Abstract
A new method for the rapid and efficient coupling of homopolymers to yield di- and triblock copolymers as well as cyclic polymers using the 3 + 2 π Huisgen copper catalyzed cyclo-addition reaction has been developed. This facile method utilizes commercially available Cu nanoparticles that are tolerant to O2, easily removable and recyclable.
Keywords: Click chemistry, block copolymer, cyclic polymers, heterogeneous catalysis
Introduction
The copper-catalyzed Huisgen-dipolar cycloaddition reaction of azides with alkynes (CuAAC), which follows all of the original tenants of Click chemistry as detailed by Sharpless,1 has emerged as an important synthetic tool in the preparation of functional materials. This robust and efficient coupling reaction has been exploited in a wide range of different polymer platforms2,3 for the preparation of functionalized materials4 in areas ranging from semiconductor processing5 to drug delivery6. The efficiency of this process offers particular appeal for a major challenge in polymer synthesis – the coupling of chain end functionalized homopolymers to give block copolymers and cyclic polymers in high yield.7–9 Prior work has demonstrated success in the synthesis of block copolymers by CuAAC, however the reduced reactivity of polymeric chain ends leads to long reaction times, oxygen poisoning, and difficultly in removing the ligands/copper catalyst.7,8,10 This lowered reactivity limits many of the examples in the literature to low molecular weight block copolymers, typically 5–10 kDa with selected examples as high as 20 kDa being reported. 7,8,10 As a result there is a need to optimize the efficiency of CuAAC chemistry for polymer chain end coupling and to develop systems that operate under atmospheric conditions with facile removal of the Cu catalyst. Herein, the use of commercially available and recyclable copper nanoparticles (CuNPs) in combination with microwave reaction conditions leads to the fast and efficient synthesis of high molecular weight block and cyclic copolymers.
Results and Discussion
Following the discovery that copper metal can be a source of catalytic species for CuAAC,11 increasing attention has been devoted to the application of solid or supported Cu species as catalytic entities in order to reduce both the catalyst loading and the reaction times.12–17 In this study, commercially available Cu nanoparticles (nominal size < 50 nm)18 that can be removed by centrifugation and have low sensitivity to oxygen are shown to be a versatile and robust catalyst for producing advanced macromolecular architectures such as block and cyclic copolymers.
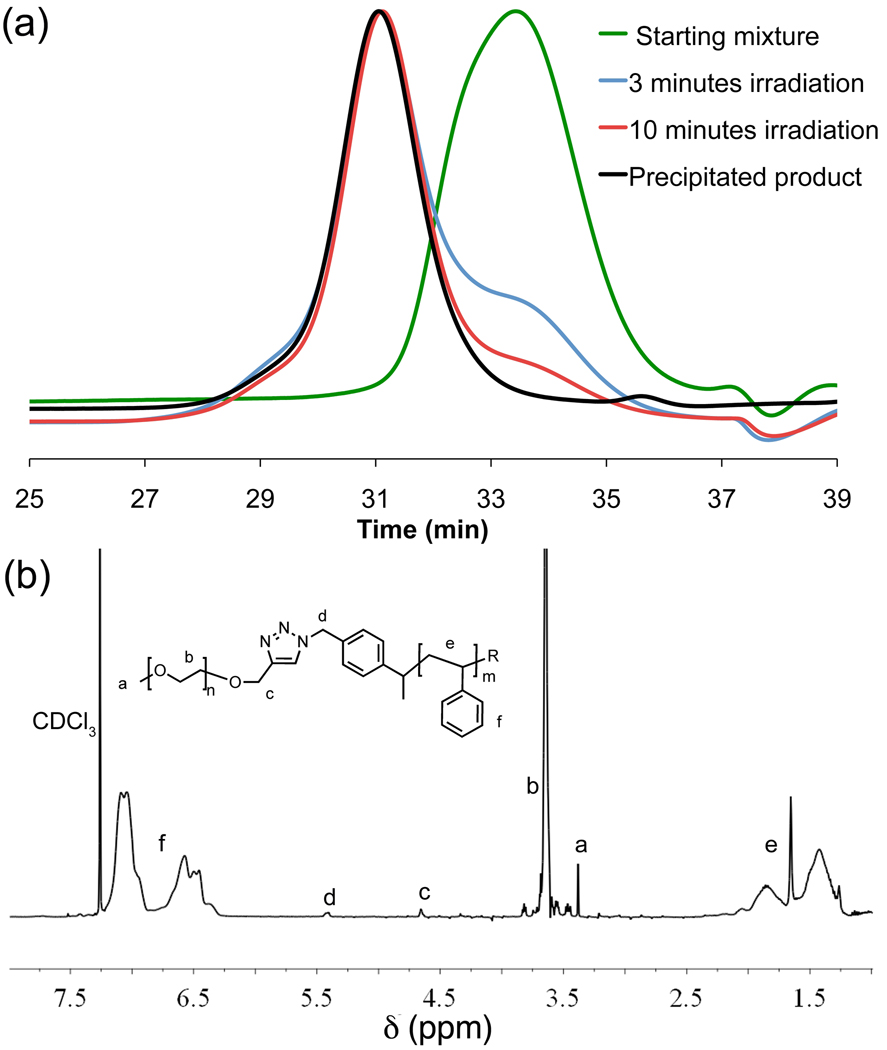
Scheme 1 shows the general procedure for production of macromolecular architectures, such as diblock and triblock copolymers, using click chemistry with microwave irradiation in the presence of CuNPs. Key to the success of these strategies is the preparation of well-defined, chain end functionalized macromolecules prepared by either living radical or ring-opening polymerization (Scheme 2). In a typical diblock copolymer synthesis (Scheme 2), 5.0 kDa poly(ethylene glycol)-acetylene (PEG-alkyne, 1) and 6.5 kDa polystyrene-azide (PS-N3, 5, 1.2 – 1.5 eq) homopolymers were dissolved in anisole (1 mL/50mg PEG). CuNPs (2 mg/50 mg PEG) were added, and the reaction mixture subjected to microwave irradiation. As the reaction is unaffected by atmospheric oxygen, no purging or inert atmosphere is required and the conversion of the coupling reaction can be easily monitored by gel permeation chromatography (GPC). GPC traces for the CuNPs catalyzed reaction of 1 and 5 demonstrates that full conversion is achieved after only 10 minutes (Figure 1). Furthermore the use of CuNPs simplifies purification as the CuNPs can be easily removed by centrifugation at 3000g for 5 min (Figure 2), followed by decantation, with the block copolymer (9) being isolated in ~90% yield after precipitation. The successful coupling of PEG and PS blocks was confirmed by multi-detector GPC as well as 1H-NMR spectroscopy, which showed the expected resonances that are unique for each block. Additionally the level of residual copper in the purified products was examined by atomic absorption spectroscopy and show to be less than 200 ppm, which is significantly lower than that observed for both traditional soluble and insoluble catalysts (ca. 500–2000 ppm)4 and represents removal of >97% of the copper with a single centrifugation step.
Figure 1.
(a) GPC elution profiles for coupling of 5.0 kDa PEG-acetylene, 1, and 6.5 kDa PS-N3, 5, to yield PEG-b-PS, 9. (b) 1H-NMR spectrum of polymer 9.
Figure 2.
Representative image showing separation of CuNPs by centrifugation.
The success of this initial block copolymer coupling reaction prompted an examination of the synthesis of higher molecular weight PEO-b-PS and PS-b-PMMA diblock and triblock copolymers using CuNPs and microwave irradiation. As shown in Table 1, formation of di- and triblocks with moderate molecular weights (ca. 8kDa – 24 kDa) typically required irradiation for 10 minutes to reach completion (8–11). In the case of the higher molecular weight, PS-b-PMMA diblock copolymer, 12, longer reaction times (4 hr) were required to achieve high coupling yields due to the low concentration and steric hindrance of chain end groups.
Table 1.
Results for the formation of di and tri-block copolymers.
| Polymer | Mn(Da)a | Mw(Da)a | PDIa | Time |
|---|---|---|---|---|
| 1 PEG5k-alkyne | 11.4k | 11.9k | 1.04 | -- |
| 2 PEG10k-alkyne | 22.8k | 24.6k | 1.08 | -- |
| 3 alkyne-PEG10k-alkyne | 23.9k | 26.1k | 1.09 | -- |
| 4 PS3k-N3 | 3.0k | 3.9k | 1.30 | -- |
| 5 PS7k-N3 | 6.5k | 8.0k | 1.24 | -- |
| 6 PS44k-N3 | 44.0k | 56.6k | 1.29 | -- |
| 7 PMMA60k-alkyne | 60.8k | 78.2k | 1.29 | -- |
| 8 PS3k-b-PEG5k | 14.2k | 15.4k | 1.08 | 10 min |
| 9 PS7k-b-PEG5k | 18.3k | 20.6k | 1.13 | 10 min |
| 10 PS7k-b-PEG10k | 25.2k | 29.9k | 1.19 | 10 min |
| 11 PS7k-b-PEG10k-b-PS7k | 32.8k | 39.6k | 1.21 | 10 min |
| 12 PS44k-b-PMMA60k | 83.1k | 110k | 1.32 | 4 hr |
GPC calibrated to PS standards.
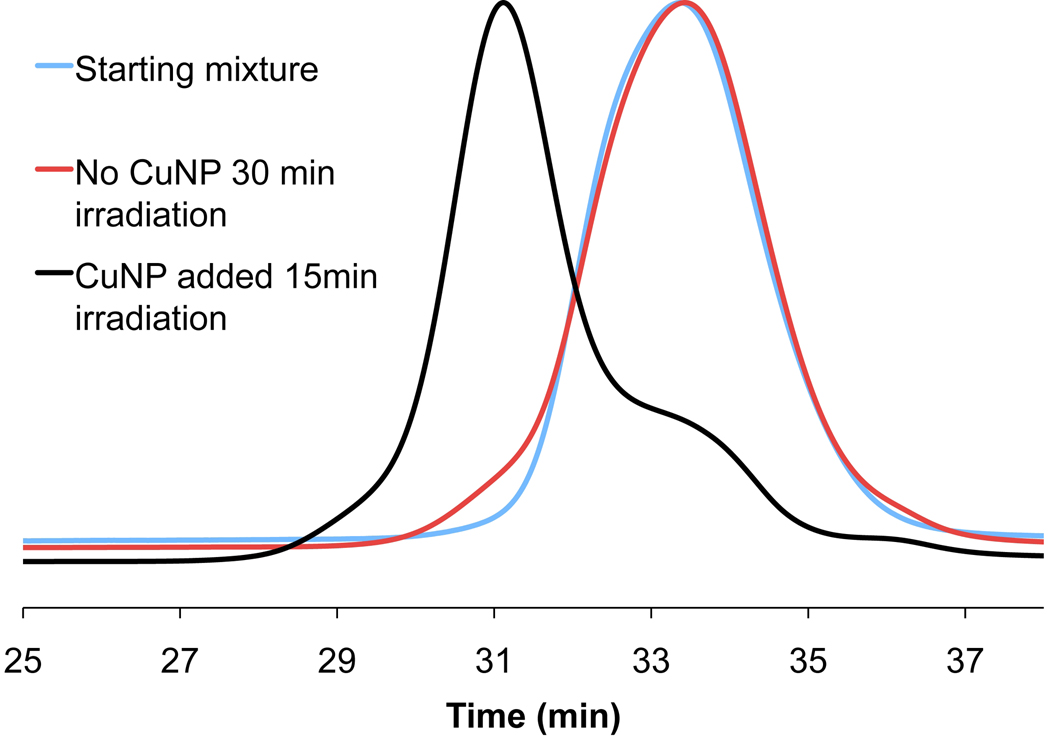
Another advantage of the CuNPs is their tolerance to atmospheric and dissolved oxygen, which allows for catalyst recycling. To examine the level of insensitivity of the reaction to oxygen, the coupling of 5 and 1 in the presence of recycled CuNPs was examined (Table 2). Recycling of the catalyst was performed by centrifugation of the initial reaction solution, removal of the supernatant, addition of additional aliquots of the starting homopolymer reaction mixture to the CuNP residue, stirring to redisperse followed by repetition of the microwave irradiation. Significantly, no decrease in reaction rate was observed after four recycling reactions. In addition, control reactions without CuNPs were performed and negligible coupling was observed demonstrating the absence of any background thermal reaction under these conditions (Figure 3). Interestingly, addition of CuNPs to these previously irradiated control samples led to essentially complete coupling under standard reaction conditions, demonstrating that both the azido and acetylene chain ends are stable under microwave irradiation and do not undergo reaction in the absence of the CuNP catalyst (Figure 3).
Table 2.
Results for click reactions with recycled copper catalyst.
| Polymer | Mn(Da)a | Mw(Da)a | PDIa | Timeb |
|---|---|---|---|---|
| 13.1 PS3k-b-PEG5k | 14.5k | 15.4k | 1.06 | 15 min |
| 13.2 PS3k-b-PEG5k | 14.4k | 16.5k | 1.15 | 15 min |
| 13.3 PS3k-b-PEG5k | 14.2k | 16.0k | 1.13 | 15 min |
| 13.4 PS3k-b-PEG5k | 14.2k | 16.0k | 1.13 | 15 min |
GPC calibrated to PS standards;
Essentially complete conversion was observed.
Figure 3.
GPC elution profiles for the control reaction of 5.0 kDa PEG alkyne (1) with 6.5 kDa PS-azide (5, 1.2 eq) at 160°C without CuNPs (30 min, crude) followed by addition of CuNPs and reaction at 160°C (15 min, crude).
The efficiency and recyclability of CuNPs for the synthesis of di- and tri-block copolymers also suggested their use in the formation of cyclic polymers (Scheme 3). The suitability of Cu nanoparticles for cyclic polymer formation arises from the heterogeneous nature of the catalyst, which may allow the acetylene or azide chain end to bind to the nanoparticle surface with cyclization being favored due to the partial immobilization of the chain. Additionally, the insensitivity of the CuNPs to deactivation permits stepwise or continuous addition of a concentrated hetero-bi-functional linear polymer solution to the reaction mixture.19–20 The speed of the reaction coupled with the inert nature of the cyclic product then alleviates the ultra-dilute conditions normally required for cyclic polymer formation. By recycling the catalyst and repetitively, adding more aliquots of linear polymer every 15 minutes, moderate to high yields could be achieved in a few hours. This allows for higher concentrations and shorter reaction times providing significant advantage over the conventional Click route to cyclic polymers, which requires large amounts of oxygen sensitive CuBr complex and extremely dilute solutions.21
When compared to prior, batch approaches, this repetitive addition strategy leads to increased final cycle concentrations coupled with a dramatic improvement in the time needed for cycle formation. For example, ~15–20 mg of cyclic polymer could be produced in 5–10 mL of solvent in <1 h under our conditions. In direct contrast, it takes more than 24 hrs to produce less than ~5 mg of cyclic polymer per 10 mL of solvent using the traditional high dilution strategy.21 Recently, Lonsdale et al. have produced cyclic polymers in higher concentration using homogenous catalysts and preparative GPC.22 Characterization of the products (Table 3) obtained from the repetitive addition of hetero-bi-functional linear PEG showed the products to be pure cyclic polymer with little or no evidence of unreacted linear polymer or higher molecular weight materials by a combination of GPC, NMR and MALDI-TOF mass spectrometry (Figures 4 and 5).
Table 3.
Results for click reactions with recycled copper catalyst.
| Polymer | Mn(Da)a | Mw(Da)a | PDIa | Time |
|---|---|---|---|---|
| 14 N3-PEG2k-alkyne | 2.1k | 2.4k | 1.14 | -- |
| 15 N3-PEG4.5k-alkyne | 4.4k | 4.6k | 1.05 | -- |
| 16 Cyc-PEG2k | 1.5k | 1.8k | 1.20 | 9 × 10 min |
| 17 Cyc-PEG4.5k | 3.4k | 3.8k | 1.12 | 5 × 20 min |
GPC calibrated to PEG standards.
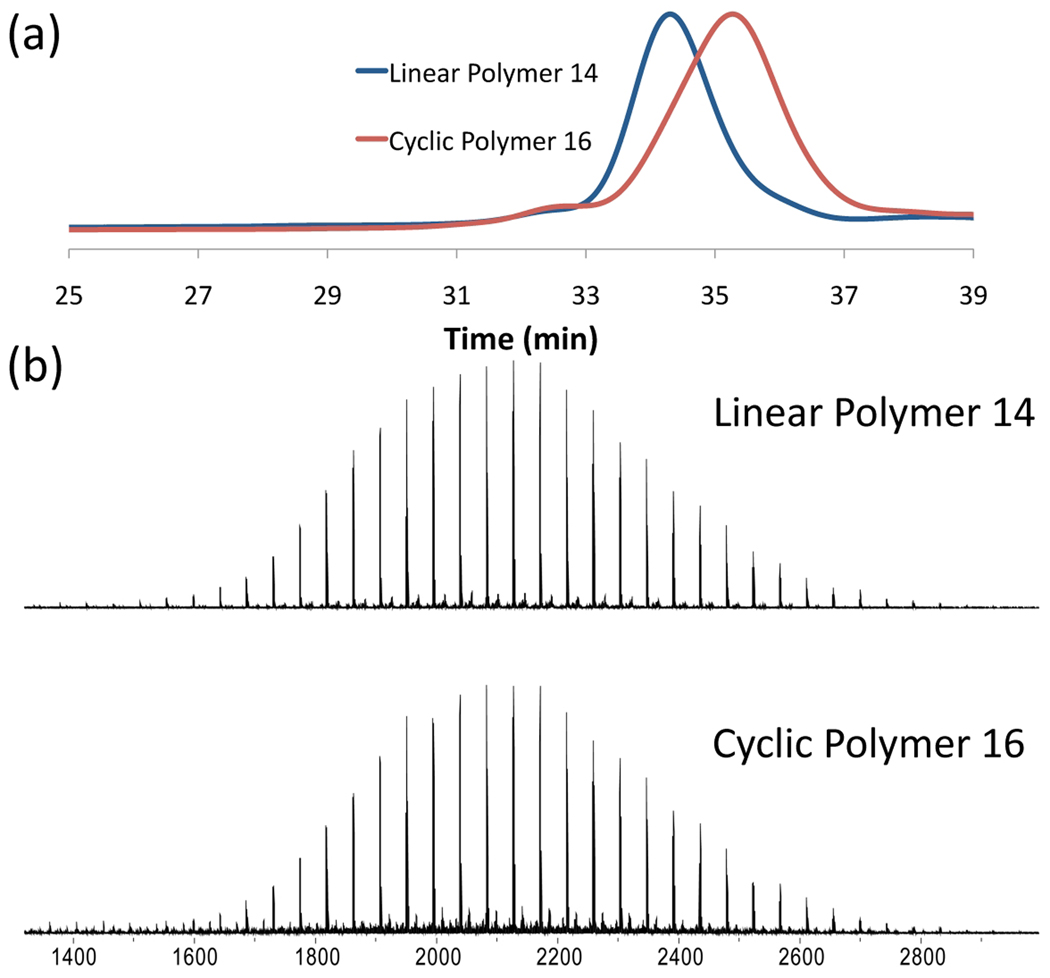
Figure 4.
Cyclization of 2 kDa PEG: (a) GPC traces, (b) full MALDI spectra showing conservation of molecular weight for the linear precursor (14) and cyclic (16) polymer.
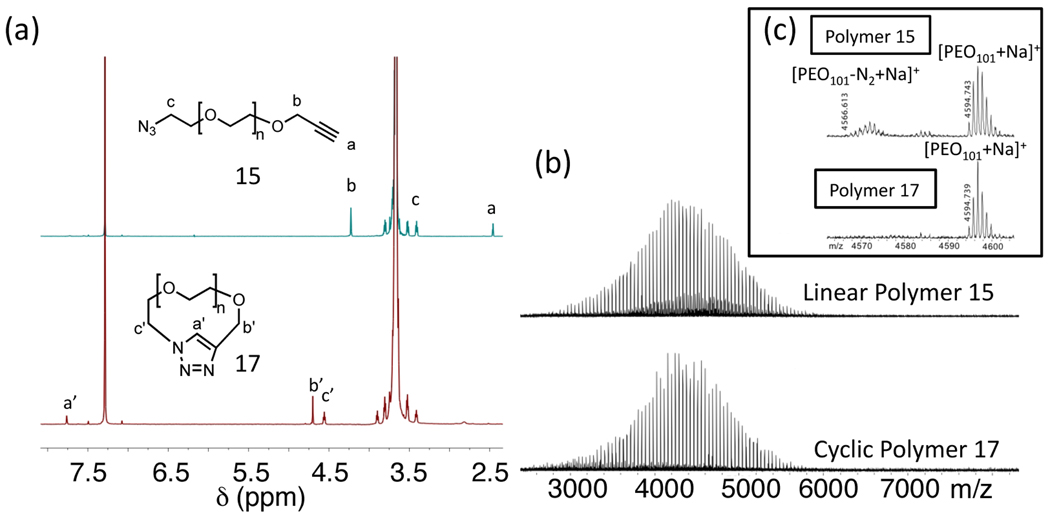
Figure 4.
Cyclization of 4.5 kDa PEG: (a) NMR spectra, (b) full MALDI and (c) zoomed-in MALDI spectra showing change in end groups, conservation of molecular weight and transformation of the azide group for linear (15) to the triazole ring of the cyclic (17) polymer.
As shown in Figure 5a, NMR spectroscopy showed the complete disappearance of resonances for the acetylene and azide end groups of the starting linear polymer and appearance of the expected triazole resonance at 7.80 ppm. Similarly, comparison of the MALDI mass spectra for the starting linear polymer with the cyclic product revealed the expected conservation of molecular weight (Figures 4b and 5b) while high resolution MALDI analysis (Figure 5c) shows loss of N2 for the linear polymer with an azide end group and only the molecular ion for the cyclic analog. Final confirmation of cyclization was obtained by comparison of the GPC elution profiles for the linear and cyclic polymers which showed the characteristic longer retention time for a cyclic polymer when compared to its linear analog (Figure 4a).23,24
Experimental
Materials
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification unless otherwise stated. Functionalized poly(ethylene glycol) (PEG) derivatives were obtained from Intezyne Technologies, (Tampa, FL, USA). PEG-alkyne,5 chloro-functional NMP initiator 4a,25 alkynyl RAFT agent26 and 4-pentynoic anhydride27 were prepared as previously reported.
Instrumentation
Polymeric materials were characterized by 1H nuclear magnetic resonance (NMR) spectroscopy using a Bruker 500 MHz spectrometer with the residual solvent signal as an internal reference. Gel permeation chromatography (GPC) was performed in DMF on a Waters system (Millford, MA, USA) equipped with four 5-mm Waters columns (300 × 7.7 mm) connected in series with increasing pore size (102, 103, 104, and 106 Å). Waters 410 differential refractometer index and Waters 996 photodiode array detectors were employed. The molecular weights of the polymers were calculated relative to linear PS standards. MALDI-TOF mass spectroscopy was conducted on a Bruker UltraFlex MALDI-TOF MS with SCOUT-MTP Ion Source (Bruker Daltonics, Bremen) equipped with a N2-laser (337nm), a gridless ion source and reflector design. All spectra were acquired using a reflector-positive method with an acceleration voltage of 25kV and a reflector voltage of 26.3kV. The detector mass range was set to 1,000–9,000 Da in order to exclude high intensity peaks from the lower mass range. The laser intensity was set to the lowest value possible to acquire high resolution spectra. The obtained spectra were analyzed with FlexAnalysis Bruker Daltonics, Bremen, version 2.2. The instrument was calibrated using SpheriCal™ calibrants. The calibrants were purchased from Polymer Factory. Infrared spectra were recorded on a Perkin Elmer Spectrum 100 with a Universal ATR sampling accessory.
PS-N3
All PS-N3 polymers (4, 5 and 6) were synthesized under similar conditions. For example, chloro-functional NMP initiator 4a25 (377 mg, 1.01 mmol) was dissolved in styrene (10.0g, 96 mmol) degassed via 3 freeze-pump-thaws and heated at 125°C for 1.5 h to 30% conversion and then precipitated into methanol to achieve PS3k-Cl (4b, 2.83g). Polymer 4b (2.44 g, 0.8 mmol) and NaN3 (230 mg, 3.5 mmol) were dissolved in 7 mL DMF and heated at 50°C overnight. The reaction was diluted with dichloromethane (DCM) and extracted with water (3×), dried over MgSO4, and precipitated in MeOH, yield 1.57 g (64%). 1H NMR (500 MHz, CDCl3) δ 7.26 – 6.30 (m, 5Ar-H), 4.31 – 4.25 (m, CH2-Cl), 3.54 – 3.50 (m, NCH(Ph)iPr), 2.25 – 0.90 (m, CH and CH2 of polymer backbone). FT-IR, ν (cm−1): 3025, 2922, 2097, 1492, 1451, 754, 695. GPC Mn 3.0k, Mw 3.9k, PDI 1.30 (PS standards).
PMMA60k-alkyne (7)
AIBN (0.82 mg, 0.005 mmol), acetylene-RAFT agent26 (16.3 mg, 0.05 mmol) and MMA (10.0 g, 100 mmol) were mixed in a schlenk flask and deoxygenated by 3 freeze-pump-thaws and heated at 70°C for 12 hr followed by precipitation into methanol to yield 5.6 g of polymer 7. 1H NMR (500 MHz, CDCl3) δ 3.62 (br, CH3O), 1.88 – 1.56 (m, CH2 of polymer backbone), 1.09 – 0.79 (m, CH3 of polymer backbone). FT-IR, ν (cm−1): 2950, 1724, 1435, 1145. GPC Mn 60.8k, Mw 78.2k, PDI 1.29 (PS standards).
PS3k-b-PEG5k (8)
All Click polymer-coupling reactions were performed under similar conditions for the times denoted in Table 1. For example, PS3k-N3 4 (405 mg, 0.135 mmol), PEG5k-alkyne 1 (450 mg, 0.090 mmol) and CuNPs (20 mg) were dissolved in 10 mL of anisole in a microwave tube, sealed and reacted in a Biotage microwave reactor for 10 min at a nominal temperature of 160°C. The Cu nanoparticles were then separated by centrifugation at 3000g for 5 min and the supernatant precipitated into cyclohexane, yield 594 mg (83%). 1H NMR (400 MHz, CDCl3) δ 7.87 – 7.63 (m, triazole-H) 7.26 – 6.27 (m, 5Ar-H), 5.46 – 5.36 (m, Ar-CH2-triazole), 4.71 – 4.60 (m, O-CH2-triazole), 3.64 (br, CH2 of PEG backbone), 3.38 (s, CH3O), 2.40 – 0.87 (m, CH and CH2 of PS backbone). FT-IR, ν (cm−1): 3025, 2884, 1492, 1452, 1342, 1279, 1241, 1104, 962, 842, 756, 696. GPC Mn 14.2k, Mw 15.4k, PDI 1.08 (PS standards).
N3-PEG2k-alkyne (14)
Commercially available Boc-NH-PEG2k-N3 (1.12 g) was deprotected in 10% (TFA/DCM) solution overnight, concentrated and precipitated in diethyl ether and dried on high vacuum to give TFA-NH2-PEG2k-N3 (1.00 g, 89%). TFA-NH2-PEG2k-N3 (372 mg) was dissolved in 2 mL DCM and 100 µL TEA was added followed by 80 mg 4-pentynoic anhydride and let stir overnight followed by 2× washes with NaHSO4 and 1× wash H2O. The organic phase was then concentrated, precipitated in ether and dried under high vacuum to give N3-PEG2k-alkyne (210 mg, 57%). 1H NMR (500 MHz, CDCl3) δ 6.55 (br, NH), 3.87 – 3.46 (br, CH2 of PEG backbone), 3.41 (t, J = 5.1 Hz, CH2N3), 2.58 – 2.53 (m, alkyne-CH2CH2-), 2.44 (t, J = 7.3 Hz, -CH2CH2CONH-), 2.04 (t, J = 1.8 Hz, alkyne-H). FT-IR, ν (cm−1): 2886, 2099, 1654, 1339, 1278, 1239, 1100, 1059, 945, 840. GPC Mn 2.1k, Mw 2.4k, PDI 1.14 (PEG standards).
N3-PEG4.5k-alkyne (15)
Starting with the acetylene-PEO derivative (476 mg) and following the reported procedure28 of mesylation and then reaction with sodium azide, 235 mg of polymer 15 was obtained (overall yield = 50%). 1H NMR (500 MHz, CDCl3) δ 4.22 (d, J = 2.4 Hz, alkyne-CH2O), 3.58 (br, CH2 of PEG backbone), 3.41 (t, J = 5.1 Hz, CH2N3), 2.46 (t, J = 2.4 Hz, alkyne-H). FT-IR, ν (cm−1): 2880, 2098, 1341, 1279, 1240, 1100, 1059, 946, 841. GPC Mn 4.4k, Mw 4.6k, PDI 1.05 (PEG standards).
Cyclic PEG2k (16)
1.0 g of CuNPs were dispersed in 10 mL of anisole in a microwave tube. 18.6 mg N3-PEG2k-alkyne (14) was dissolved in 900 µL anisole and 100 µL aliquots of this solution sequentially added to the reaction mixture followed by irradiation at 160°C for 10 min. The reaction mixture was purified by centrifugation at 5000g for 5 min to remove the CuNPs, the supernatant was then decanted and solvent was removed in vacuo. 1H NMR (500 MHz, CDCl3) δ 7.56 (s, triazole-H), 6.54 (br, NH), 4.51 (t, J = 5.2 Hz, triazole(N)-CH2), 3.90 – 3.38 (br, CH2 of PEG backbone), 3.07 (t, J = 7.4 Hz, triazole(C)-CH2), 2.63 (t, J = 7.4 Hz, -CH2CH2CONH-). FT-IR, ν (cm−1): 3445, 2868, 1651, 1348, 1297, 1249, 1101, 948, 849. GPC Mn 1.5k, Mw 1.8k, PDI 1.20 (PEG standards).
Cyclic PEG4.5k (17)
0.50 g of CuNPs were dispersed in 10 mL of anisole in a microwave tube. 4.0 mg aliquots of N3-PEG4.5k-alkyne (15) dissolved in 100 µl of anisole were added to the microwave tube for 5 consecutive microwave reactions at 160°C for 20 min (total of 20 mg). The reaction mixture was then purified by centrifugation at 5000g for 5 min and the supernatant decanted and solvent removed in vacuo. 1H NMR (500 MHz, CDCl3) δ 7.77 (s, triazole-H), 4.71 (s, triazole(C)-CH2), 4.56 (t, J = 5.1 Hz, triazole(N)-CH2), 3.93 – 3.40 (br, CH2 of PEG backbone). FT-IR, ν (cm−1): 2861, 1348, 1288, 1259, 1098, 949, 859. GPC Mn 3.4k, Mw 3.8k, PDI 1.11 (PEG standards).
Conclusion
In conclusion, we have introduced an easy and simple method for the formation of di- and tri-block copolymers, as well as cyclic polymers using Cu nanoparticles as a catalyst system coupled with microwave irradiation. Advantages of this procedure are the speed of the reaction, its tolerance to O2, and the ability to easily separate and recycle the Cu nanoparticles.
Supplementary Material
Schematic representation of the synthesis of di- and triblock copolymers via chain end coupling of azide and alkyne homopolymers in the presence of CuNPs.
Synthesis of end-functionalized homopolymers and the resultant diblock copolymer.
Schematic Representation of CuNP catalyzed cyclization of polymers.
Acknowledgements
Dr. Hunaid Nulwala from National Energy and Technology Laboratory (DoE) is thanked for helpful scientific discussions. Dr. Michael Malkoch and Dr. Per Antoni are thanked for assistance with MALDI analysis. This material is based upon work supported by the National Heart, Lung and Blood Institute of the National Institutes of Health as a Program of Excellence in Nanotechnology (HHSN268201000046C), the MRSEC Program of the National Science Foundation under Award No. DMR 05-20415 and the NSF Chemistry Program under award CHE 09-57492.
References
- 1.Kolb HC, Finn MG, Sharpless KB. Angewandte Chemie. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Quemener D, Davis TP, Barner-Kowollik C, Stenzel MH. Chem Comm. 2006;48:5051–5053. doi: 10.1039/b611224b. [DOI] [PubMed] [Google Scholar]
- 3.a) Malkoch M, Vestberg R, Gupta N, Mespouille L, Dubois P, Mason AF, Hedrick JL, Liao Q, Frank CW, Kingsbury K, Hawker CJ. Chem Comm. 2006:2774–2776. doi: 10.1039/b603438a. [DOI] [PubMed] [Google Scholar]; b) Durmaz H, Dag A, Hizal G, Tunca U. J Polym Sci Part A: Polym Chem. 2010;48:5083–5091. [Google Scholar]; c) Liu Z, Hu J, Sun J, Liu G. J Polym Sci Part A: Polym Chem. 2010;48:4922–4928. [Google Scholar]; d) Durmaz H, Dag A, Gursoy D, Demirel AL, Hizal G, Tunca U. J Polym Sci Part A: Polym Chem. 2010;48:1557–1564. [Google Scholar]; e) Schulz M, Tanner S, Barqawi H, Binder WH. J Polym Sci Part A: Polym Chem. 2010;48:671–680. [Google Scholar]
- 4.Urbani CN, Bell CA, Whittaker MR, Monteiro MJ. Macromolecules. 2008;41:1057–1060. [Google Scholar]
- 5.a) Tang CB, Sivanandan K, Stahl BC, Fredrickson GH, Kramer EJ, Hawker CJ. ACS Nano. 2010;4:285–291. doi: 10.1021/nn901330q. [DOI] [PubMed] [Google Scholar]; b) Tang CB, Lennon EM, Fredrickson GH, Kramer EJ, Hawker CJ. Science. 2008;322:429–432. doi: 10.1126/science.1162950. [DOI] [PubMed] [Google Scholar]
- 6.Ting SRS, Granville AM, Quemener D, Davis TP, Stenzel MH, Barner-Kowollik C. Aust J Chem. 2007;60:405–409. [Google Scholar]
- 7.Opsteen JA, van Hest JCM. Chem Comm. 2005:57–59. doi: 10.1039/b412930j. [DOI] [PubMed] [Google Scholar]
- 8.Durmaz H, Dag A, Altintas O, Erdogan T, Hizal G, Tunca U. Macromolecules. 2007;40:191–198. [Google Scholar]
- 9.Fournier D, Hoogenboom R, Schubert US. Chem Soc Rev. 2007;36:1369–1380. doi: 10.1039/b700809k. [DOI] [PubMed] [Google Scholar]
- 10.Iha RK, Wooley KL, Nyström AM, Burke D, Kade M, Hawker CJ. Chem Rev. 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Díaz DD, Punna S, Holzer P, McPherson AK, Sharpless KB, Fokin VV, Finn MG. J Polym Sci Part A: Polym Chem. 2004;42:4392–4403. [Google Scholar]
- 12.Molteni G, Bianchi CL, Marinoni G, Santo N, Ponti A. New J Chem. 2006;30:1137–1139. [Google Scholar]
- 13.Alonso F, Moglie Y, Radivoy G, Yus M. Tet Letters. 2009;50:2358–2362. [Google Scholar]
- 14.Lee B, Yi M, Chu SY, Lee JY, Kwon HR, Lee KR, Kang D, Kim WS, Lim HB, Lee J, Youn H-J, Chi DY, Hur NH. Chem Comm. 2010;46:3935–3937. doi: 10.1039/c001255f. [DOI] [PubMed] [Google Scholar]
- 15.Paxton WF, Spruell JM, Stoddart JF. J Am Chem Soc. 2009;131:6692–6694. doi: 10.1021/ja9015974. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs M, Goessler W, Pilger C, Kappe CO. Adv Syn And Cat. 2010;352:323–328. [Google Scholar]
- 17.Lipshutz BH, Taft BR. Ange Chem. 2006;118:8415–8418. [Google Scholar]
- 18.Sigma Aldrich, USA. Cu nanopowder, product # 684007, < 50 nm particle size (TEM).
- 19.Kato K, Uchidab E, Kangc E-T, Uyamaa Y, Ikada Y. Prog in Polym Sci. 2003;28:209–259. [Google Scholar]
- 20.Harth E, Horn BV, Lee VY, Germack DS, Gonzales CP, Miller RD, Hawker CJ. J Am Chem Soc. 2002;124:8653–8660. doi: 10.1021/ja026208x. [DOI] [PubMed] [Google Scholar]
- 21.Laurent BA, Grayson SM. J Am Chem Soc. 2006;128:4238–4239. doi: 10.1021/ja0585836. [DOI] [PubMed] [Google Scholar]
- 22.a) Lonsdale DE, Montiero MJ. [Google Scholar]; b) Lonsdale DE, Monteiro MJ. J Polym Sci Part A: Polym Chem. 2010;48:4496–4503. [Google Scholar]; c) Lonsdale DE, Monteiro MJ. Chem Comm. 2010:7945–7947. doi: 10.1039/c0cc02904a. [DOI] [PubMed] [Google Scholar]
- 23.Laurent BA, Grayson SM. Chem Soc Rev. 2009;38:2202–2213. doi: 10.1039/b809916m. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Hoskins JN, Sreerama SG, Grayson SM. Macromolecules. 2010;43:6225–6228. doi: 10.1021/ma100599n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodlert M, Harth E, Rees I, Hawker CJ. J Polym Sci Part A: Polym Chem. 2000;38:4749–4763. [Google Scholar]
- 26.O'Reilly RK, Joralemon MJ, Hawker CJ, Wooley KL. J Polym Sci Part A: Polym Chem. 2006;44:5203–5217. [Google Scholar]
- 27.a) Malkoch M, Schleicher K, Drockenmuller E, Hawker CJ, Russell TP, Wu P, Fokin VV. Macromolecules. 2005;38:3663–3678. [Google Scholar]; b) Greene AC, Grubbs RB. J Polym Sci Part A: Polym Chem. 2009;47:6342–6352. [Google Scholar]
- 28.Gao H, Matyjaszewski K. J Am Chem Soc. 2007;129:6633–6639. doi: 10.1021/ja0711617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic representation of the synthesis of di- and triblock copolymers via chain end coupling of azide and alkyne homopolymers in the presence of CuNPs.
Synthesis of end-functionalized homopolymers and the resultant diblock copolymer.
Schematic Representation of CuNP catalyzed cyclization of polymers.