Abstract
Although growth limitation of trees at Alpine and high-latitude timberlines by prevailing summer temperature is well established, loss of thermal response of radial tree growth during last decades has repeatedly been addressed. We examined long-term variability of climate-growth relationships in ring width chronologies of Stone pine (Pinus cembra L.) by means of moving response functions (MRF). The study area is situated in the timberline ecotone (c. 2000 – 2200 m a.s.l.) on Mt. Patscherkofel (Tyrol, Austria). Five site chronologies were developed within the ecotone with constant sample depth (≥ 19 trees) throughout most of the time period analysed. MRF calculated for the period 1866-1999 and 1901-1999 for c. 200 and c. 100 yr old stands, respectively, revealed that mean July temperature is the major and long-term stable driving force of Pinus cembra radial growth within the timberline ecotone. However, since the mid 1980s, radial growth in timberline and tree line chronologies strikingly diverges from the July temperature trend. This is probably a result of extreme climate events (e.g. low winter precipitation, late frost) and/or increasing drought stress on cambial activity. The latter assumption is supported by a < 10 % increase in annual increments of c. 50 yr old trees at the timberline and at the tree line in 2003 compared to 2002, when extraordinary hot and dry conditions prevailed during summer. Furthermore, especially during the second half of the 20th century, influence of climate variables on radial growth show abrupt fluctuations, which might also be a consequence of climate warming on tree physiology.
Keywords: Climate warming, moving response function, Pinus cembra, temperature sensitivity, tree ring
Introduction
It is well established that tree growth at high elevations and at northern ‘high’ latitudes is mainly limited by low temperatures throughout the growing season (Wardle 1971; Tranquillini 1979; Körner 1998; Wieser and Tausz 2007). Several dendroecological and dendroclimatological studies conducted throughout these ecotones have shown that the radial growth of trees is primarily limited by summer temperature (e.g., Eckstein and Aniol 1981; Briffa et al. 1990; Schweingruber et al. 1993; Carrer and Urbinati 2004; Oberhuber 2004; Büntgen et al. 2005; Frank and Esper 2005a). Additionally, several authors report that tree species have been facing radial growth increases during the 20th century, which can be related to climate warming (e.g., Graumlich et al. 1989; Peterson et al. 1990; Jacoby and D’Arrigo 1997; Rolland et al. 1998; Oberhuber and Kofler 2003; Bunn et al. 2005). However, long-term variability of the relationship between key climate variables and tree growth (ring width and/or maximum latewood density) was reported by several authors (for a review see D’Arrigo et al. 2007), which raise concern about accurate reconstruction of past climate, overestimation of carbon uptake and sequestration and tree line advance due to climate warming (Briffa et al. 1998a,b; Smith et al. 1999; Barber et al. 2000; Vogel and Schweingruber 2001; Wilmking et al. 2004; Wilson and Elling 2004). Recently, studies conducted by Büntgen et al. (2006a) and Carrer and Urbinati (2006) at subalpine regions in the European Alps also documented nonstationary responses of tree-ring growth of Norway spruce (Picea abies (L.) Karst.) and European larch (Larix decidua Mill.), respectively, to summer temperature. Long-term variability in growth/climate response of these widespread alpine timberline species were related to temperature-induced increase in late-summer drought stress coincidently with the recent warming trend for Norway spruce (Büntgen et al. 2006a), whereas a discrimination between covarying forcing factors of radial tree growth (i.e., temperature, precipitation, nitrogen deposition, atmospheric CO2 concentration) was not possible for European larch (Carrer and Urbinati 2006). Long-term variability in growth sensitivity to climate of subalpine Stone pine (Pinus cembra L.), which is the dominant conifer within the Central Eastern Alps at the timberline (Ellenberg 1988), have also been related to development of drought stress due to climate warming in recent decades (Oberhuber 2004). While previous work on temporal changes in climate sensitivity of Stone pine were based on evaluation of extreme radial growth (Oberhuber 2004; Pfeifer et al. 2005), this paper examines the long-term variability of the response of Stone pine at the timberline and tree line to key climate variables by means of moving response functions including a bootstrap procedure to test statistical significance, which is considered to be the most appropriate method to establish climate-growth relationships (Fritts 1976; Briffa and Cook 1990; Guiot 1991; Biondi 1997). Additionally, radial growth response to the heat-wave in 2003 (Beniston 2004) was evaluated.
Material and Methods
Study area
The sampled trees are located at Mt. Patscherkofel (2246 m a.s.l.) near Innsbruck, in western Austria (47°20′N, 11°30′E) within the timberline ecotone at north-, south- and west-facing slopes (slope angle 20 – 40 °). Timberline and tree line sites stretched from 2000 to 2080 m a.s.l. and 2110-2200 m a.s.l., respectively, whereby the timberline and tree line was defined as the upper limit of the closed forest and the upper limit of trees > 2 m high, respectively (cf. Körner 1998). At the tree line, trees were isolated in small groups and 2 – 4 m high, whereas stand height at the timberline ranged between 10 – 15 m. Mt. Patscherkofel is situated within an inner Alpine dry zone, where the local climate is strongly influenced by warm and dry southerly winds (Foehn), which most frequently occur in spring (Fliri 1975). During the period 1967-2000 mean annual precipitation at the top of Mt. Patscherkofel was 888 mm with a maximum during summer (June-August: 358 mm) and minimum in winter (December-February: 149 mm). Mean annual temperature was −0.1 °C and the coldest and warmest months were February (−4.2 °C) and July (11.4 °C), respectively.
Pinus cembra is the dominant and widespread tree species at the timberline of the Central Eastern Alps. Within the study area Norway spruce (Picea abies (L.) Karst.) and European larch (Larix decidua Mill.) are scattered at some locations. Soils are podzols (according to nomenclature of World reference base for soil resources, FAO 2006) formed on siliceous bedrock (Neuwinger 1970).
Field collection, sample preparation, chronology development and statistics
Chronology development was focused on establishing homogeneous and comparable age classes at the timberline and tree line. Therefore, more than 150 trees free of major stem or crown anomalies due to lightning, wind or snow breakage were sampled (two radii/tree). The tree species line (krummholz limit) was not included in this study. Two core samples were extracted with an increment borer at 1.3 m (timberline trees) and about 0.5 m (tree line trees) height from opposite sides of each tree. In the laboratory, they were mounted on sample holders and the surface was prepared with a sharp razor blade (Pilcher 1990). Ring widths were measured to the nearest 0.01 mm using an incremental measuring table. Tree ring series where grouped into three age classes (c. 200 yr, c. 100 yr and c. 50 yr) and those ring width series, which deviated strongly from mean age of all other trees were eliminated from further analysis. In this way, homogeneous age classes with minimal changes in variance due to changes in sample depth could be developed at the timberline and tree line (see Table 1 and Fig. 2). The correct dating of measured tree ring series was checked by using COFECHA (Holmes 1983; Grissino-Mayer 2001), which identifies segments within each ring width series that may have erroneous cross-dating or measurement errors. As independent references, P. cembra chronologies from Mt. Patscherkofel (LaMarche and Fritts 1971; Oberhuber 2004) and Ötz-valley (Siebenlist-Kerner 1984) were used.
Table 1.
Chronology-statistics of selected stands within the timberline ecotone. The chronology code is based on the site location within the timberline ecotone and on the mean tree age (TB = timberline, TR = tree line; RW = ring width, mean ± SD; SD = standard deviation; Autocorr = lag-1 autocorrelation; EPS = expressed population signal; PC1 = % variance explained by the first principal component; for details see Material and Methods).
| Site code |
n trees1 |
Age2 (years) |
RW3 (1/100 mm) |
SD3 | Autocorr3 | EPS3 | PC13 (%) |
|---|---|---|---|---|---|---|---|
| TB200 | 25 | 234±8 | 78±19 | 0.18 | 0.67 | 0.89 | 42.6 |
| TB100 | 20 | 113±8 | 158±37 | 0.22 | 0.42 | 0.88 | 53.3 |
| TB50 | 19 | 59±9 | 214±42 | 0.15 | 0.51 | 0.94 | 50.3 |
| TR100 | 20 | 115±17 | 76±21 | 0.22 | 0.71 | 0.87 | 58.1 |
| TR50 | 20 | 55±7 | 119±37 | 0.20 | 0.57 | 0.93 | 45.3 |
Each tree with two radii from opposite sides and parallel to the contourline.
Mean values±SD; cambial age at breast height (timberline) or c. 50 cm above ground (tree line).
Calculated prior to prewhitening, i.e. removing of serial autocorrelation.
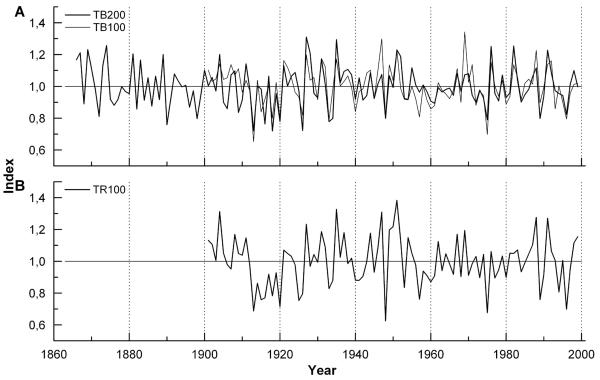
Fig. 2.
Total ring width chronologies and sample depth (= number of trees included in chronologies) of Pinus cembra stands selected at timberline and tree line. Period of divergent trend in radial growth and July temperature since the mid 1980s and annual increments in 2003 of c. 50 yr old stands are emphasized by a hatched box and filled circles, respectively.
Residual chronologies were calculated using ARSTAN (Cook and Holmes 1984; Cook 1985) with the purpose to enhance the climate signal in ring width series. First, a negative exponential curve or a linear regression line was fit to the ring series. The second step used a cubic smoothing spline with a frequency-response cut-off set at two-thirds of the length of each series. In this way most of the low-frequency variability in each ring series that is assumed to be unrelated to climate like tree aging and forest stand development (Cook 1987) was removed. Dimensionless indices were formed by dividing the observed ring width value by the predicted ring width value. This process creates stationary time series for each tree with a mean of 1 and a homogeneous variance. Residual chronologies were derived from ARMA modelling, with a robust mean value function applied to discount the effect of statistical outliers (Cook 1985; Holmes 1994). Residual chronologies are commonly used in dendroclimatic studies because removal of serial autocorrelation is required for some statistical analysis.
To permit a direct comparison of temperature and ring width series standardized indices zi were calculated by z-transformation according to the formula:
where v is the recorded temperature or ring width (after removing low-frequency variability and autocorrelation), m is the mean and std is the standard deviation of the entire series length.
Several statistics were calculated for the standardized chronologies, prior to autoregressive modelling. The standard deviation (SD) measures the variability of the measurements at all wave lengths. The first-order autocorrelation assesses relationships with previous growth (Fritts 1976). The expressed population signal (EPS) quantifies the degree to which a particular sample chronology portrays the hypothetically perfect chronology, which may in turn be regarded as the potential climate signal (Wigley et al. 1984). Though a specific range of EPS values cannot be given, Wigley et al. (1984) suggest a threshold of 0.85 as an acceptable statistical quality. Common variance was estimated by the percentage of variance explained by the first component in principal component analysis. Higher common variance indicates a greater climatic influence on tree growth (Fritts 1976; Briffa and Jones 1990).
Climate data sets
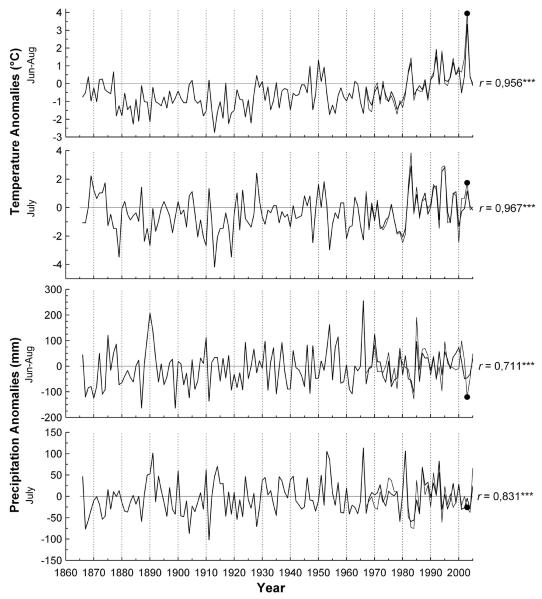
Total monthly precipitation and mean monthly temperatures were collected at a meteorological station in Innsbruck (582 m a.s.l), reaching back to 1866 (Böhm et al. 2001). Hence, climate-growth relationships and moving response functions (see below) were determined for the periods 1866-1999 and 1901-1999 for c. 200 yr and c. 100 yr old stands, respectively. Climate records gathered at the top of Mt. Patscherkofel (2246 m a.s.l.) were not used due to short length of series (first records in 1967) and our focus on analysis of long-term variability of key climate variables on radial growth, rather than on general evaluation of climate-growth relationships (see Oberhuber 2004). Pearson correlation analysis between key climate variables from both stations within the overlapping time interval (i.e. 1967-2005) revealed highly significant coefficients (Fig.1).
Fig. 1.
Time-series plots of key climate variables (anomalies relative to the 1967-2005 average). Climate data from Innsbruck (thick lines) and Mt. Patscherkofel (thin lines) reach back to 1866 and 1967, respectively. Correlation coefficients (r) between climate variables from Innsbruck and Mt. Patscherkofel are indicated (*** = p < 0.001). Climate anomalies in 2003 within the study area are marked by filled circles.
Moving response function analysis
Climate-growth relationships have been tested by the elaboration of ‘response functions’, which is a form of principal component regression designed to account for collinearity of monthly climate predictors (Fritts et al. 1971; Briffa and Cook 1990). Statistical calibrations between ring width series and monthly climate variables were tested for temporal changes by applying the software package DENDROCLIM2002 (Biondi 1997; Biondi and Waikul 2004) that computes bootstrapped response and correlation functions for single and multiple intervals. Moving response functions (MRF) are based on progressively shifting the period of a fixed number of years across time to compute the response coefficients. To provide a sufficient number of degrees of freedom, the length of the calibration period was 100 % more than the number of predictors, which was 28 in this study, i.e. only periods ≥ 56 yr were considered. Hence, MRF could not be performed for c. 50 yr old stands because of insufficient number of years. MRF produce a temporal set of coefficients for each monthly predictor, whereby statistical significance at p ≤ 0.05 was tested using a bootstrap procedure (Guiot 1991; Biondi and Waikul 2004). MRF were arbitrarily plotted against the last year of the period.
The climatic data set included mean monthly air temperature (°C) and total monthly precipitation (mm) from August of the year prior growth to September of the year of growth, i.e. 28 predictors. Coefficients must also be computed between ring indices and climate variables for several months before the growing season, because the width of an annual ring is an integration of climatically influenced processes occurring over a longer period (Fritts 1976). Response functions were determined using the residual chronologies calculated by the program ARSTAN.
Results
Chronology descriptive statistics
Five growth chronologies were developed within the timberline ecotone (Fig. 2), whereby most ring series showed an age-related exponential decrease in ring width. The influence of tree age on growth is also expressed in higher mean ring widths of younger stands. Within the same age class mean ring width was reduced by about 50 % at the tree line compared to the timberline (Table 1). High first-order autocorrelation indicates that radial growth throughout the timberline ecotone was strongly influenced by conditions in the preceding year. Principal component (PC) analyses on the individual samples from each chronology showed that the variance accounted for by PC1 ranged between 43 – 58 %. All chronologies show EPS-values, which exceed the suggested threshold of 0.85 indicating a strong climate signal in site chronologies.
Climate forcing of radial growth
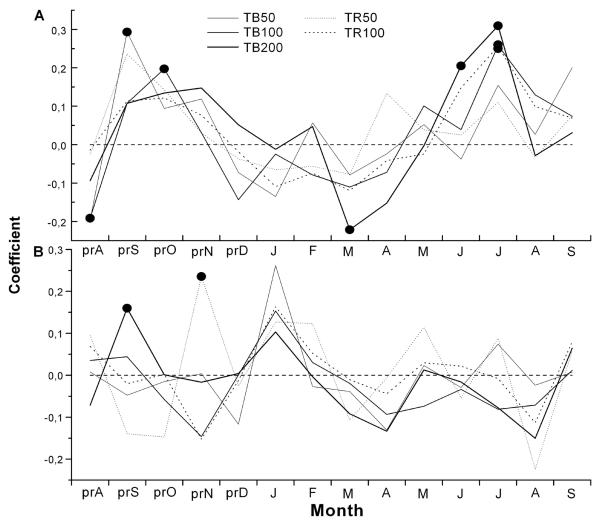
Response function coefficients depicted in Fig. 3 show that the dominant climatic factor controlling tree ring widths of selected stands was temperature in July. Above average June temperature also favoured radial growth of c. 200 yr old trees at the timberline. Furthermore, a negative temperature coefficient in previous August and current March were found for c. 100 yr and c. 200 yr timberline stands, respectively. At the timberline, significant direct relationships were also observed between precipitation in previous September and temperature in previous October.
Fig. 3.
Response function analysis between residual chronologies and monthly mean temperature (A) and monthly precipitation (B) for the periods 1866-1999 (TB200), 1901-1999 (TB100, TR100) and 1950-1999 (TB50, TR50), respectively. Filled circles indicate significant relationships at p < 0.05 (pr = previous year climate variable).
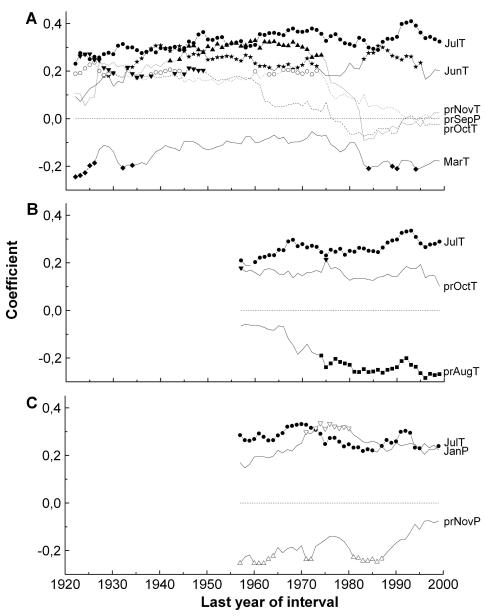
Residual ring width chronologies used for calculation of MRF are depicted in Fig. 4. The period prior to 1866 is not considered in order to match the length of the climate record used. Temporally stable and unstable key climate variables of c. 200 yr and c. 100 yr old stands were uncovered by comparing results of moving response functions (Fig. 5). The strongest and long-term stable climatic signal at the timberline and tree line was a direct response to July temperature. June temperature was also a largely temporally stable predictor of radial growth of c. 200 yr old timberline trees. Previous October temperature significantly influenced radial growth, when the calibration interval ended before 1950. Previous November temperature showed a significant influence on radial growth, which lasted almost 40 yr around the middle period of the investigated time interval. Both, mean temperature of previous October and November were not significantly related to growth over the whole period (1866-1999). Significant coefficients of previous September precipitation were scattered up to mid 1970s. When the calibration period ended after the mid 1970s, response coefficients of climate predictors related to previous autumn (temperature in October-November and September precipitation) showed a striking decrease. March temperature only sporadically showed a significant influence on radial growth.
Fig. 4.
A Timberline (TB200, TB100) and B tree line (TR100) ring width chronologies used as predictand in moving response functions.
Fig. 5.
Moving 56-yr response functions between key climate variables and residual chronologies. A TB200, B TB100, C TR100 (pr = previous year climate variable, T = mean temperature, P = precipitation).
Current mean July temperature was a significant and constant predictor of radial growth of c. 100 yr old trees at the timberline. The strength of the negative relationship between previous August temperature and the ring width series increased in recent decades and became significant when the calibration interval ended in the 1970s. In contrast, previous October temperature was only sporadically significantly related to growth. At the tree line, July and previous November temperatures and precipitation in current January showed a temporarily significant negative and direct relationship to radial growth. Regression estimates of previous November temperatures on ring width decreased strongly during recent decades. Over the whole period (1901-1999) these climate variables did not significantly influence radial growth at the tree line.
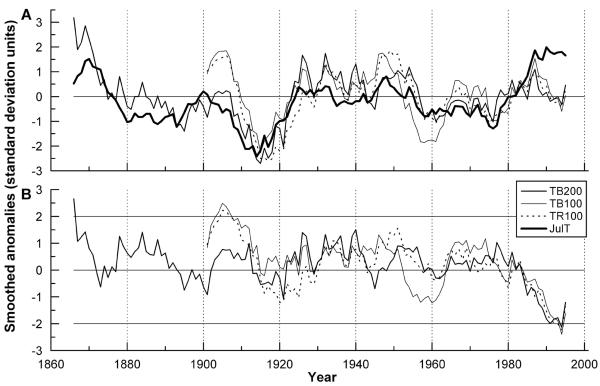
Residual ring width series of stands with mean age > 100 yr were plotted in Fig. 6A with mean July temperature after a z-transformation and after smoothing by 11-yr running averages. Low-frequency variability of temperature and radial tree growth within the timberline ecotone deviates less than one standard deviation throughout most of the time period compared. However, at the beginning of the last century and in the late 1980s, July temperature and tree growth curves strikingly diverge by > 2 standard deviation units (Fig. 6B). Annual increments of young stands (c. 50 yr) at the timberline and at the tree line show a comparable growth response during last decades (see Fig. 2).
Fig. 6.
A Smoothed residual chronologies and mean July temperature for the period 1866-1999 (TB200) and 1901-1999 (TB100, TR100), respectively. Curves represent z-transformed 11-yr running averages with equal weights (see Material and Methods). B Difference series (growth minus mean July temperature).
Discussion
Recently, Büntgen et al. (2005) reconstructed alpine summer temperatures back to ad 951 by including Pinus cembra ring width series in a multi-species Alpine tree-ring network. Applying split calibration and verification periods (1864-1933 and 1934-2002), they found no indications of a temporal shift in climate-growth relationship. Analysis of MRF of ring width series developed within the timberline ecotone of the Central Austrian Alps also revealed that radial growth response of Pinus cembra to July temperature was long-term stable throughout the last c. 150 yr. However, we detected a divergent trend in response between radial tree-growth of Pinus cembra at the timberline and at the tree line to July temperature since the mid 1980s. In addition, we miss an expected growth response to the extraordinary hot summer in 2003 (cf. Büntgen et al. 2006).
Weak growth responses in 2003 (< 10 % increase in annual increments with respect to 2002) were found in ring width chronologies of c. 50 yr old Norway spruce stands (Picea abies (L.) Karst.) from the timberline and tree line within the study area (Fig. 2) and at two Pinus cembra stands with mean age of c. 150 yr from timberline sites (c. 1950 m a.s.l.) in the Eastern Central Alps (Staller Sattel, Italy) and Dolomites (Olang, Italy; data not shown). In Siberia, Vaganov et al. (1999) found that changing climate sensitivity of high-latitude or high-altitude tree growth since the 1950s was linked to delayed snow melt due to an increasing winter precipitation trend. Other explanations suggested for the observed change in temperature sensitivity of Northern Hemisphere tree-growth are e.g. growth limiting effects of enhanced ultra violet radiation (UV-B) as a consequence of falling concentrations of stratospheric ozone Briffa et al. (2004) or temperature-induced drought stress (Barber et al. 2000; Wilmking et al. 2004; Büntgen et al. 2006a). Additionally, nonlinearity in the climate-growth response of timberline trees to climate warming has to be considered (Fritts 1976; Carrer et al. 1998; Wilmking et al. 2004; for a review see D’Arrigo et al. 2007).
Although a decreasing trend in summer precipitation is not obvious within the study area in recent decades (Wieser 2004), the divergence of radial growth and July temperature since the mid 1980s might be due to the impact of temporary drought during the growing season on radial tree growth. In addition, carry-over effects from previous year(s), e.g. due to restricted root growth and/or storage of reserve substances, may also be considered. This is further supported by significant negative relationships between previous August temperature and annual increments of c. 100 yr old trees at the timberline during last decades. Generally, leaf gas exchange studies revealed that at timberline in the Alps the growing season moisture regime is quite favourable to photosynthesis and hence regarded to be of minor importance for limiting tree growth (Tranquillini 1979; Wieser and Tausz 2007). However, Anfodillo et al. (1998) reported that (i) soils at the timberline could become physiologically dry during the growing period and (ii) that high temperatures and vapour pressure deficits have a negative effect on the radial growth of Pinus cembra. Also, soil water content and summer temperature are correlated, since temperature influences evapotranspiration. These indications suggest that temporary mild soil water stress may reduce radial growth of Pinus cembra and might be responsible for missing adequate response to temperature detected in this study.
Since radial growth of Pinus cembra stands situated within inner alpine regions is also strongly controlled by environmental conditions prevailing prior to and during winter (Oberhuber 2004), Pfeifer et al. (2005) suggested that within the study area synergistic effects of several growth limiting climate factors, e.g. cold autumn, low winter precipitation and drought during the growing season might be the trigger for radial growth depressions that are not reflected in records of July temperature. The fact that radial stem increment of Pinus cembra does not follow the trend in air temperature during some periods was also reported by Paulsen et al. (2000). One of the limitations of using monthly temperature and precipitation data in analysing climate-growth relationships is that short-term, extreme climatic events are not well represented. Stressful events such as drought, early or late frosts, and temperature fluctuations often occur over a period of only several days or weeks, but might substantially affect the physiological behaviour of trees and hence may also cause abrupt growth reductions (Innes 1994; Graumlich and Brubaker 1995; Neuwirth et al. 2003). Since the combined influence of various unfavourable weather factors is not necessarily linear, synergistic impacts of growth limiting climate variables might be responsible for decreased sensitivity to temperature since mid 1980s.
A nonlinear growth response to warming and the existence of a temperature threshold was suggested by Wilmking et al. (2004) for white spruce (Picea glauca (Moench (Voss)) at tree line in Alaska. This might also be valid for Pinus cembra, as supported by the only moderate increases in 2003 (Fig. 2), when the long-term mean summer temperature was exceeded by c. 4 °C (Fig. 1). However, July temperature in 2003 was just c. 1.5 °C above long-term mean, but is the climate factor most highly correlated to tree growth within the study area. This might also explain the weak growth response to the 2003 summer heat-wave. In addition, an increase in whole plant respiration for cellular maintenance and active transport mechanisms in response to higher temperatures might be involved (Atkin and Tjoelker 2003). On the other hand, evidence for shortening of the growing period due to an increasing trend of winter precipitation associated with delayed snow melt is not obvious from snow depths records gathered within the study area. In contrast, there are numerous studies which have demonstrated a lengthening of the growing season in Europe due to climate warming (e.g., Menzel and Fabian 1999; Walther et al. 2002; Menzel et al. 2006). Wieser (2004) reported that within the study area on Mt. Patscherkofel the growing season extended from 168 ± 12 days during the period 1972 to 1985 to 196 ± 23 days during the years 1994 to 2004.
Climate-growth response patterns denote the influence of additional climate variables besides July temperature on radial increment growth of Pinus cembra. While climate conditions during the previous year (direct influence of October temperature) have a major impact on current year radial growth by influencing carbon storage, root growth, sensitivity against winter stress, and water supply (Wardle 1971; Baig and Tranquillini 1980; Kozlowski and Pallardy 1997), current year climate conditions during winter (inverse relationship to March temperature) are related to effects of frost desiccation on tree growth (Tranquillini 1963; Hadley and Smith 1983). MRF analysis, however, revealed long-term variability of these climate variables, which is especially pronounced in the second half of the 20th century. This lack of temporal stability could primarily be due to changes in temperature and/or precipitation patterns but also due to changes in frequency and intensity of “Foehn” conditions, i.e. occurrence of strong, warm and dry southerly winds. Wind exposure of Pinus cembra is known to influence water relations and growth processes throughout the year (Grace 1977; Kronfuss and Havranek 1999).
Conclusion
Although tree-ring records developed within the timberline ecotone on Mt. Patscherkofel show a consistent relationship between annual increments of Pinus cembra and July temperature over at least the past 100 years, our data indicate that short-term climate extremes and/or the presence of temperature thresholds may have been important factors limiting radial growth in recent decades. However, since Wilson and Topham (2004), Frank and Esper (2005b) and Büntgen et al. (2006b) found no recent decrease in summer temperature sensitivity of stands located at timberlines in the Alps, the degree to which geographical and species-specific limitations apply, requires further investigation.
Acknowledgments
This work was supported by the Austrian Scientific Fonds (P14554-BOT “Variability of the growth-climate relationship of Cembra pine (Pinus cembra L.) at the alpine timberline ecotone” and P18819-B03 “Temperature dependence of Pinus cembra (L.) stem growth and respiration along an altitudinal transect”). We also thank Hypo-Tirol Bank, Innsbruck, and Swarovski, Wattens, for financial support. Climate data were provided by “Zentralanstalt für Meteorologie”, Innsbruck, which is greatly acknowledged. We also thank two anonymous reviewers for valuable suggestions on improving the manuscript.
References
- Anfodillo T, Carrer M, Rento S, Urbinati C. Long and short term growth dynamics of Picea abies (L.) Karst, Larix decidua (Mill.), Pinus cembra (L.) and climatic factors: first results of an integrated study at the timberline in eastern Italian Alps. Écologie. 1998;29:253–259. [Google Scholar]
- Atkin OK, Tjoelker MG. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science. 2003;8(7):343–351. doi: 10.1016/S1360-1385(03)00136-5. [DOI] [PubMed] [Google Scholar]
- Baig MN, Tranquillini W. The effects of wind and temperature on cuticular transpiration of Picea abies and Pinus cembra and their significance in desiccation damage at the alpine tree line. Oecologia. 1980;47:252–256. doi: 10.1007/BF00346828. [DOI] [PubMed] [Google Scholar]
- Barber VA, Juday GP, Finney BP. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature. 2000;405:668–673. doi: 10.1038/35015049. [DOI] [PubMed] [Google Scholar]
- Beniston M. The 2003 heat wave in Europe: A shape of things to come? An analysis based on Swiss climatological data and model simulations. Geophys Res Lett. 2004;31:2022–2026. [Google Scholar]
- Biondi F. Evolutionary and moving response functions in dendroclimatology. Dendrochronologia. 1997;15:139–150. [Google Scholar]
- Biondi F, Waikul K. DENDROCLIM2002: A C++ program for statistical calibration of climate signals in tree-ring chronologies. Comp Geosci. 2004;30:303–311. [Google Scholar]
- Böhm R, Auer I, Brunetti M, Maugeri M, Nanni T, Schöner W. Regional temperature variability in the European Alps: 1760-1998 from homogenized instrumental time series. Int J Climatol. 2001;21(14):1779–1801. [Google Scholar]
- Briffa KR, Cook ER. Methods of response function analysis. In: Cook ER, Kairiukstis L, editors. Methods of Dendrochronology. Kluwer Academic Publishers; Dordrecht: 1990. pp. 165–178. [Google Scholar]
- Briffa KR, Jones PD. Basic chronology statistics and assessment. In: Cook ER, Kairiukstis L, editors. Methods of Dendrochronology. Kluwer Academic Publishers; Dordrecht: 1990. pp. 137–153. [Google Scholar]
- Briffa KR, Bartholin TS, Eckstein D, Jones PD, Karlén W, Schweingruber FH, Zetterberg P. A 1,400-year tree-ring record of summer temperatures in Fennoscandia. Nature. 1990;346:434–439. [Google Scholar]
- Briffa KR, Schweingruber FH, Jones PD, Osborn TJ, Shiyatov SG, Vaganov EA. Reduced sensitivity of recent tree-growth to temperature at high northern latitudes. Nature. 1998a;391:678–682. [Google Scholar]
- Briffa KR, Schweingruber FH, Jones PD, Osborn TJ, Harris IC, Shiyatov SG, Vaganov EA, Grudd H. Trees tell of past climates: but are they speaking less clearly today. Philos Trans R Soc London B. 1998b;353:65–73. [Google Scholar]
- Briffa KR, Osborn TJ, Schweingruber FH. Large-scale temperature inferences from tree rings: a review. Global Planet Change. 2004;40:11–26. [Google Scholar]
- Bunn AG, Graumlich LJ, Urban DL. Trends in twentieth-century tree growth at high elevations in the Sierra Nevada and White Mountains, USA. The Holocene. 2005;15(4):481–488. [Google Scholar]
- Büntgen U, Esper J, Frank DC, Nicolussi K, Schmidhalter M. A 1052-year tree-ring proxy for Alpine summer temperature. Clim Dyn. 2005;25(2-3):141–153. [Google Scholar]
- Büntgen U, Frank DC, Schmidhalter M, Neuwirth B, Seifert M, Esper J. Growth/climate response shift in a long subalpine spruce chronology. Trees. 2006a;20:99–110. [Google Scholar]
- Büntgen U, Frank DC, Nievergelt D, Esper J. Summer temperature variations in the European Alps, AD 755-2004. J Clim. 2006b;19:5606–5623. [Google Scholar]
- Carrer M, Anfodillo T, Urbinati C, Carraro V. High altitude forest sensitivity to global warming: results from long-term and short-term analyses in the Eastern Italian Alps. In: Beniston M, Innes JL, editors. The impacts of climate variability on forests. Springer; Berlin, Heidelberg, New York: 1998. pp. 171–189. (Lecture notes in earth sciences, Vol 74). [Google Scholar]
- Carrer M, Urbinati C. Age-dependent tree-ring growth response to climate in Larix decidua and Pinus cembra. Ecology. 2004;85(3):730–740. [Google Scholar]
- Carrer M, Urbinati C. Long-term change in the sensitivity of tree-ring growth to climate forcing in Larix decidua. New Phytol. 2006;170:861–872. doi: 10.1111/j.1469-8137.2006.01703.x. [DOI] [PubMed] [Google Scholar]
- Cook ER, Holmes RL. Program ARSTAN User Manual. Laboratory of Tree Ring Research, University of Arizona; Tucson, USA: 1984. [Google Scholar]
- Cook ER. A time series analysis approach to tree-ring standardization. University of Arizona; Arizona: 1985. PhD thesis. [Google Scholar]
- Cook ER. The decomposition of tree-ring series for environmental studies. Tree-Ring Bull. 1987;47:37–59. [Google Scholar]
- D’Arrigo R, Wilson R, Liepert B, Cherubini P. On the ‘divergence problem’ in Northern forests: a review of the tree-ring evidence and possible causes. Global Planetary Change. 2007 (in press) [Google Scholar]
- Eckstein D, Aniol RW. Dendroclimatological reconstruction of the summer temperatures for an alpine region. Mitt Forstl Bundesvers, Wien. 1981;142:391–398. [Google Scholar]
- Ellenberg H. Vegetation ecology of Central Europe. Cambridge University Press; New York, USA: 1988. [Google Scholar]
- FAO . World reference base for soil resources. FAO; Rome: 2006. (World Soil Resources Reports 103). [Google Scholar]
- Fliri F. Das Klima der Alpen im Raume von Tirol Universitätsverlag Wagner. Innsbruck, Austria: 1975. [Google Scholar]
- Frank D, Esper J. Characterization and climate response patterns of a high-elevation, multi-species tree-ring network in the European Alps. Dendrochronologia. 2005a;22:107–121. [Google Scholar]
- Frank D, Esper J. Temperature reconstructions and comparisons with instrumental data from a tree-ring network for the European Alps. Int J Climatol. 2005b;25:1437–1454. [Google Scholar]
- Fritts HC, Blasing TJ, Hayden BP, Kutzbach JE. Multivariate techniques for specifying tree-growth and climate relationships and for reconstructing anomalies in paleoclimate. J Appl Meteor. 1971;10:845–864. [Google Scholar]
- Fritts HC. Tree rings and climate. Academic Press; London: 1976. [Google Scholar]
- Grace J. Plant response to wind. Academic Press; London: 1977. [Google Scholar]
- Graumlich LJ, Brubaker LB, Grier CC. Long-term growth trends in forest net primary productivity: Cascade Mountains, Washington. Ecology. 1989;70:405–410. [Google Scholar]
- Graumlich LJ, Brubaker LB. Long-term records of growth and distribution of conifers: Integration of paleoecology and physiological ecology. In: Smith WK, Hinckley TM, editors. Ecophysiology of coniferous forests. Academic Press; San Diego: 1995. pp. 37–62. [Google Scholar]
- Grissino-Mayer HD. Evaluating crossdating accuracy: a manual and tutorial fpr the computer program COFECHA. Tree-Ring Res. 2001;57:205–221. [Google Scholar]
- Guiot J. The bootstrapped response function. Tree-Ring Bull. 1991;51:39–41. [Google Scholar]
- Hadley JL, Smith WK. Influence of wind exposure on needle desiccation and mortality for timberline conifers in Wyoming, USA. Arct Alp Res. 1983;15:127–135. [Google Scholar]
- Holmes RL. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983;43:69–78. [Google Scholar]
- Holmes RL. Dendrochronology program library user’s manual. Laboratory of Tree-Ring Research University of Arizona; Tucson, USA: 1994. [Google Scholar]
- Innes JL. Climatic sensitivity of temperate forests. Env Poll. 1994;83:237–243. doi: 10.1016/0269-7491(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Jacoby GC, D’Arrigo RD. Tree rings, carbon dioxide, and climate change. Proc Natl Acad Sci USA. 1997;94:8350–8353. doi: 10.1073/pnas.94.16.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. A re-assessment of high elevation tree line positions and their explanation. Oecologia. 1998;115:445–459. doi: 10.1007/s004420050540. [DOI] [PubMed] [Google Scholar]
- Kozlowski TT, Pallardy SG. Physiology of woody plants. Academic Press; San Diego, California USA: 1997. [Google Scholar]
- Kronfuss H, Havranek MK. Effects of elevation and wind on the growth of Pinus cembra L in a subalpine afforestation. Phyton. 1999;39:99–106. [Google Scholar]
- LaMarche VC, Fritts HC. Tree rings, glacial advance, and climate in the Alps. Z Gletscherk Glazialgeol. 1971;7:125–131. [Google Scholar]
- Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659. [Google Scholar]
- Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kübler K, Bissolli P, Braslavska O, Briede A, Chmielewski FM, Crepinsek Z, Curnel Y, Dahl Å , Defila C, Donnelly A, Filella Y, Jatczak K, Mage F, Mestre A, Nordli Ø , Penuelas J, Pirinen P, Remisova V, Scheifinger H, Striz M, Susnik A, Van Vliet AJH, Wielgolaski F-E, Zach S, Zust A. European phenological response to climate change matches the warming pattern. Global Change Biol. 2006;12(10):1969–1976. [Google Scholar]
- Neuwinger I. Böden der subalpinen und alpinen Stufe in den Tiroler Alpen. Mitt Ostalpin-Dinarische Ges Vegetationsk. 1970;11:135–150. [Google Scholar]
- Neuwirth B, Esper J, Schweingruber FH, Winiger M. Site ecological differences to the climatic forcing of spruce pointer years from the Lötschental, Switzerland. Dendrochronologia. 2003;21:69–78. [Google Scholar]
- Oberhuber W, Kofler W. Effects of climate and slope aspect on radial growth of Cembran Pine (Pinus cembra L.) at the alpine timberline ecotone on Mt. Patscherkofel (Tyrol, Austria) Austr J For Sci. 2003;120:39–50. [Google Scholar]
- Oberhuber W. Influence of climate on radial growth of Pinus cembra within the alpine timberline ecotone. Tree Physiol. 2004;24:291–301. doi: 10.1093/treephys/24.3.291. [DOI] [PubMed] [Google Scholar]
- Paulsen J, Weber UM, Körner C. Tree growth near treeline: abrupt or gradual reduction with altitude? Arctic, Antarctic Alp Res. 2000;32:14–20. [Google Scholar]
- Peterson DL, Arbaugh MJ, Robinson LJ, Dederian BI. Growth trends of whitebark pine and lodgepole pine in a subalpine Sierra Nevada forest, California, USA. Arctic Alp Res. 1990;22:233–243. [Google Scholar]
- Pfeifer K, Kofler W, Oberhuber W. Climate related causes of distinct radial growth reductions in Pinus cembra during the last 200 yr. Veget Hist Archaeobot. 2005;14:211–220. [Google Scholar]
- Pilcher JR. Sample preparation, cross-dating and measurement. In: Cook ER, Kairiukstis L, editors. Methods of Dendrochronology. Applications in the Environmental Sciences. Kluwer Academic Publishers; Dordrecht: 1990. pp. 40–51. [Google Scholar]
- Rolland C, Petitcolas V, Michalet R. Changes in radial tree growth for Picea abies, Larix decidua, Pinus cembra and Pinus uncinata near the alpine timberline since 1750. Trees. 1998;13:40–53. [Google Scholar]
- Schweingruber FH, Briffa KR, Nogler P. A tree-ring densitometric transect from Alaska to Labrador: comparison of ring-width and maximum-latewood-density chronologies in the conifer belt of northern North America. Int J Biometeor. 1993;37:151–169. [Google Scholar]
- Siebenlist-Kerner V. Der Aufbau von Jahrringchronologien für Zirbelkiefer, Lärche und Fichte eines alpinen Hochgebirgsstandortes. Dendrochronologia. 1984;2:9–29. [Google Scholar]
- Smith KT, Cufar K, Levanic T. Temporal stability and dendroclimatology in Silver Fir and Red Spruce. Phyton. 1999;39(3):117–122. [Google Scholar]
- Tranquillini W. Climate and water relations of plants in the sub-alpine region. In: Rutter AJ, Whitehead FH, editors. The water relations of plants. Blackwell; Oxford: 1963. pp. 153–166. [Google Scholar]
- Tranquillini W. Physiological ecology of the alpine timberline. Tree existence in high altitudes with special reference to the European Alps. Springer; Berlin Heidelberg New York: 1979. (Ecological studies, Vol 31). [Google Scholar]
- Vaganov EA, Hughes MK, Kirdyanov AV, Schweingruber FH, Silkin PP. Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature. 1999;400:149–151. [Google Scholar]
- Vogel RB, Schweingruber FH. Centennial variability of tree-ring width of spruce, fir and oak in relation to climate in Switzerland for the last 450 years. Dendrochronologia. 2001;19(2):197–209. [Google Scholar]
- Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin O, Hoegh-Guldberg J-M, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Wardle P. An explanation for alpine timberline. New Zeal J Bot. 1971;9:371–402. [Google Scholar]
- Wieser G. Seasonal variation of soil respiration in a Pinus cembra forest at the upper timberline in the Central Austrian Alps. Tree Physiol. 2004;24:475–480. doi: 10.1093/treephys/24.4.475. [DOI] [PubMed] [Google Scholar]
- Wieser G, Tausz M. Trees at their upper limit. Treelife limitation at the alpine timberline. Springer; Berlin: 2007. (Series: Plant Ecophysiology). [Google Scholar]
- Wigley TM, Briffa KR, Jones PD. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J Clim Appl Meteor. 1984;23:201–213. [Google Scholar]
- Wilmking M, Juday GP, Barber VA, Zald HJ. Recent climate warming forces contrasting growth responses of white spruce at treeline in Alaska through temperature thresholds. Global Change Biol. 2004;10:1724–1736. [Google Scholar]
- Wilson R, Elling W. Temporal instability in tree-growth/climate response in the Lower Bavarian Forest region: implications for dendroclimatic reconstruction. Trees. 2004;18:19–28. [Google Scholar]
- Wilson R, Topham J. Violins and climate. Theor Appl Clim. 2004;77:9–24. [Google Scholar]