cell model (Fig. 3A). Quantitative analysis demonstrated that htt levels were not significantly altered in wild-type or mutant striatal cells after exposure to 40 µM Mn(II) for 30 hours (Fig 3B). As previously reported, mutant htt levels are decreased in the STHdhQ111/Q111 cells relative to wild-type protein levels in the wild-type cell line (Fig. 3) (Trettel et al. 2000).
Fig. 3.
Mn(II) exposure does not alter htt protein levels in striatal cells. (A) representative blot showing lysates from STHdhQ7/Q7 (wt) or STHdhQ111/Q111(mut) cells after 30 hours of 40µM Mn(II) chloride exposure (+) were analyzed by western blot. The htt protein from mutant animals runs at a higher molecular weight due to the glutamine-tract expansion. (B) Quantification of htt protein expression in striatal cell lines relative to actin. Mean values are plotted as a percentage of the vehicle exposed wild-type cells (± SEM. n=7 independent samples). Statistical analysis of htt protein levels by two-way univariate ANOVA found a significant effect of genotype (F(1,28)=28.1, p<0.001), but indicated no significant effect of Mn(II) exposure (F(1,28)=1.13, p=0.298) or a genotype by exposure interaction (F(1,28)=1.16, p=0.292). Significant differences in htt protein levels between genotypes were detected for both vehicle and Mn(II) exposed cells (p<0.05 post-hoc t-test).
Expression of mutant HTT is sufficient for the manganese-resistance phenotype
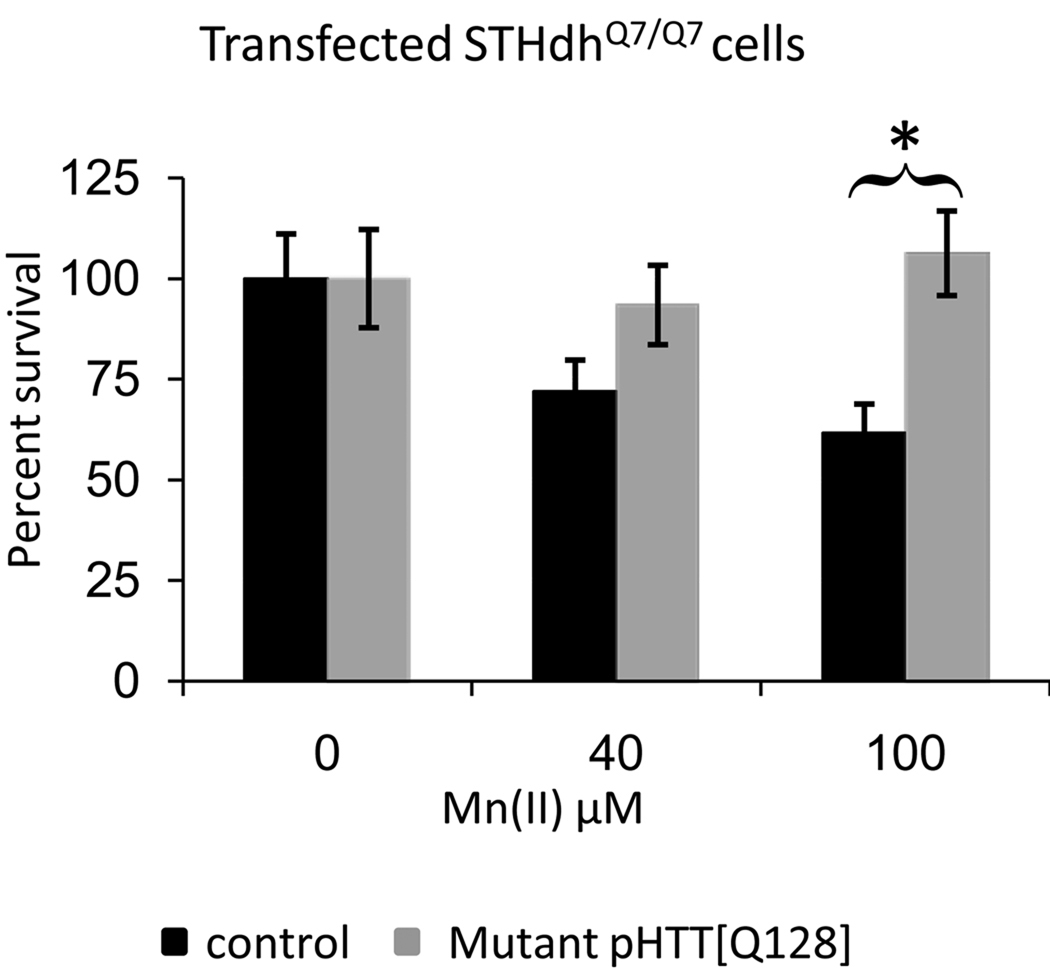
The wild-type and mutant striatal cell lines are independent lines. Thus differences in their response to Mn(II) exposure may be due to expression of mutant htt or other inherent differences between the two lines. To determine if the Mn(II) resistance phenotype is due to expression of mutant htt we transiently transfected full-length human mutant HTT with 128 repeats (HTT[128Q]) or a control vector into the wild-type STHdhQ7/Q7 cells. Transfected cells were exposed to vehicle, 40µM Mn(II), or 100 µM Mn(II) and cell survival assessed by microscopy (Fig. 4). Statistical analysis by two-way univariate ANOVA revealed a significant difference between HTT[128Q] and control transfected cells (F(1,108)=7.33, p=0.008) and a significant two-way interaction between Mn(II) exposure and transfection (F(2,108)=3.92, p=0.023). Post-hoc analysis indicated significantly higher cell survival following Mn(II) exposure in the HTT[128Q] transfected cells relative to control cells (Fig. 4). Therefore, expression of mutant HTT is sufficient to confer resistance to manganese toxicity.
Fig. 4.
Expression of mutant HTT confers Mn-resistance phenotype. The vectors pHTT[128Q] and pEGFP-N1 were transiently expressed in the STHdhQ7/Q7 cell line and compared to a control vector plus pEGFP-N1 transfection. Following exposure to Mn(II) chloride for 30 hours, cells were stained with DAPI then analyzed by fluorescence microscopy. The number of surviving cells per visual field (18 images per transfection/exposure group, 9 images from 2 independent coverslips) was counted by automated image analysis using NIH ImageJ software. Mean values are plotted as the percentage of cells relative to the average number of cells (± SEM) in vehicle exposed controls for each transfection condition, n=18 images. Significant differences in survival (* p<0.05 post-hoc t-test) between HTT[128Q] expressing and control cells are indicated.
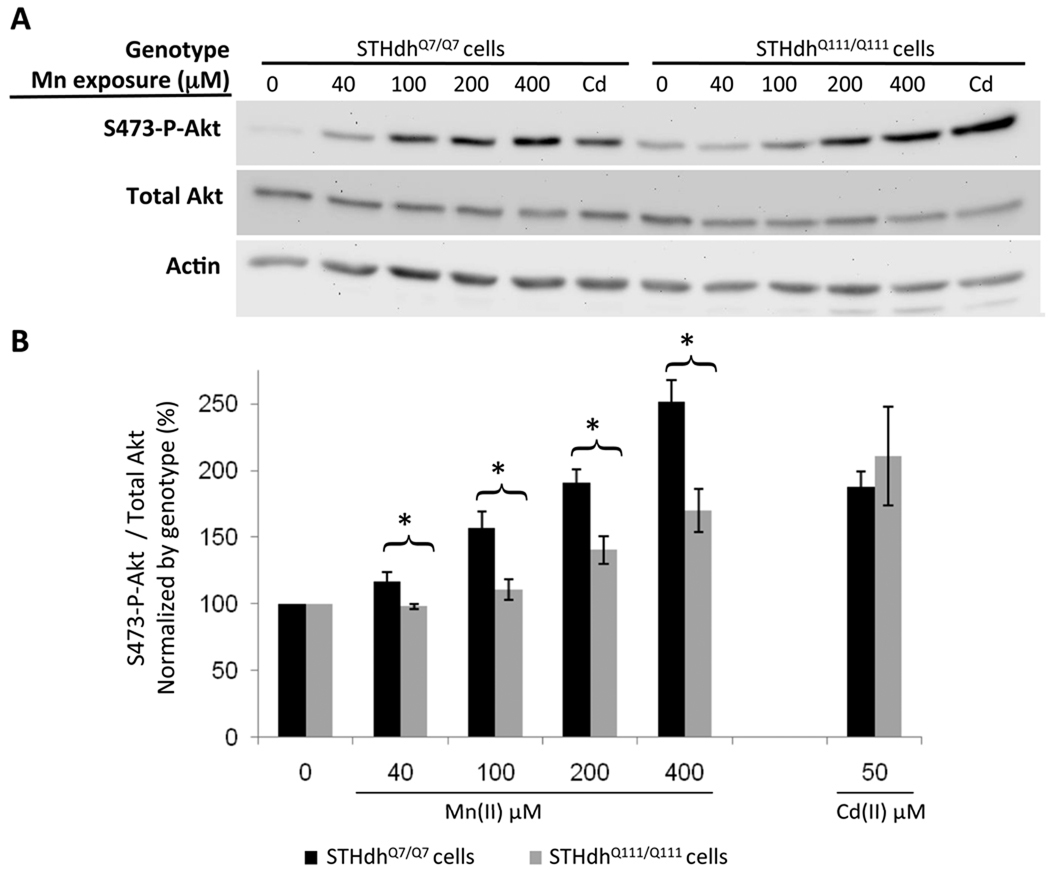
Diminished Mn(II)-dependent Akt activation in HD striatal cells
As changes in S473-phosphorylated Akt (S473-P-Akt) levels are associated with both Mn(II) toxicity and HD, we hypothesized that this signaling pathway would show disease-toxicant interactions (Bae et al. 2006, Colin et al. 2005, Gines et al. 2003a, Humbert et al. 2002, Lee et al. 2009, Liao et al. 2007, Warby et al. 2005). To begin we quantified S473-P-Akt levels following a 30 hour exposure to 40µM Mn(II). This exposure led to a manganese-dependent increase in S473-P-Akt levels (p<0.05) in wild-type cells, yet mutant cells showed no detectable change in S473-P-Akt levels relative to untreated mutant cells (data not shown). As expected from previous work, S473-P-Akt levels were elevated in the mutant line versus wild-type (Gines et al. 2003a). Thus, the lack of a manganese-dependent increase in S473-P-Akt in the mutant cells may be due to a general inability of these cells to increase S473-P-Akt levels beyond their already higher basal levels. To determine if higher levels of Mn(II) are capable of increasing S473-P-Akt levels, we exposed cells for a shorter time point (3 hours) to allow us to collect protein before significant cell death. At this time point, other studies reported strong manganese-dependent increases in S473-P-Akt levels (Bae et al. 2006, Lee et al. 2009). We found that mutant STHdhQ111/Q111 cells were capable of increasing S473-P-Akt levels in response to high concentrations of Mn(II). However, while 40µM Mn(II) was sufficient to significantly raise S473-P-Akt above basal levels in wild-type cells (p<0.05), we did not detect a significant increase in S473-P-Akt above its higher basal levels in mutant cells until 200µM Mn(II) or higher (p<0.05) (Fig. 5 and Fig. S2). Interestingly, at exposures of 100µM and above, the levels of S473-P-Akt in the wild-type cells increased sufficiently to equal the levels seen in mutant cells exposed at the same Mn(II) concentration (Fig. S2). Controlling for the elevated S473-P-Akt levels in the mutant cells by normalizing the basal levels of each genotype to 100%, mutant cells exhibited a significant decrease in S473-P-Akt activation relative to wild-type cells across all tested Mn(II) concentrations (p<0.05) (Fig. 5).
Fig. 5.
Diminished manganese-dependent Akt phosphorylation in HD striatal cells. (A) Lysates harvested from STHdhQ7/Q7 (wild-type) or STHdhQ111/Q111 (mutant) cells after 3 hours of manganese chloride exposure were analyzed by western blot for phosphorylated Akt (S473-P-Akt), total Akt and actin. Representative blots are shown. (B) Quantification of S473-P-Akt/total Akt expression in striatal cell lines. Mean values were normalized by genotype to the vehicle-only control (± SEM, n=4 independent samples). Significant differences in protein levels (* p<0.05 post-hoc t-test) between genotypes are indicated for each exposure.
Enhanced Cd(II)-dependent Akt activation in HD striatal cells
To evaluate whether the differential phosphorylation of Akt following Mn(II) exposure in mutant cells is a specific marker of the manganese-HD interaction, we tested the effect of Cd(II) exposure on S473-P-Akt levels in the STHdh cell lines. We chose to test cadmium given its opposite effect from manganese on cell survival (Fig 1). Published evidence has demonstrated that Cd(II) exposure leads to phosphorylation of Akt at S473 in a variety of cell types (Thevenod 2009). A 3 hour exposure of STHdh cells to 50µM Cd(II) increased S473-P-Akt levels in both wild-type and mutant lines (Fig. 5 and S2). Indeed, despite the elevated basal levels of S473-P-Akt, Cd(II) exposed mutant STHdhQ111/Q111 cells had significantly higher levels of S473-P-Akt than wild-type cells (p<0.05) (Fig. S2). Controlling for the difference in basal S473-P-Akt levels, mutant and wild-type cells both had a similar ∼2-fold increase in S473-P-Akt levels (Fig. 5). Thus the elevated basal S473-P-Akt levels in the mutant cells do not limit per se the capacity of mutant cells to increase the S473-P-Akt to total Akt ratio.
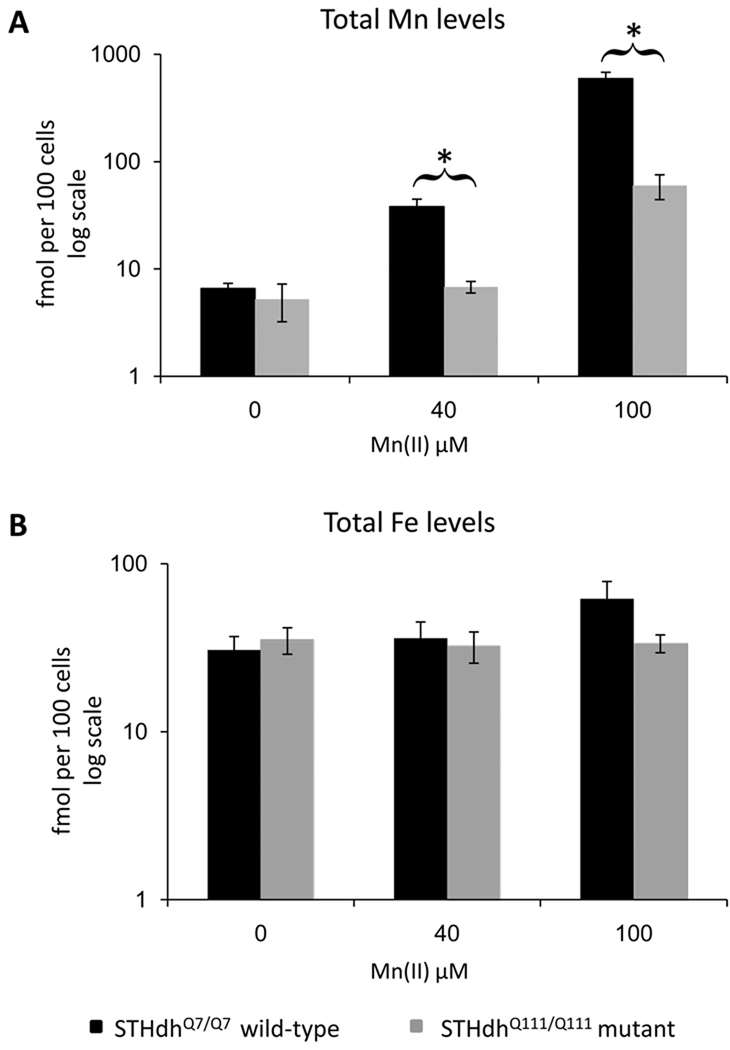
Cellular manganese accumulation is substantially impaired by mutant htt
Given the severely blunted S473-P-Akt response of the mutant cells to Mn(II) exposure, we tested the hypothesis that the expression of mutant htt impedes the cellular stress response to Mn(II) by decreasing accumulation of manganese during exposure. We measured total intracellular manganese and iron levels following Mn(II) exposure in the STHdhQ7/Q7 and STHdhQ111/Q111 cell lines by GFAAS (Garcia et al. 2007). We observed that the STHdhQ111/Q111 cells accumulated 4-fold (p<0.005) and 10-fold (p<0.001) less manganese after a 30 hour exposure to 40µM Mn(II) and 100µM Mn(II) respectively (Fig. 6A). No difference in basal manganese levels was seen, however, the manganese levels in the vehicle-only samples were near the lower detection limit of the GFAAS. Examination of total iron levels in these cells revealed no differences between wild-type and mutant cells at basal state or 40µM Mn(II) exposure, though wild-type cells showed a trend towards elevated total iron at the 100µM Mn(II) exposures (p=0.13) not seen in the mutant cells (Fig. 6B). Thus differences in manganese accumulation between wild-type and mutant cells did not correlate with significant changes in iron accumulation.
Fig. 6.
Substantial decrease in manganese accumulation in HD striatal cells. Measurement of total intracellular manganese in (black) STHdhQ7/Q7 or (grey) STHdhQ111/Q111 cell lines after application of indicated concentrations of Mn(II) chloride for 30 hours. (A) The average amount of intracellular manganese is plotted on log scale (± SEM, n=5 independent samples). (B) Measurement of total intracellular iron levels after application of Mn(II) chloride for 30 hours. The average amount of intracellular iron is plotted on log scale (± SEM, n=5 independent samples). Significant differences in metal levels (* p<0.05 post-hoc t-test) between wild-type and mutant STHdh cells for each exposure are indicated.
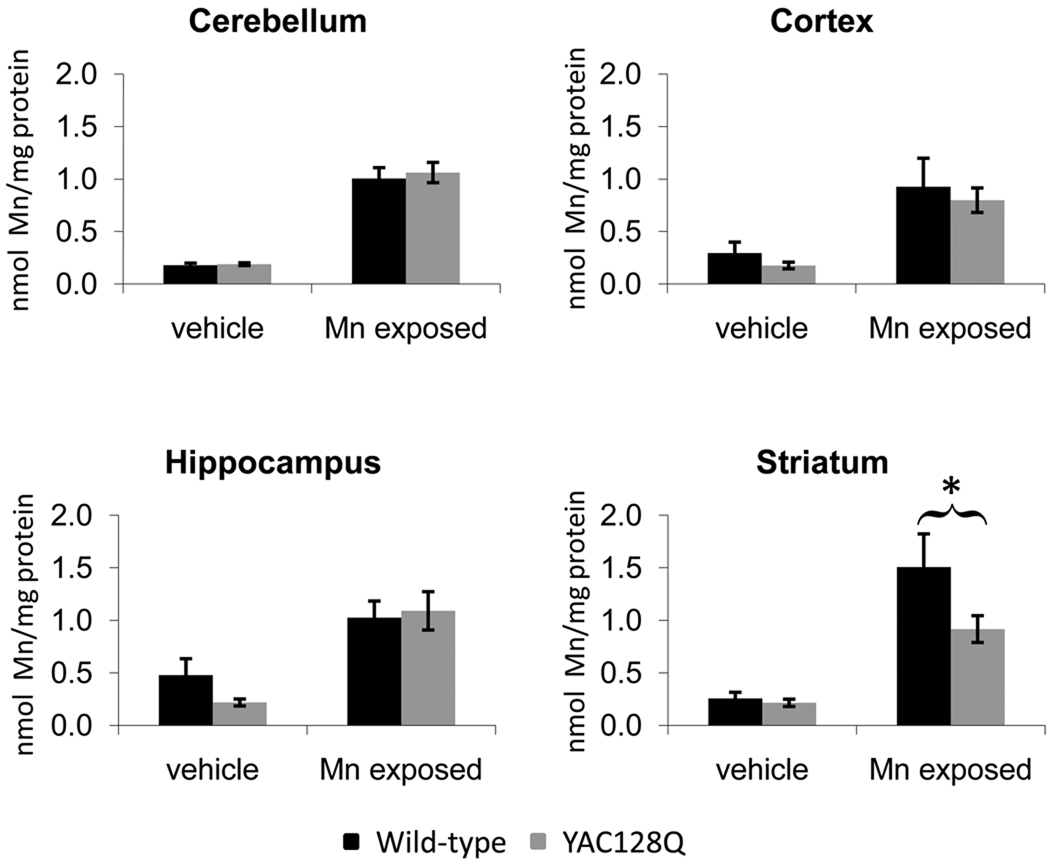
Striatal specific deficit in total manganese accumulation in YAC128Q HD mouse model
To determine if expression of mutant HTT influences manganese accumulation in vivo, we exposed 3-month (presymptomatic) YAC128Q HD mice and wild-type littermates to Mn(II) using a subcutaneous manganese exposure paradigm (Dodd et al. 2005, Slow et al. 2003). Loss of striatal volume in the YAC128Q HD mouse model is absent as late as 6 months of age, and is first detected at 9 months of age (Slow et al. 2003). Analyses of cerebellar, cortical, hippocampal, and striatal manganese levels by GFAAS indicated that Mn(II) exposed wild-type animals had increased total manganese levels in all four brain regions with the striatum having the greatest accumulation (Fig. 7). Cerebellum, cortex and hippocampus showed similar increases in manganese levels between wild-type and mutant animals, while striatum from wild-type Mn(II) exposed animals showed higher levels than mutant (1.51 nmol Mn/mg protein versus 0.92 nmol Mn/mg protein). A multivariate two-way ANOVA was used to analyze regional manganese levels (Table S1). ANOVA found a significant effect of Mn(II) exposure for each of the four brain regions (p<0.05). A significant effect of genotype and a genotype by exposure two-way interaction was found for striatum only (p<0.05). Post-hoc analysis of striatal manganese levels showed significantly less (p<0.05) manganese accumulation in mutant versus wild-type Mn(II) exposed animals (Fig. 7). We also examined iron levels in the same brain tissues. Data were analyzed by multivariate two-way ANOVA model (Fig. S3 and Table S2). This analysis failed to find a significant effect of Mn(II) exposure or a two-way genotype by Mn(II) exposure interaction for iron levels in any brain region, though a significant genotype difference in cerebellar iron levels was seen (p=0.024). Finally, we have confirmed the impaired striatal manganese accumulation in the YAC128Q HD animals in an independent set of animals by inductively coupled plasma mass spectrometry (data not shown). Therefore, a mouse model of HD that recapitulates the selective degeneration of the corpus striatum observed in patients, also exhibited an early and selective deficit in striatal manganese accumulation after Mn(II) exposure.
Fig. 7.
Reduced striatal manganese uptake in the YAC128Q HD mouse model. Wild-type and YAC128Q HD mice at 3 months of age were exposed to Mn(II) by subcutaneous injection on Day 0, 3, and 7. Brain regions, tails, and whole blood were harvested on Day 8, and total manganese levels per mg of protein were determined by GFAAS. Mean manganese levels ± SEM are shown (n = 12 to 14 samples for each genotype-exposure group; 53 animals total). Total iron levels are shown in Fig. S3. ANOVA analysis of total manganese levels showed a significant effect of Mn(II) exposure for all four brain regions (p<0.05). A significant genotype and genotype by Mn(II) exposure two-way interaction was found for the striatum only (p<0.05). Post-hoc analysis of the exposure effect showed a significant increase in manganese levels in Mn(II)-exposed animals compared to vehicle-only animals of the same genotype (p<0.05 post-hoc t-test, not indicated). Post-hoc analysis of the genotype effect found a significant difference in striatal manganese accumulation between wild-type and mutant animals as indicated (* p<0.05 post-hoc t-test).
Discussion
Our study has used a disease-toxicant interaction screen to evaluate toxicants that may share pathophysiological mechanisms underlying HD neurodegenerative processes. The approach exploited the diverse toxicology of metals to reveal processes that may underlie gene-environment interactions in disease. Metals were the toxicant of choice for this screen because they are known to alter cellular pathways also implicated in HD pathology. We tested eight metals and the mitochondrial complex II inhibitor 3NPA for modification of cell survival in the presence of either wild-type htt or a polyglutamine-expanded form of the protein. 3NPA was a positive control showing increased toxicity in the presence of the glutamine-expansion as previously reported (Fig. S1). The wild-type and HD mutant cell lines showed no difference in their response to the metals Cu(II), Fe(III), Pb(II), Co(II), Ni(II) or Zn(II) (Fig. 1). In contrast, the mutant HD cell line was found to have enhanced sensitivity to Cd(II) toxicity, and resistance to Mn(II) toxicity. The selective effect of these two metals strongly suggests that the HD mutation can alter the influence of specific environmental toxicants on striatal neurons and validates the utility of a disease-toxicant interaction screen to identify pathways of gene-environment interactions.
Oxidative stress is known to be a key factor in copper and iron mediated toxicity and is a potential mechanism of HD pathology. The lack of a significant difference in toxicity between genotypes with these metals is consistent with an earlier study that found no difference in vulnerability to oxidative stress in another HD cell model (Fig. 1) (Snider et al. 2003). Furthermore, it suggests that a change in sensitivity to oxidative stress is not the source of the altered vulnerability of mutant STHdhQ111/Q111 cells to cadmium, manganese or 3NPA (Fig. 1 and S1).
Cadmium and 3NPA exert their toxic effects directly on the mitochondria (Li et al. 2003, Mao et al. 2006). The mutant STHdhQ111/Q111 cells are reported to have an increased susceptibility to multiple mitochondrial stressors (Gines et al. 2003b, Seong et al. 2005, Oliveira et al. 2006). More recently, Cd(II) toxicity has been linked to aberrant activation of mitogen-activated protein kinases (MAPK) and the mammalian target of rapamycin (mTOR) cell signaling pathways (Lopez et al. 2006, Chen et al. 2008). Disruption of these signaling pathways has also been suggested in HD (Apostol et al. 2006, Ravikumar et al. 2004). Further investigation is needed to determine the relative contribution of these and other mechanisms to the increased sensitivity of STHdhQ111/Q111 cells to Cd(II) toxicity.
Activation of Akt via S-473 phosphorylation has been associated with neuroprotection (Brunet et al. 2001). Yet, we found that the increased sensitivity of mutant STHdhQ111/Q111 cells to Cd(II) toxicity correlated with increased S473-P-Akt levels relative to wild-type exposed cells, while the decreased sensitivity to Mn(II) toxicity correlated with a blunted S473-P-Akt response. While this observation does not rule out a neuroprotective role for Akt signaling in Mn(II) or Cd(II) toxicity, it does demonstrate that enhanced Akt activation between wild-type and HD mutant cells does not correlate with improved cell survival following exposure to these environmental toxicants in the STHdh cellular model. Future studies are needed to explore changes in Akt signaling following exposure to these metals in animal models of HD. Besides Akt, several other kinases are reported to be activated upon Mn(II) exposure including ERK (Extracellular signal-regulated kinase), p38, and JNK (c-Jun N-terminal kinase) (Bae et al. 2006, Moreno et al. 2008, Lee et al. 2009). Additionally, Cd(II) has been shown to affect MAPK/ERK signaling in hippocampal slices (Rigon et al. 2008). It will be interesting to evaluate how HD influences activation of these and other signaling systems that are modulated by Mn(II) and Cd(II).
We report the discovery that expression of the disease-causing allele of htt suppresses Mn(II) toxicity (Fig. 1, 2 and 4). This neuroprotective interaction was highly metal specific, suggesting a unique relationship between mutant htt and Mn(II) toxicity. We found no evidence that htt protein levels are altered by 40µM Mn(II) exposure, indicating that survival differences between cells does not depend on changes in the expression of the toxic mutant htt protein. However, it remains possible that higher levels of Mn(II) exposure may be able to alter the expression of htt. Importantly, dosimetry studies have revealed that manganese concentrations in rodent striatum are normally between 4µM to 18µM, and can increase to as high as 70µM in Mn(II) exposed animals (Aschner et al. 2005). Thus, our observation of a htt-manganese gene-environment interaction at Mn(II) exposures in this range are potentially pathologically relevant. Further work is needed to evaluate the relationship between manganese homeostasis and HD.
The significant decrease in net manganese accumulation in the mutant cells justifies the strong Mn(II) resistance phenotype, and suggests a manganese homeostatic defect due to mutant HTT. Of particular note, a comparison of cell survival and total manganese accumulation reveals that wild-type cells exposed to 40µM Mn(II) have statistically indistinguishable cell survival and total manganese levels relative to mutant cells exposed at 100µM Mn(II) (Fig. 2 versus Fig. 6A). These data strongly suggest that impairment in manganese accumulation may contribute, at least in part, to the Mn(II) resistance phenotype of STHdhQ111/Q111 cells. Furthermore, the cellular disease-toxicant interaction accurately predicted a defect in manganese accumulation in the striatum of the YAC128Q HD mouse model. Indeed, the in vivo study showed a manganese-accumulation deficit in the very brain region most vulnerable in HD (Fig. 7). However, additional work is needed to determine if the specific nature of the manganese-accumulation defect is similar between the cellular and animal models (e.g. extracellular versus intracellular accumulation). We propose three possible general mechanisms for the deficient manganese accumulation in the mutant STHdhQ111/Q111 cells: (1) a decrease in manganese uptake, (2) an increase in manganese export, and (3) a decrease in manganese storage capacity. Future studies will explore the kinetics of the manganese transport defect to elucidate the cellular mechanism and transporters involved.
Supplementary Material
Acknowledgments
We would like to thank Marcy MacDonald, Ph.D. (Massachusetts General Hospital) for generous gifts of the STHdh cell lines; Juan Botas, Ph.D. (Baylor College of Medicine) for providing us with a full length Huntingtin cDNA. We are also grateful to Drs. Pat Levitt and Beth Ann McLaughlin (Vanderbilt University) for use of equipment. We are grateful to Heather Tanner for technical assistance. We also thank Roger Colbran, Doug Mortlock and Diana Neely for insightful discussions. Funding was provided through the Center for Molecular Toxicology at Vanderbilt University NIH 5P30 ES00026 (ABB). This work was supported by NIH/NIEHS RO1ES016931 (ABB) and RO1ES10563 (MA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or NIH.
Abbreviations
- 3NPA
3-Nitroproprionic Acid
- ERK
Extracellular signal-regulated kinase
- GFAAS
graphite furnace atomic absorption spectroscopy
- HD
Huntington’s disease
- HTT
Full-length Huntingtin gene or protein
- HTT[128Q]
Full-length HTT with 128 glutamines in the polyglutamine domain
- JNK
c-Jun N-terminal kinase
- MAPK
Mitogen-activated protein kinases
- mTOR
Mammalian target of rapamycin
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide salt
- S473-P-Akt
Activated Akt phosphorylated at S473
- pHTT[128Q]
HTT[128Q] mammalian expression construct
- STHdhQ111/Q111
Striatal cell line expressing mutant mouse huntingtin
- STHdhQ7/Q7
Striatal cell line expressing wild-type mouse huntingtin
- YAC128Q
HD animal model expressing mutant Huntingtin with 128 repeats
References
- Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Extracellular norepinephrine, norepinephrine receptor and transporter protein and mRNA levels are differentially altered in the developing rat brain due to dietary iron deficiency and manganese exposure. Brain Res. 2009;1281:1–14. doi: 10.1016/j.brainres.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol BL, Illes K, Pallos J, et al. Mutant huntingtin alters MAPK signaling pathways in PC12 and striatal cells: ERK1/2 protects against mutant huntingtin-associated toxicity. Hum Mol Genet. 2006;15:273–285. doi: 10.1093/hmg/ddi443. [DOI] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Bae JH, Jang BC, Suh SI, Ha E, Baik HH, Kim SS, Lee MY, Shin DH. Manganese induces inducible nitric oxide synthase (iNOS) expression via activation of both MAP kinase and PI3K/Akt pathways in BV2 microglial cells. Neurosci Lett. 2006;398:151–154. doi: 10.1016/j.neulet.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins BG, et al. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13:4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman AB, Lam YC, Jafar-Nejad P, et al. Duplication of Atxn1l suppresses SCA1 neuropathology by decreasing incorporation of polyglutamine-expanded ataxin-1 into native complexes. Nat Genet. 2007;39:373–379. doi: 10.1038/ng1977. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Bush AI. Metals and neuroscience. Curr Opin Chem Biol. 2000;4:184–191. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Cattaneo E, Conti L. Generation and characterization of embryonic striatal conditionally immortalized ST14A cells. J Neurosci Res. 1998;53:223–234. doi: 10.1002/(SICI)1097-4547(19980715)53:2<223::AID-JNR11>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Luo Y, Huang S. MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J Neurochem. 2008;105:251–261. doi: 10.1111/j.1471-4159.2007.05133.x. [DOI] [PubMed] [Google Scholar]
- Colin E, Regulier E, Perrin V, Durr A, Brice A, Aebischer P, Deglon N, Humbert S, Saudou F. Akt is altered in an animal model of Huntington's disease and in patients. Eur J Neurosci. 2005;21:1478–1488. doi: 10.1111/j.1460-9568.2005.03985.x. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114(Pt 4):1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- Dodd CA, Ward DL, Klein BG. Basal Ganglia accumulation and motor assessment following manganese chloride exposure in the C57BL/6 mouse. Int J Toxicol. 2005;24:389–397. doi: 10.1080/10915810500366500. [DOI] [PubMed] [Google Scholar]
- Ehrich M, Sharova L. In Vitro Methods for Detecting Cytotoxcity: Current Protocols in Toxicology. 2000 doi: 10.1002/0471140856.tx0206s03. [DOI] [PubMed] [Google Scholar]
- Fox JH, Kama JA, Lieberman G, et al. Mechanisms of Copper Ion Mediated Huntington's Disease Progression. PLoS ONE. 2007;2:e334. doi: 10.1371/journal.pone.0000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M. Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol Sci. 2007;95:205–214. doi: 10.1093/toxsci/kfl139. [DOI] [PubMed] [Google Scholar]
- Gines S, Ivanova E, Seong IS, Saura CA, MacDonald ME. Enhanced Akt signaling is an early pro-survival response that reflects N-methyl-D-aspartate receptor activation in Huntington's disease knock-in striatal cells. J Biol Chem. 2003a;278:50514–50522. doi: 10.1074/jbc.M309348200. [DOI] [PubMed] [Google Scholar]
- Gines S, Seong IS, Fossale E, Ivanova E, Trettel F, Gusella JF, Wheeler VC, Persichetti F, MacDonald ME. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington's disease knock-in mice. Hum Mol Genet. 2003b;12:497–508. doi: 10.1093/hmg/ddg046. [DOI] [PubMed] [Google Scholar]
- Hilditch-Maguire P, Trettel F, Passani LA, Auerbach A, Persichetti F, MacDonald ME. Huntingtin: an iron-regulated protein essential for normal nuclear and perinuclear organelles. Hum Mol Genet. 2000;9:2789–2797. doi: 10.1093/hmg/9.19.2789. [DOI] [PubMed] [Google Scholar]
- Humbert S, Bryson EA, Cordelieres FP, Connors NC, Datta SR, Finkbeiner S, Greenberg ME, Saudou F. The IGF-1/Akt pathway is neuroprotective in Huntington's disease and involves Huntingtin phosphorylation by Akt. Dev Cell. 2002;2:831–837. doi: 10.1016/s1534-5807(02)00188-0. [DOI] [PubMed] [Google Scholar]
- Imarisio S, Carmichael J, Korolchuk V, et al. Huntington's disease: from pathology and genetics to potential therapies. Biochem J. 2008;412:191–209. doi: 10.1042/BJ20071619. [DOI] [PubMed] [Google Scholar]
- Kreutz C, Bartolome Rodriguez MM, Maiwald T, Seidl M, Blum HE, Mohr L, Timmer J. An error model for protein quantification. Bioinformatics. 2007;23:2747–2753. doi: 10.1093/bioinformatics/btm397. [DOI] [PubMed] [Google Scholar]
- Lee ES, Yin Z, Milatovic D, Jiang H, Aschner M. Estrogen and tamoxifen protect against Mn-induced toxicity in rat cortical primary cultures of neurons and astrocytes. Toxicol Sci. 2009;110:156–167. doi: 10.1093/toxsci/kfp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Xia T, Jiang CS, Li LJ, Fu JL, Zhou ZC. Cadmium directly induced the opening of membrane permeability pore of mitochondria which possibly involved in cadmium-triggered apoptosis. Toxicology. 2003;194:19–33. doi: 10.1016/s0300-483x(03)00327-5. [DOI] [PubMed] [Google Scholar]
- Liao SL, Ou YC, Chen SY, Chiang AN, Chen CJ. Induction of cyclooxygenase-2 expression by manganese in cultured astrocytes. Neurochem Int. 2007;50:905–915. doi: 10.1016/j.neuint.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Lopez E, Arce C, Oset-Gasque MJ, Canadas S, Gonzalez MP. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic Biol Med. 2006;40:940–951. doi: 10.1016/j.freeradbiomed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Mao Z, Choo YS, Lesort M. Cystamine and cysteamine prevent 3-NP-induced mitochondrial depolarization of Huntington's disease knock-in striatal cells. Eur J Neurosci. 2006;23:1701–1710. doi: 10.1111/j.1460-9568.2006.04686.x. [DOI] [PubMed] [Google Scholar]
- Moreno JA, Sullivan KA, Carbone DL, Hanneman WH, Tjalkens RB. Manganese potentiates nuclear factor-kappaB-dependent expression of nitric oxide synthase 2 in astrocytes by activating soluble guanylate cyclase and extracellular responsive kinase signaling pathways. J Neurosci Res. 2008;86:2028–2038. doi: 10.1002/jnr.21640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JM, Chen S, Almeida S, et al. Mitochondrial-dependent Ca2+ handling in Huntington's disease striatal cells: effect of histone deacetylase inhibitors. J Neurosci. 2006;26:11174–11186. doi: 10.1523/JNEUROSCI.3004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Rigon AP, Cordova FM, Oliveira CS, et al. Neurotoxicity of cadmium on immature hippocampus and a neuroprotective role for p38 MAPK. Neurotoxicology. 2008;29:727–734. doi: 10.1016/j.neuro.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Lesort M, MacDonald ME, Johnson GV. Striatal cells from mutant huntingtin knock-in mice are selectively vulnerable to mitochondrial complex II inhibitor-induced cell death through a non-apoptotic pathway. Hum Mol Genet. 2004;13:669–681. doi: 10.1093/hmg/ddh082. [DOI] [PubMed] [Google Scholar]
- Seong IS, Ivanova E, Lee JM, et al. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum Mol Genet. 2005;14:2871–2880. doi: 10.1093/hmg/ddi319. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Casale M, Alcon B, Pham N, Narayan N, Lynch G. Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington's disease. Glia. 2007;55:1074–1084. doi: 10.1002/glia.20526. [DOI] [PubMed] [Google Scholar]
- Slow EJ, van Raamsdonk J, Rogers D, et al. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet. 2003;12:1555–1567. doi: 10.1093/hmg/ddg169. [DOI] [PubMed] [Google Scholar]
- Snider BJ, Moss JL, Revilla FJ, Lee CS, Wheeler VC, Macdonald ME, Choi DW. Neocortical neurons cultured from mice with expanded CAG repeats in the huntingtin gene: unaltered vulnerability to excitotoxins and other insults. Neuroscience. 2003;120:617–625. doi: 10.1016/s0306-4522(03)00382-8. [DOI] [PubMed] [Google Scholar]
- Thevenod F. Cadmium and cellular signaling cascades: to be or not to be? Toxicol Appl Pharmacol. 2009;238:221–239. doi: 10.1016/j.taap.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Trettel F, Rigamonti D, Hilditch-Maguire P, Wheeler VC, Sharp AH, Persichetti F, Cattaneo E, MacDonald ME. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum Mol Genet. 2000;9:2799–2809. doi: 10.1093/hmg/9.19.2799. [DOI] [PubMed] [Google Scholar]
- Warby SC, Chan EY, Metzler M, Gan L, Singaraja RR, Crocker SF, Robertson HA, Hayden MR. Huntingtin phosphorylation on serine 421 is significantly reduced in the striatum and by polyglutamine expansion in vivo. Hum Mol Genet. 2005;14:1569–1577. doi: 10.1093/hmg/ddi165. [DOI] [PubMed] [Google Scholar]
- Ying HS, Gottron FJ, Choi DW. Assessment of Cell Viability in Primary Neuronal Cultures: Current Protocols in Neuroscience. John Wiley & Sons, Inc; 2000. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res. 1999;833:125–132. doi: 10.1016/s0006-8993(99)01558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.